Cellulose Ionogel Regeneration with Hexagonal Boron Nitride Nanoparticles: A Novel Approach for Reinforcing Cellulose Cryogels
Bolaji Sadiku1, Md Intaqer Arafat1, Adesewa Maselugbo1, Jeffrey Alston1,2, *
1Joint School of Nanoscience and Nanoengineering, North Carolina A & T State University, USA
2Joint School of Nanoscience and Nanoengineering, University of North Carolina, Greensboro, USA
*Corresponding author: Jeffrey Alston, Joint School of Nanoscience and Nanoengineering, North Carolina A & T State University, USA.
Received: 09 May 2023; Accepted: 16 May 2023; Published: 18 September 2023
Article Information
Citation:
Bolaji Sadiku, Md Intaqer Arafat, Adesewa Maselugbo, Jeffrey Alston. Cellulose Ionogel Regeneration with Hexagonal Boron Nitride Nanoparticles: A Novel Approach for Reinforcing Cellulose Cryogels. Journal of Nanotechnology Research. 5 (2023): 14-21.
DOI: 10.26502/jnr.2688-85210038
View / Download Pdf Share at FacebookAbstract
This study focuses on producing ionogel derived cellulose cryogel nanocomposites with hexagonal boron nitride (hBN) nanoparticles to explore improvement in thermomechanical properties. Cellulose cryogels were produced using microcrystalline cellulose dissolved in ionic liquid (1-butyl 3-methyl imidazolium chloride) to form ionogels. The hBN is dispersed within the cellulose matrix during the ionogel formation while the cellulose cryogel composite is formed in situ through regeneration, solvent exchange, and freeze-drying steps. This study investigated the effect of varying concentrations of hBN on the properties of the produced cellulose cryogel nanocomposites. The results show that adding hBN significantly improved the thermomechanical properties of the cellulose cryogels, making them more suitable for various real-world applications. This research contributes to the development of sustainable and eco-friendly cryogel composites, which have great potential in various fields such as thermal insulation, biosensors, tissue engineering, and drug delivery.
Keywords
<p>Cellulose; Nanocomposites; Ionogel; Biopolymer; Cryogel; Aerogel</p>
Article Details
1. Introduction
Low-density polymer networks like cryogels and aerogels are highly sought-after materials due to their unique combination of low-density, high porosity, and large surface areas. These properties make aerogels and cryogels ideal for a wide range of applications, including thermal insulation, biosensors, tissue engineering, and drug delivery [1]. Depending on the starting materials, aerogels and cryogels can be organic, inorganic, or hybrid [2]. An essential subset of organic-polymer low-density structures are those formed from biopolymers. Cellulosic gels were some of the first “aerogels” reported, prepared from cellulose and nitrocellulose ‘‘jellies” which were solvent exchanged and dried in supercritical propane [3]. Biopolymer-based structures are particularly attractive due to their renewability, sustainability, biocompatibility, biodegradability, and eco-friendliness, and because of the abundance of source materials biopolymers can produce various aerogel properties. An extensive review of biopolymer aerogels was compiled by Zhao, S. et al in 2018, and is an invaluable reference for biopolymer aerogel properties [4]. Cellulose is, by far, the most abundant biopolymer and has been of significant interest in aerogel research since 1931[3]. Cellulosic aerogels, including natural cellulose aerogels, regenerated cellulose aerogels, and cellulose derivative aerogels, have shown promising properties such as renewability, sustainability, biodegradability, and environmental friendliness[5]. Producing cellulose aerogels typically involves the dissolution of cellulose or its derivatives in a suitable solvent, followed by solvent exchange, gelation, and drying, all while trying to retain the 3D porous structure[5]. Early work showed that complete dissolution of the cellulose is not necessary, however the choice of drying method has a significant impact on the final properties of the aerogel[6]. Freeze-drying has been shown to produce cellulosic fibers with higher crystallinity index, crystal size, compressive modulus, and yield stress. At the same time, some evidence suggests that supercritical drying reduces these attributes for cellulose-based aerogels[7, 8]. An aerogel is formed after a super-critical drying process, whereas a cryogel is formed through freeze-drying.
Despite the promising properties of cellulose aerogels and cryogels, their structural strength is often not sufficient for many real-world applications[4]. One promising approach to improve the properties of cellulose cryogels is through reinforcement to form a composite. Nanocomposite aerogels and cryogels can be produced by combining various polymer matrices, inorganic aerogels, or aerogels with fibers, nanoparticles, or textiles[9]. Hexagonal boron nitride (hBN) is a two-dimensional, layered nanomaterial similar to graphene. It has received significant attention in the field of advanced materials due to its unique combination of mechanical, thermal, and electrical properties. hBN has a high thermal stability, high thermal conductivity, and low thermal expansion coefficient, while remaining electrically insulating with a wide bandgap (~ 5 to 6 eV), making it attractive for use as a reinforcement material in composites[10]. In recent years, hBN has been incorporated into various polymer matrix composites to enhance their properties, such as mechanical strength, thermal conductivity, and flame resistance. For example, hBN has been shown to improve the mechanical properties of polymer matrix composites significantly. Efforts to form cellulose-hBN nanocomposites have been previously demonstrated. Wu, K. et al used ball milling to functionalize hBN with hydroxyl groups and to mix the hBN with cellulose nanofibers (CNF)[11], and recently chemically functionalized lignan was used as a stabilizer to disperse hBN in a CNF dispersion[12]. These efforts have resulted in nanocomposites that show improved thermal conductivity. However, the products are not accurate demonstrations of hBN dispersed in a cellulose matrix, but rather the aggregation of CNF particles with hBN particles. In this study, we have analysed the resulting thermomechanical properties of cellulose cryogels after producing cellulose cryogel nanocomposites.
The composites formed in this study are the results of hBN dispersed in a solution of cellulose dissolved in ionic liquid. These viscous solutions are regenerated to form a cellulose polymer matrix with hexagonal boron nitride nanoparticle reinforcement. Dissolution of cellulose is achieved using 1-Butyl-3-methylimidazolium chloride (BmimCl) to swell and dissolve microcrystalline cellulose (MCC) to form a highly viscous cellulose gel in BmimCl, an ionogel. Lignocellulosic gels from ionic liquids, “ionogels”, were first studied in 2009, demonstrating a straightforward method of cellulose gel production via an ionic liquid dissolution-regeneration route. BmimCl is a non-derivatizing cellulose solvent with a relatively high solvating power (5 – 25 wt. % cellulose)[13], has good recyclability, low vapor pressure, and low cytotoxicity[14]. The hBN nanoparticles are introduced into the matrix by adding a dimethylformamide (DMF) dispersion of hBN into the ionogel. Afterward, the cellulose-hBN nanocomposite is formed through regeneration of the cellulose followed by solvent exchange and freeze-drying. The effects of varying hBN concentration in the ionogel on the properties of the produced cellulose cryogel were investigated.
Materials and Methods
Materials
Microcrystalline cellulose (MCC), and 1-Butyl-3-methylimidazolium chloride (BmimCl), 98%, were purchased from Millipore-Sigma (USA) and were used without purification and dried in a vacuum oven for at least 24 hours at 60 °C before dissolution to remove excess adsorbed water. N, N-Dimethylformamide (DMF) and methanol, also purchased at Millipore-Sigma (USA) were reagent grade (99.8%) and used as received. Ultrapure deionized water (18 MΩ) was verified by conductivity.
Dissolution of cellulose in IL
In 20 ml vials, 10 g of vacuum dried BmimCl was heated to 100 °C using heating blocks set on a hot plate. An overhead stirrer was used for continuous stirring and uniform torque. Seven units of 1% cellulose (0.1g) were added sequentially, following the absence of visible cellulose particles in the IL-cellulose solution. To ensure that all the cellulose is dissolved, solutions were stirred for an additional hour after the final 1 wt.% addition and after no visible particles were noticed. This procedure was repeated with four additional samples, and each sample was done in triplicate.
hBN dispersion in cellulose
To achieve a 5 mg/ml hBN concentration in DMF, 50 mg of hBN was dispersed in 10 ml of DMF for 5 minutes using a probe sonicator with 20 W of power. The original hBN dispersion was further diluted with DMF, resulting in dispersion concentrations of 2.5 mg/ml, 1.25 mg/ml, and 0.63 mg/ml. To each vial of cellulose-IL solution, 2.4g of varying concentrations of hBN dispersed in DMF was added. Dilution reduced the overall cellulose concentration in the cellulose/IL/hBN/DMF solution from 7wt% to 5wt.%. The temperature utilized for this phase is 40°C to prevent the evaporation of DMF and all solutions were stirred for 30mins to ensure homogeneity. The hBN/DMF concentrations used were 5mg/ml, 2.5mg/ml, 1.25mg/ml, 0.625mg/ml and 0mg/ml which produced 1.79, 0.90, 0.45, 0.23 and 0.00 wt.% hBN in cellulose respectively. Table 1 details the ionic liquid, cellulose, hBN and DMF contents of the ionogels and the weight percentage of hBN and cellulose in each regenerated sample.
|
SAMPLE |
|||||
|
1 |
2 |
3 |
4 |
5 |
|
|
Ionogel Content (g) |
|||||
|
BmimCl (g) |
10 |
10 |
10 |
10 |
10 |
|
hBN conc (g/mL) |
0.005 |
0.0025 |
0.00125 |
0.00063 |
0 |
|
Mass of hBN/DMF soln (g) |
2.4 |
2.4 |
2.4 |
2.4 |
2.4 |
|
density DMF (g/mL) |
0.94 |
0.94 |
0.94 |
0.94 |
0.94 |
|
hBN mass (g) |
0.013 |
0.0064 |
0.0032 |
0.0016 |
0 |
|
MCC cellulose (g) |
0.7 |
0.7 |
0.7 |
0.7 |
0.7 |
|
Ionogel Content (wt%) |
|||||
|
BmimCl |
76.26% |
76.26% |
76.26% |
76.26% |
76.26% |
|
cellulose |
5.34% |
5.34% |
5.34% |
5.34% |
5.34% |
|
hBN |
0.10% |
0.05% |
0.02% |
0.01% |
0.00% |
|
DMF |
18.30% |
18.30% |
18.30% |
18.30% |
18.30% |
|
Regenerated Solid (wt%) |
|||||
|
cellulose |
98.21% |
99.10% |
99.55% |
99.77% |
100.00% |
|
hBN |
1.79% |
0.90% |
0.45% |
0.23% |
0.00% |
Table 1: Ionogel content and regenerated hBN/cellulose wt.% for samples with varying hBN content
Regeneration of hBN-cellulose
Regeneration was performed in premade silicon molds of bar shapes appropriate for mechanical characterization using dynamic mechanical analyzer (DMA) and the universal testing machine. Warm cellulose/IL/hBN/DMF solutions were poured and allowed to cool in these molds. After placing the molds into larger containers, methanol was carefully poured into the larger container to submerge the entire mold. The samples were allowed to regenerate for 48 hours, then gently pushed out of the molds and into fresh methanol. The samples were left for another 48 hours before the regeneration fluid was replaced with DI water. The regeneration fluid was exchanged to DI water because water can easily be used in standard freeze-drying methods. The DI water was replaced twice at 48-hour intervals to guarantee complete methanol removal.
Drying
Labconco freezone 6 lyophilizer was used for the freeze-drying process. Before placing the samples in the lyophilizer, they were placed in conical flasks and frozen for 16 hours in a deep freezer to keep their shape when placed in liquid nitrogen. The samples were then submerged in liquid nitrogen for 5 minutes before being placed in the lyophilizer for 48 hours of drying.
Characterization
Dynamic Mechanical Analysis (DMA)
Parker’s DMA 8000 in dual cantilever mode was used for mechanical characterization of the sample. Samples were made in preformed molds with an internal dimension of 26mm×7.70mm×0.80 mm. Thermal scanning mode within the temperature range -40°C to 250°C was used to determine all samples' storage modulus and transition temperatures. Operational frequency was also varied at 1Hz, 3Hz, 5Hz, and 10Hz to analyze the effect of frequencies on cellulose cryogel properties.
Mechanical Characterization
Universal Testing Machine (INSTRON 5900R) was employed to test the stress-strain property of hBN-cellulose hybrid cryogel. The standard sample dimension was ASTM D-638 type 5 and tested at a maximum load of 500 N with a strain rate 0.002 s-1 at room temperature.
Thermogravimetric analysis
TA Q500 thermogravimetric analyzer was used to conduct thermogravimetric analysis within the temperature range of 30 to 700 °C and at a heating rate of 10°C/min. The experiments were conducted in a platinum crucible with a 50 ml/min flow rate of dry nitrogen gas. The sample sizes ranged from 10.0 to 18.0 mg. The characteristic parameters of the TGA curves were determined, i.e., the onset of degradation (Tonset) at which the sample begins to degrade, and the ash content at 400 °C.
Scanning electron microscopy (SEM).
Zeiss Auriga field-emission scanning electron microscope was used to examine the morphology of cellulose/hBN cryogels (FESEM). 5 kV was the acceleration voltage. Before the observations, all the samples were sputter coated with platinum at a thickness of 7 nm.
Results
Thermomechanical Properties
The thermomechanical parameters of cellulose cryogels containing varying amounts of hBN were studied using dynamic mechanical analysis (DMA). The storage modulus (E’), a measure of the energy required to distort a material, and the loss modulus (E”), which accounts for the viscous component of the complex modulus, were measured at temperatures ranging from -40°C to 250°C (Figure 1 a & b). The results showed that the storage modulus decreased with increasing temperature in all samples due to the loss of stiffness in the cryogels. At a low frequency of 1Hz, the rate of shear is likewise low; the material’s ability to keep its initial strength is therefore high [15]
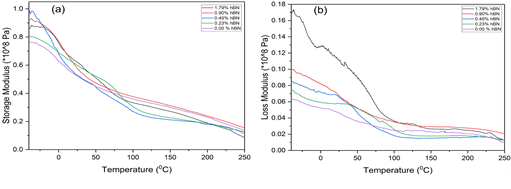
Figure 1: The thermomechanical relationship between a) Storage modulus and b) loss modulus and temperature for regenerated cellulose samples with varying hBN loading.
The loss modulus showed a significant difference in the range of -40°C to 80°C, with increased hBN loading significantly increasing the loss modulus by as much as 125% at room temperature and even higher at lower temperatures. The energy dissipation in the samples increased with increasing hBN loading from sub-zero temperatures until 80°C, but all samples showed similar loss modulus above 80°C. At room temperature (20°C), as depicted in Figure 2, both storage and loss modulus generally increased with increasing hBN loading, with the increased storage modulus indicating higher energy required to deform a hBN-loaded cellulose cryogel.
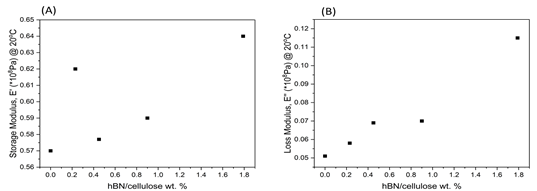
Figure 2: The relationship between hBN loading content in hBN reinforced cellulose cryogels and a) storage modulus and b) loss modulus at room temperature.
The mechanical damping, tan δ, was also studied, and the results in Figure 3 shows the presence of three of the four known cellulose glass transitions (α1, α2, and β). The increase in peak height of tan δ with hBN loading suggests increased stiffness of the hBN/cellulose hybrid cryogel.
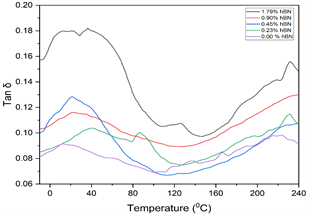
Figure 3: Mechanical damping of varying cellulose/hBN cryogel compositions with temperature.
The storage modulus increased with increasing frequency from 1 to 10 Hz for sample 1 (Figure 4), but no apparent trend was observed for loss modulus with respect to frequency. The storage modulus increased with frequencies from 1 to 10 Hz for the cellulose- 1.79% hBN cryogel but the increment from 1 to 5Hz is much stronger than from 5-10Hz. However, damping (reduction of the amplitude of free vibration (oscillation) as energy drains off from a system) is in the downward direction of all the composites. A lower reduction in damping is observed for the pure cellulose, and a steeper reduction in damping is observed for the cellulose- 1.79% hBN cryogel composite.
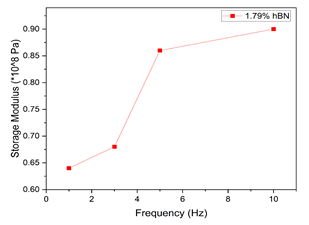
Figure 4: The relationship between frequency and storage modulus. Representation of multi-frequency scan for 1.79 wt.% hBN in cellulose cryogel
Mechanical Properties
The maximum strain at failure quantifies the degree of ductility of a material. The maximum strain at failure for all samples is shown in Figure 5. Maximum strain at failure for the samples increased as hBN loading increased and this increment was about 500% for an hBN loading differential of 1.79%.
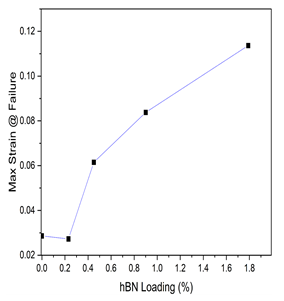
Figure 5: Maximum strain at failure for cellulose cryogels with different hBN loading.
SEM images of all samples revealed remarkably similar profiles, with the cellulose-1.79% hBN cryogel sample serving as the representative example in Figure 6. Figure 6a depicts the sample's smooth and nonporous exterior surface, while Figure 6b depicts the sample's cross-sectional view. Micropores in the samples can be attributed to the expansion of water during freezing in the freeze-drying process. Upom freezing water expands, resulting in greater crystal sizes and the enlargement of some pores at the expense of others. As seen in Figure 6b, the enlarged pores transform into huge micropores, while others collapse. Figure 6c, a magnification of Figure 6b, depicts the alignment of the cellulose layers and the huge pores associated with the samples. Large ice crystals are responsible for the compression and loss of numerous tiny hydrogel holes in the cellulose cryogel. These aligned cellulose layers may explain why freeze-dried cellulose cryogels have better mechanical properties than supercritical dried cellulose aerogels [8].

Figure 6: SEM images of a) surface view b) cross-sectional view c) zoomed cross-sectional view for freeze-dried hBN/cellulose cryogel with 1.79%hBN loading.
TGA curves for both MCC and hybrid cellulose cryogels, adjusted for water content, are displayed in Figure 7. All samples and MCC show a similar degradation onset, although the ash content at 400 °C varies significantly. The ash content of MCC at 400 °C was the lowest of any material tested. The ash content rose in tandem with the hBN loading and was as high as 34% in samples with hBN compared to that without hBN (19%).
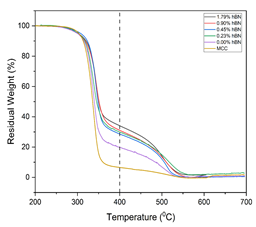
Figure 7: Thermogravimetric plot of the residual weight of hBN/cellulose cryogels with increased temperature. At the Tash (400°C) the residual weight of the cellulose cryogels was compared.
Discussion
The study's results showed that adding hBN impacted the thermomechanical properties of cellulose cryogels. The increased storage modulus at room temperature with increasing hBN loading indicated higher energy required to deform the cryogel, possibly due to stronger interactions between the hBN and cellulose components. The significant increase in loss modulus in the range of -40°C to 80°C with hBN loading suggests improved energy dissipation in the cryogel, which could have applications in damping and shock absorption. Tan δ is an excellent metric for determining phase transition temperatures in samples (from temperature values of the peaks) and for determining the degree of crosslinking of the system. In highly crystalline cellulose or the crystalline regions of cellulose, the relaxation state does not change until the melting point, which is above the thermal decomposition of cellulose. However, in the amorphous region of cellulose, these domains show numerous isophase transitions over a wide temperature range below thermal decomposition temperature. A variety of methods have shown that cellulose exhibits α1, α2, β and γ isophase transitions from as low as -93 °C to as high as 227 °C. The primary α1 glass transition temperature ranges anywhere from 217 °C to 227 °C, the secondary α2 glass transition ranges from 117 °C to 137 °C, β transition associated with small segments can be found anywhere from 7 °C to 27 °C and γ glass transition associated with the transition of hydoxymethyl groups can be found in the -93 °C to -73 °C range[16]. The presence of three of the four known cellulose glass transitions in the mechanical damping result supports previous studies on cellulose and its transitions. The increase in peak height of tan δ with hBN loading suggests increased stiffness of the hBN/cellulose hybrid cryogel, which could be due to improved crosslinking in the system. The significantly increased strain at failure at less than 2% hBN loading indicates that hBN induces ductility in the usually very fragile and brittle cellulose cryogels thus showing great potential in eliminating this challenge associated with aerogels and cryogels. The multi-frequency analysis showed increased storage modulus with frequency for 1.79% hBN loaded sample, suggesting improved energy storage ability in these cryogels. Looking into the thermal profiles of the regenerated celluloses, the presence of hBN in the cryogels shows the potential to significantly enhance the thermal degradation properties of cellulose cryogels and aerogels. With less than 2% hBN added to the first sample, we saw a significantly higher ash content as the material degrades under heat which could widen the application scope of low-density cellulose cryogels for higher temperature applications.
Conclusions
In this study, we investigated the impact of adding hexagonal boron nitride to cellulose cryogels on their thermomechanical properties. The results of dynamic mechanical analysis showed that the storage modulus increased with increasing hBN loading at room temperature, indicating a higher energy required to deform the cryogel. The loss modulus showed a significant increase within the temperature range of -40 °C to 80 °C with hBN loading, suggesting improved energy dissipation in the cryogel. The mechanical damping results showed the presence of three of the four known cellulose glass transitions and an increase in peak height of tan δ with hBN loading, indicating increased stiffness of the hBN/cellulose cryogel nanocomposite. The multi-frequency analysis showed increased storage modulus with frequency for cellulose-1.72% hBN cryogel, suggesting improved energy storage ability in these cryogels. The results of this study provide new insight into the thermomechanical properties of low-density cellulose composites and demonstrate the potential of hBN to improve these properties. The findings from this study contribute to the development of cellulose cryogels and aerogels and their potential applications in fields such as energy storage and damping. Our results indicate the possibility of creating hybrid cellulose cryogels with superior thermomechanical characteristics compared to their conventional counterparts. Future research directions could include studying the effects of different hBN loadings and investigating the chemical interactions between hBN and cellulose in cryogels and aerogels. Additionally, exploring the potential applications of these cellulose composites in fields such as energy storage and damping could also be of interest. The findings from this study contribute to the understanding of cellulose aerogels and their properties. They could lead to further developments in using these materials in various applications.
Funding
This work was performed in whole at the Joint School of Nanoscience and Nanoengineering, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (Grant ECCS-2025462)
Acknowledgments
We thank Uddin Mohammed (ORCiD: 0009-0006-7180-370X) for his assistance with the mechanical measurements of the cellulose aerogels.
Conflicts of Interest
“The authors declare no conflict of interest.”
References
- Smirnova I, P Gurikov. Aerogel production: Current status, research directions, and future opportunities. The Journal of Supercritical Fluids 134 (2018): 228-233.
- Novak BM, D Auerbach, C Verrier. Low-density, mutually interpenetrating organic-inorganic composite materials via supercritical drying techniques. Chemistry of materials 6 (1994): 282-286.
- Kistler SS. Coherent Expanded Aerogels and Jellies. Nature 127 (1931): 741-741.
- Zhao S, Malfait Wim J, Guerrero-Alburquerque Natalia, et al. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angewandte Chemie International Edition 57 (2018): 7580-7608.
- Zaman A, Huang F, Jinag M, et al. Preparation, Properties, and Applications of Natural Cellulosic Aerogels: A Review. Energy and Built Environment 1 (2020): 60-76.
- Aaltonen O, O Jauhiainen. The preparation of lignocellulosic aerogels from ionic liquid solutions. Carbohydrate Polymers 75 (2009): 125-129.
- Mo W, Knong F, Chen K, et al. Relationship between freeze-drying and supercritical drying of cellulosic fibers with different moisture contents based on pore and crystallinity measurements. Wood Science and Technology (2022): 1-16.
- Buchtova N, Pradille C, Bouvard JL, et al. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 15 (2019): 7901-7908.
- Smirnova I, P Gurikov. Aerogels in Chemical Engineering: Strategies Toward Tailor-Made Aerogels. Annu Rev Chem Biomol Eng 8 (2017): 307-334.
- Maselugbo AO, HB Harrison, JR Alston. Boron nitride nanotubes: A review of recent progress on purification methods and techniques. Journal of Materials Research (2022).
- Wu K, Liao P, Du R, et al. Preparation of a thermally conductive biodegradable cellulose nanofiber/hydroxylated boron nitride nanosheet film: the critical role of edge-hydroxylation. Journal of Materials Chemistry A 6 (2018): 11863-11873.
- Hu Z, Liu Y, Lin X, et al. Pyrene-Functionalized Alkali Lignin to Disperse Hydroxylated Boron Nitride Nanosheets in Cellulose Nanofibers for Thermal Management. ACS Applied Nano Materials 6 (2023): 200-211.
- Swatloski RP, Spear S, Holbrey JD, et al. Dissolution of Cellose with Ionic Liquids. Journal of the American Chemical Society 124 (2002): 4974-4975.
- Rogers RD, KR Seddon. Chemistry. Ionic liquids--solvents of the future? Science 302 (2003): 792-793.
- Ansari IA, Gupta G, Ramkumar J, et al., Fly ash-mixed polymeric media for abrasive flow machining process, in Handbook of Fly Ash, K.K. Kar, Editor., Butterworth-Heinemann (2022): 681-713.
- Ioelovich M. Isophase transitions of cellulose–a short review. Athens J Sci 3 (2016): 309-322.

 Impact Factor: * 2.9
Impact Factor: * 2.9 Acceptance Rate: 78.36%
Acceptance Rate: 78.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 