Fabrication of the Carbon Nanorods by β-Cyclodextrins with the Diathermic Oil Emulsification Process
Yu Liao, Ranran Zhang, Jun Qian*
School of Printing and Packaging, Wuhan University, Wuhan, China
*Corresponding Authors: Jun Qian, School of Printing and Packaging, Wuhan University, Wuhan 430072, China
Received: 29 July 2019; Accepted: 09 August 2019; Published: 16 August 2019
Article Information
Citation:
Yu Liao, Ranran Zhang, Jun Qian. Fabrication of the Carbon Nanorods by β-Cyclodextrins with the Diathermic Oil Emulsification Process. Journal of Nanotechnology Research 1 (2019): 088-094.
DOI: 10.26502/jnr.2688-8521007
View / Download Pdf Share at FacebookAbstract
A low-cost and environment-friendly method using β-cyclodextrins (β-CD) and diathermic oil emulsification process is applied to synthesize the carbon nanorods with a high production. The morphological and structural properties of the carbon nanorods are characterized by SEM and Raman. The chemical elemental analysis is discussed by means of FTIR and XPS. The results confirm that the nanomaterials are amorphous carbon with a diameter of approximate 100 nm. The surface of the obtained carbon nanorods are full of –OH, -C-O, -C=O functional groups, which can greatly improve the hydrophilicity and chemical reactivity of the carbon nanorods.
Keywords
<p>Carbon materials, Nanoparticles, Diathermic oil, Emulsification, Carbonization</p>
Article Details
1. Introduction
Carbon nanomaterials such as carbon nanorods, carbon nanotubes, carbon nanofibers and carbon nanosheets, hold a great technological promise for a variety of applications because of their excellent stability, outstanding electrical and mechanical characteristics, light weight etc. [1-4]. They can be applied to fuel cell [5, 6], polymeric composites [7, 8], hydrogen storage and supercapacitors [9-11]. In particularly, carbon nanorod has attracted intensive attentions with its appropriately electrical, thermal and mechanical properties, which has a potential application in electronic devices, interconnects, reinforcement materials [12-14]. However, the fabrication procedures of the carbon naorod usually involve the noble metal materials as catalyst and complicated equipments, resulting in high-cost consumption. For example, Wang et al. [15] had employed 20nm gold nanofilms as the catalyst to prepare carbon nanorods and grapheme-like nanosheets by PTHFCVD and HFCVD. Shakirullah et al. [4] reported the preparation of carbon nanorods from soot samples by heating at 800°C for 5 hours and 10 hours under nitrogen atmosphere in the presence of iron as catalyst. Yan et al. [16] reported that nitrogenated carbon nanorods catalytically were synthesized on the silicon substrate by gold films in a plasma-enhanced hot filament chemically vapor deposition system, which could be used as the large-area flat panel displays or ultrasensitive electrochemical sensors for the determination of dopamine. These procedures are complicated, and leading to high-costs and pollutions. Comparing with these synthesis routes, the diathermic oil emulsification procedure has many advantages, low pressure, simple-operation, low-cost, controllable temperature, uniform heat transfer, etc. [17].
In this work, we demonstrate a new strategy for the preparation of carbon nanorods. In this approach, diathemic oil is selected as continuous phase and an excellent heat transfer medium, which is a convenient and novel way to fabricate carbon nanomaterials with less time-consuming and a high production. The emulsions are formed by small water droplets dispersed in a continuous oil phase (water-in-oil emulsion). The emulsions are thermodynamically instable, which need to append surfactants to maintain the stability [18-21]. It is the first report to synthesize carbon nanorods using diathermic oil emulsification treatment.
2. Experimental
2.1 Preparation of diathermic oil mother liquor
The preparation of mother liquor was carried out as following procedure. Firstly, 6 g of oleic acid was added into diathermic oil (65 g) and the mixture was stirred at 60°C for 1 h. Subsequently, 1 g of Monoethanolamine was dropwised into the mixture. The emulsification of diathermic oil could be obtained with stronger stirring for 10 min. The colour of the mixture presented ivory-white.
2.2 Synthesis of carbon nanorods
The carbon nanorods were fabricated by an emulsion heating technology of diathemic oil. In a typical synthesis process, 2M HCl aqueous solution (2 ml), 1g of ?-cyclodextrins (?-CD) and 1g of amphiphilic emulsifier were dispersed in distilled water (20 ml) with a stirring procedure at 60°C for 2 h to obtain water mixture. Subsequently, the mixed water solution was added into the diathermic oil mother liquor drop by drop with high speed stirring for 5 min to achieve uniform water-in-oil emulsion. Then, the water-in-oil emulsion was transferred to a three-necked bottle with a stirring procedure and treated for 1h at 280°C by electric-heated jacket. The obtained solid product was recovered via filtration and dried at 80°C. Finally, carbonization was carried out in a tubular furnace at 700°C for 2 h with the protection of argon gas.
3. Results and Discussion
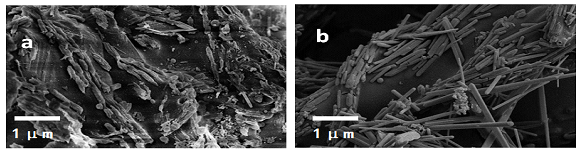
SEM images for the pre-carbonization of ?-CD and the carbon nanorods after carbonization at 700°C are presented in Figure 1 (a) and (b), respectively. It can be seen that the products from pre-carbonization via diathermic oil exhibit rod-like structures with rough surfaces and diameters ~200 nm as shown in Figure 1 (a). With the carbonization at 700°C for 2h, the carbon nanorods are formed with homogenous size and regularity. The diameters of these carbon nanorods are approximately 100nm and the surfaces become smooth as shown in Figure 1 (b).
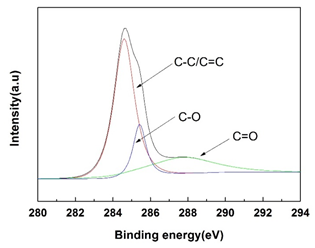
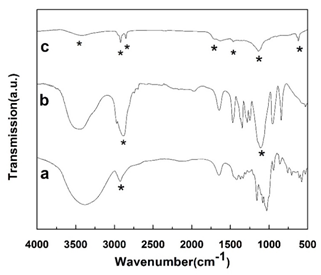
The X-ray photoelectron binding energy spectrum of C1s is given in Figure 2. The spectrum shows that the carbonaceous materials mainly consists of three kinds of carbon groups, including C-C/C=C (aliphatic or aromatic), C-O(hydroxyl or ether) and C=O(carboxyl or ester), which correspond to binding energies at 284.6 eV, 285.4 eV and 289.6 eV, respectively [22]. These functional groups are resulted from the aromatization, dehydration, and etherification, etc. The FTIR spectra in Figure 3 indicate that the surface of the obtained nanorods carbonized at 700°C rich in functional groups [23, 24] as well. A broadband at 3015-3700 cm-1 can be seen, which can be attributed to the O-H stretching vibrations of the hydroxyl or carboxyl groups. The peaks appear at 2850 cm-1 indicates the stretching vibration of aliphatic C-H functional groups. The brands of 1700 cm-1 and 1140 cm-1 suggest the C=O vibrations corresponding to carboxyl or ester and the stretching vibration of aromatic C-O-C groups, respectively. The FTIR results are consistent with that of XPS.
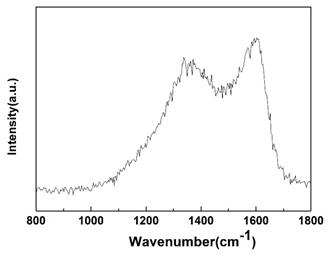
In order to confirm the structure of the synthesized carbon nanorods, Raman spectrometer is used to investigate the carbon structure. The Raman spectra of carbon nanorods carbonized at 700°C is shown in Figure 4. A distinct pair of broad band at 1594 cm-1 (G band) and 1348 cm-1 (D band) can be observed, which is a typical spectrum of carbonized materials [25]. The D band indicates the existence of defects/disordered structures and corresponds to the vibration of sp3 C-C groups. G peak demonstrates the E2g zone center mode of the crystalline graphite [26, 27] and is resulted from the vibration of sp2 C-C groups. The peak position at 1348 cm-1 (D band) is moved to high frequency compared to the diamond (1332 cm-1) because of the structural defects of microcrystal and the unsaturated carbon atoms. Moreover, the existence of oxygen-containing functional groups leads to the increased intensity of D peak. The ratio ID/IG can reflect the degree of graphitization of carbon materials. The Raman spectra reveal that the materials have numerous amorphous carbon.
4. Conclusion
Carbon nanorods have been successfully synthesized by ?-cyclodextrins with diathermic oil emulsification process.
The process is carried out in a low-cost and environment-friendly way with high yield. Diathermic oil is used as a heating medium, which has a higher heating efficiency and uniform heating performance. The obtained nanorods are about 100nm in diameter. Furthermore, the surface of these nanomaterials has plenty of functional groups, which can improve the hydrophilicity of the carbon nanorods. In a word, this paper demonstrate an effective method to fabricate the carbon nanorods with kinds of functional groups, which has promising applications in reinforced materials, and electronic materials, etc.
Acknowledgements
This work is financially supported by Hubei Science and Technology Supported Project (YJG0261).
References
- Liang F, Yanping Xie, Yueyue Wang, et al. Facile synthesis of hierarchical porous carbon nanorods for supercapacitors application. Applied Surface Science 464 (2019): 479-487.
- Manish Kumar, Maiju Hietala, Kristiina Oksman. Lignin-Based Electrospun Carbon Nanofibers. Frontiers in Materials 6 (2019): 62.
- Wang JC, Kaskel S. KOH activation of carbon-based materials for energy storage. Journal of Materials Chemistry 22 (2012): 23710-23715.
- Shakirullah M, Ahamd I, Zada N, et al. Thermocatalytic Conversion of Coal Soot to Carbon Nanorods. Fullerenes Nanotubes and Carbon Nanostructures 21 (2013): 171-182.
- Elliot Padgett, Venkata Yarlagadda, Megan E Holtz, et al. Mitigation of PEM Fuel Cell Catalyst Degradation with Porous Carbon Supports. Journal of The Electrochemical Society 166 (2019): 198-207.
- Ritikos R, Rahman SA, Gani SMA, et al. Catalyst-free formation of vertically-aligned carbon nanorods as induced by nitrogen incorporation. Carbon 49 (2011): 1842-1848.
- Xiaohui Ma, Bin Shen, Lihua Zhang, et al. Porous superhydrophobic polymer/carbon composites for lightweight and self-cleaning EMI shielding application. Composites Science and Technology 158 (2018): 86-93.
- Junwei Gu, Yongqiang Guo, Xutong Yang, et al. Synergistic improvement of thermal conductivities of polyphenylene sulfide composites filled with boron nitride hybrid fillers. Composites: Part A 95 (2017): 267-273.
- Adam Slesinski, Camelia Matei-Ghimbeu, Krzysztof Fic, et al. Self-buffered pH at carbon surfaces in aqueous supercapacitors. Carbon 129 (2018): 758-765.
- Tu CH, Wu CH, Chen CH, et al. Direct growth of hollow carbon nanorods on porous graphenic carbon film without catalysts. Carbon 84 (2015): 272-279.
- Doudou N Luta, Atanda K Raji, Optimal sizing of hybrid fuel cell-supercapacitor storage system foroff-grid renewable applications. Energy 166 (2019): 530-540.
- Yang ZX, Xia YD, Zhu YQ, et al. Self-Assembled Ultralarge Millimeter-Sized Graphitic Carbon Rods Grown on Mesoporous Silica Substrate. Chemistry of Materials 19 (2007): 6317-6322.
- Hou X, Cheng W, Chen N, et al. Preparation of a high performance carbon/ carbon composite throat insert woven with axial carbon rods. New Carbon Materials 28 (2013): 355-362.
- Yu Liao, Rui Zhang, Hongxia Wang, et al. Highly conductive carbon-based aqueous inks toward electroluminescent devices, printed capacitive sensors and flexible wearable electronics. RSC Adv 9 (2019): 15184.
- Wang BB, Ostrikov K, van der Laan T, et al. Carbon nanorods and graphene-like nanosheets by hot filament CVD: growth mechanisms and electron field emission. Journal of Materials Chemistry 1 (2013): 7703-7708.
- Yan YP, Zheng K, Wang JJ, et al. Catalytic growth mechanism and catalyst effects on electron field emission of nitrogenated carbon nanorods formed by plasma-enhanced hot filament chemical vapor deposition. Vacuum 101 (2014): 283-290.
- Colangelo G, Favale E, de Risi AD, et al. Results of experimental investigations on the heat conductivity of nanofluids based on diathermic oil for high temperature applications. Applied Energy 97 (2012): 828-833.
- Gianpiero Colangelo, Ernani Favale, Paola Miglietta, et al. Thermal conductivity, viscosity and stability of Al2O3-diathermic oil nanofluids for solar energy systems. Energy 95 (2016): 124-136.
- Qiu yan Jiang, Ning Sun, Qiuhong Li, Weimeng Si, et al. Redox-Responsive Pickering Emulsions Based on Silica Nanoparticles and Electrochemical Active Fluorescent Molecules. Langmuir 35 (2019): 5848-5854.
- Lambrich U, Schubert H. Emulsification using microporous systems. Journal of Membrane Science 257 (2005): 76-84.
- Cucheval A, Chow RCY. A study on the emulsification of oil by power ultrasound. Ultrasonics sonochemistry 15 (2008): 916-920.
- Feng SS, Li W, Wang JX, et al. Hydrothermal synthesis of ordered mesoporous carbons from a biomass-derived precursor for electrochemical capacitors. Nanoscale 6 (2014): 14657-14661.
- Wu Q, Li W, Tan J, et al. Flexible cage-like carbon spheres with ordered mesoporous structures prepared via a soft-template/hydrothermal process from carboxymethylcellulose. RSC Advances 4 (2014): 61518-61524.
- Weiwei Zhu, Huan Wang, Rong Zhao, et al. In situ fabrication of nitrogen doped porous carbon nanorods derived from metal-organic frameworks and its application as supercapacitor electrodes. Journal of Solid State Chemistry 277 (2019): 100-106.
- Yang ZC, Zhang Y, Kong JH, et al. Hollow Carbon Nanoparticles of Tunable Size and Wall Thickness by Hydrothermal Treatment of ?-Cyclodextrin Templated by F127 Block Copolymers. Chemistry of Materials 25 (2013): 704-710.
- Wang HA, Qi B, Lu BP, et al. Comparative study on the electrocatalytic activities of ordered mesoporous carbons and graphene. Electrochimica Acta 56 (2011): 3042-3048.
- Zhenglu Zhu, Haibin Zuo, Shijie Li, et al. A green electrochemical transformation of inferior coals to crystalline graphite for stable Li-ion storage. J Mater Chem A 7 (2019): 7533.

 Impact Factor: * 2.9
Impact Factor: * 2.9 Acceptance Rate: 78.36%
Acceptance Rate: 78.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 