Effect on Sleep Quality and Daytime Functioning with O2 Vent Optima Oral Appliance and Expiratory Positive Pressure Accessory (ExVent) in Obstructive Sleep Apnea Patients
Sat Sharma MD1,2*, Antonella Conflitti CCPA1,2, Hilary Reiter DDS1, Ivan Valcarenghi DDS3, Barry Weinstein DDS4, Shideh Pejman DDS5, Brian Smith DDS6, Adam Teo DDS7, Bob Gibbons DDS8
1Centre for Sleep and Chronobiology, Toronto, Canada
2Windsor Sleep Disorders Clinic, Windsor, Canada
3Radiante Dental - Elmhurst (IL), USA
4Polo Park Dental Centre - Winnipeg (MB), Canada
5The Nimble Smile - North York (ON), Canada
6Burnham Dental - Peterborough (ON), Canada
7QLD Dental Sleep Therapy - Brisbane (QLD), Australia
8Future Dental - Brisbane (QLD), Australia
*Corresponding Author: Sat Sharma, Medical and Research Director, Centre for Sleep and Chronobiology, 301 – 295 College Street, Toronto, ON, Canada, M5T 1S2
Received: 06 March 2024; Accepted: 12 March 2024; Published: 21 March 2024
Article Information
Citation: Sat Sharma, Antonella Conflitti, Hilary Reiter, Ivan Valcarenghi, Barry Weinstein, Shideh Pejman, Brian Smith, Adam Teo, Bob Gibbons. Effect on Sleep Quality and Daytime Functioning with O2Vent Optima Oral Appliance and Expiratory Positive Pressure Accessory (ExVent) in Obstructive Sleep Apnea Patients. Dental Research and Oral Health. 7 (2024): 43-49.
View / Download Pdf Share at FacebookAbstract
Study Objectives: ExVent is an optional accessory for the O2Vent Optima mandibular advancement device that provides oral expiratory positive airway pressure (EPAP). Similar to nasal EPAP, oral EPAP results in passive upper airway dilatation and reduces flow limitation. Long-term benefits of combination therapy on sleep quality, excessive daytime sleepiness, daytime functioning and quality of bed partner’s sleep with the combination therapy require further study.
Methods: A retrospective survey was conducted of all patients who received O2Vent Optima MAD and ExVent in Canada and Australia since 2019. Data collected included: demographics, duration and frequency of use, excessive daytime sleepiness, reported snoring, sleep satisfaction, morning and daytime functioning, daytime tiredness/fatigue and bed partner’s sleep interruption.
Results: Out of 480 patients, 168 (35%) contacted and participated. 31 (18%) had stopped using oral appliance. Out of 137 (81%) subjects, 118 (86%) were still using ExVent Accessory, 108 (92%) used medium strength ExVent. Mean use duration was 2.7±0.9 years, mean use frequency – most nights (91%) and mean use >6 hours/night (86%). Daytime sleepiness improved to none/mild from moderate/severe (96%, p<0.05). Reported snoring improved to none/mild from moderate/severe (94%, p<0.05). Participants slept very well/reasonably well compared to not well/not well at all (82%, p<0.05), woke up more refreshed most mornings compared to some/rare mornings (86%, p<0.05), functioned very well/reasonably to not well/not well at all (88%, p<0.05), felt fatigue and tiredness none of the time compared to some/all the time (83%, p<0.05), bed partner’s sleep interruption was none/some of the time from all the time (95%, p<0.05).
Conclusions: Majority of the patients with mild to moderate OSA treated with O2Vent Optima and ExVent were compliant, experienced improved snoring, sleep quality, daytime functioning and uninterrupted bed partner’s sleep.
Keywords
<p>Obstructive sleep apnea; Mandibular advancement device; MAD; ExVent; Oral expiratory positive airway pressure; Sleep quality; O<sub>2</sub>Vent Optima</p>
Article Details
Introduction
Obstructive sleep apnea (OSA) is a complicated chronic condition, which has emerged as a very relevant public health issue because of its high prevalence and long-term effects such as cardiovascular, metabolic, cognitive, and cancer-related alterations [1-6]. Patients with OSA often suffer from low quality of life including unrefreshing sleep, daytime fatigue, memory loss, poor functioning and commonly affect bed partner’s sleep quality [7-9]. Various treatment options have been used to treat patients with OSA, including behavioral modifications, such as weight loss and alcohol avoidance; non-surgical interventions, such as continuous positive airway pressure (CPAP) and oral appliances (OA); and surgeries, such as uvulopalatopharyngoplasty (UPPP) and maxillomandibular advancement (MMA) [10-14].
Current first-line treatment for OSA is continuous positive airway pressure (CPAP), which is highly effective but not well tolerated. Greater than 50% of patients with OSA on CPAP therapy report the use of CPAP devices for less than half the night or not at all [15-18]. Mandibular advancement devices (MADs) protrude the mandible anteriorly to enlarge the upper airway volume and reduce pharyngeal collapsibility during sleep are better tolerated [19,20]. MAD therapy often yields significant reductions in OSA severity [21-23]; however, despite being better tolerated by patients with OSA, MAD therapy remains less than optimal for greater than 50% of patients (residual apnea–hypopnea index [AHI]>5) [24-26]. Because patients with OSA who do not respond to MAD therapy are also intolerant to CPAP therapy, treatment failure is associated with considerable health, safety, and financial costs [26,27]. Combination therapy with novel MAD O2Vent Optima and ExVent, an optional accessory that can be inserted into the O2Vent Optima MAD to provide upper airway support via oral expiratory positive airway pressure (EPAP) is promising [28-31]. Previous studies have demonstrated that the addition of an oral EPAP accessory, the ExVent, to the O2Vent Optima MAD effectively reduced respiratory events during sleep in patients with mild to moderate OSA [32,33].
Systemic objective measurements such as AHI and oxygen saturation correlate poorly with subjective symptomology of the disease [34]. Patient-centered outcomes of quality of life (QOL), daytime sleepiness, cognitive status, and performance in daily activities including work can be more important to individuals with OSA [35,36]. Overall, there exists limited long-term data for patient-centered outcomes of QOL and perceived functional changes for patients being treated for OSA with oral appliance therapy (OAT)OAT. Despite reducing sleep apneas, OAT has shown conflicting results on daytime sleepiness and quality of attributes [37,38]. In previous studies, daytime sleepiness measured subjectively and objective testing did not differ between active or placebo devices among patients with mild to moderate sleep apnea. Moreover, quality of life and functional outcomes of sleep did not improve in oral appliance group compared to placebo device group [35-37].
The objectives of present retrospective study were to evaluate the efficacy of O2Vent Optima MAD and ExVent accessory in improving quality of sleep and daytime functioning for patients with mild to moderate OSA. A real-life survey of subjective improvements in patients prescribed combination therapy with O2Vent Optima MAD and ExVent was conducted to evaluate their sleep quality, daytime functioning and bed partner’s sleep quality.
Methods
Device Overview
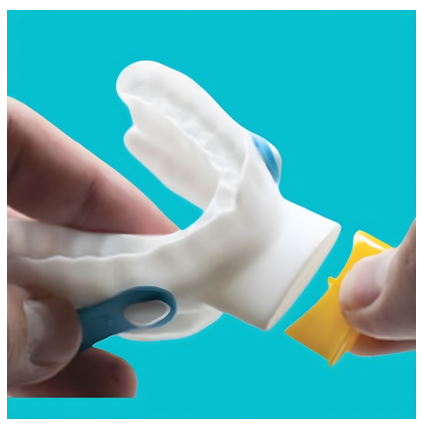
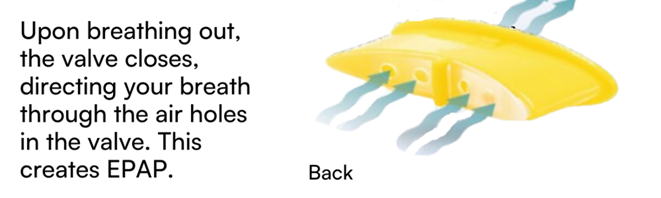
The ExVent is an oval-shaped, passive, flapper-type valve that can be inserted into the extended anterior airway inlet of the O2Vent Optima (Figure 1). When the patient is breathing through the airway, the valve fully opens during inspiration (Figure 2a) and closes upon expiration, with airflow directed through “holes” in the flapper valve, resulting in increased EPAP (Figure 2b). The ExVent is secured to the O2Vent Optima by a retention clip that allows for the easy removal of the ExVent accessory if desired. The ExVent is a single-patient, multiple-use device.
Study Design
A retrospective survey was conductedof all patients who received O2Vent Optima MAD and ExVent in Canada and Australia since 2019.Data collected included: demographics, duration and frequency of use, daytime sleepiness, reported snoring, sleep satisfaction, morning and daytime functioning, daytime tiredness/fatigue and bed partner’s sleep interruption. The questionnaire consisted of questions that required binary responses and also questions with response on a 4-point Lickert’s scale. The manufacturer’s database of approximately 4,500 patients established in 2019, identified 480 patients who had previously agreed to participate in future surveys and had their contact information available. The study coordinator obtained a verbal consent to participate and the survey was conducted as Quality Improvement Project by the respective clinical facilities. The participants were previously diagnosed with mild to moderate OSA (defined as AHI 5–29) during a Level I polysomnographic (PSG) study and were prescribed and fitted with the novel Oral appliance O2Vent Optima and ExVent. The inclusion criteria included current use of O2Vent Optima therapy with or without the ExVent accessory. The subjects were excluded from participating in the study if they did not pursue oral appliance therapy, stopped therapy or had changed therapy to another device such as CPAP. The study participants were asked questions pertaining to their snoring, sleep quality, daytime functioning, overall satisfaction with therapy and their partner’s sleep quality. The study coordinator asked the participants to recall their sleep quality prior to initiating therapy with O2Vent Optima and ExVent, and their present status. The responses were then recorded for each item of the questionnaire, tabulated and analyzed.
Statistical analysis
All data are summarized descriptively. Categorical variables are summarized as frequency and percentage, and continuous variables are summarized as the number and mean, paired-sample t-tests were used to test for significant changes across time. Means and standard deviations were analyzed and were interpreted for the t-test analyses and significance was assumed at an alpha value of 0.05.
Results
O2Vent data repository from 2019 onwards was reviewed and individuals who were prescribed O2Vent Optima and ExVent and whose contact information was available were selected. Out of 480 evaluable, 168 (35%) responded and agreed to participate in the survey. 31 (18%) stopped using oral appliance and were not included in the survey. Out of 137 (81%) survey participants, 118 (86%) were still using ExVent Accessory, 92% medium strength (yellow color). Mean duration of O2Vent Optima and ExVent use was 2.7±0.9 years, the 91% participants used the device most nights and 86% used the device for >6 hours/night (Table 1). 96% of the participants reported improvement in daytime sleepiness from moderate/severe to none/mild (p<0.05).94% of the participants reported that snoring had improved from moderate/severe to none/mild (p<0.05). 82% of the survey participants slept very well/reasonably well compared to not well/not well at all prior to using the appliance (p<0.05). The survey revealed that 86% of the respondents felt more refreshed most mornings compared to some/rare mornings (p<0.05); 88% functioned very well/reasonably to not well/not well at all (p<0.05); 83% reported fatigue and tiredness none of the time compared to some/all the time (p<0.05). Finally, 95% of the participants reported that their bed partner’s sleep interruption was none/some of the time from all the time (p<0.05) (Table 2).
|
Number of eligible patients to participate |
480 |
|
Numbers of patients could be contacted |
168 (35%) |
|
Patients who stopped using appliance |
31 (18%) |
|
Patients participated in the Survey |
137 (81%) |
|
Patients still using ExVent |
126 (92%) |
|
Duration of use (mean years) |
2.7±0.9 |
|
Mean use frequency (most nights) |
124 (91%) |
|
Average nightly use of device (>6 hrs.) |
117 (86%) |
|
ExVent accessory strength (Medium/yellow) |
126 (92%) |
|
Age (years) |
54.8±12.5 |
|
Sex (M/F) |
78/59 |
|
Previous CPAP use |
14 (10.2%) |
|
Baseline ESS |
12.6±2.1 |
|
Baseline AHI |
17.6±5.9 |
|
Baseline Severity of OSA |
|
|
Mild |
24% |
|
Moderate |
62% |
|
Severe |
14% |
|
Baseline Lowest SpO2 |
85±4.2% |
|
Mean Duration of O2Vent Optima Use |
91±3.5% |
Table 1: Participant characteristics
|
Symptom |
Baseline |
On therapy with O2Vent Optima and ExVent |
|
Number of Response out of a total of 137 |
Number of Response out of a total of 137 |
|
|
Excessive Daytime Sleepiness |
||
|
None |
0 |
84 |
|
Mild (part of the day) |
6 |
47 |
|
Moderate (most of the day) |
76 |
6 |
|
Severe (all day) |
55 |
0 |
|
Snoring reported by the bed partner or others |
||
|
None |
0 |
123 |
|
Mild (some of the night) |
6 |
9 |
|
Moderate (most of the night) |
32 |
5 |
|
Severe (all night) |
99 |
0 |
|
Most of the night, I sleep: |
||
|
Very well |
3 |
102 |
|
Reasonably well |
8 |
24 |
|
Not so well |
74 |
11 |
|
Not well at all |
53 |
0 |
|
I wake up refreshed: |
||
|
Most morning >5days/week |
5 |
117 |
|
Some mornings 3-5 days/week |
7 |
11 |
|
Rare mornings <3 days/week |
125 |
0 |
|
During the day, I function: |
||
|
Very well |
4 |
96 |
|
Reasonably well |
8 |
29 |
|
Not so well |
97 |
12 |
|
Not well at all |
25 |
0 |
|
I feel tired and fatigued during the day: |
||
|
None of the time |
4 |
113 |
|
Some of the time |
38 |
24 |
|
All the time |
95 |
0 |
|
My bed partner’s sleep is interrupted by my snoring: |
||
|
None of the time |
7 |
130 |
|
Some of the time |
46 |
7 |
|
All the time |
84 |
0 |
|
I love my Oral Appliance: |
||
|
Yes |
132 |
|
|
No |
5 |
|
Table 2: Sleep quality and daytime functioning with O2Vent Optima and ExVent therapy
Discussion
Many patients seek medical attention for obstructive sleep apnea because of snoring and daytime sleepiness. Symptoms include daytime sleepiness, poor sleep quality, headache, tiredness and fatigue. Importantly, sleep apnea patients’ bed partners also suffer from sleep interruption and poor sleep quality leading to the daytime fatigue and tiredness. Continuous positive airway pressure (CPAP) is a highly effective treatment for patients with daytime sleepiness and sleep apnea; however, adherence problems because of intrusive nature of therapy, nasal stuffiness, claustrophobia, and the risk of disturbing bed partners limit overall usefulness of this therapy. Mandibular advancement devices especially the novel oral appliance O2Vent Optima and ExVent accessory have proven efficacy as a treatment option for mild and moderate sleep apnea [30-32]. However, despite reduction in sleep apneas, the effects on daytime functioning and quality of life have not been well documented. Several previous studies evaluating symptoms resolution and QOL improvement have shown conflicting results [34-36]. Marklund et. al. measured daytime sleepiness subjectively using the Epworth Sleepiness Scale ESS and the Karolinska Sleepiness Scale (KSS), and objectively the Oxford Sleep Resistance (OSLER). The authors concluded that outcomes did not differ using active or placebo devices among patients with daytime sleepiness and snoring or mild to moderate sleep apnea [34]. Moreover, quality of life and functional outcomes of sleep were unchanged between the oral appliance group and placebo device group. Three smaller studies found no significant effect on the ESS score for an oral appliance vs a placebo device [35-37]; whereas, Gotsopoulos and colleagues, in the largest study to our knowledge, reported a small, significant reduction in a randomized, crossover study of patients with moderate sleep apnea. In this study, the proportion of patients with normal subjective sleepiness was significantly higher with the MAS than with the control device (82 versus 62%, p < 0.01), but this was not so for objective sleepiness (48 versus 34%, p = 0.08) [37]. More recently, Rangarajan et. al. demonstrated significant improvement in quality of life evaluated by the Functional Outcomes of Sleep Questionnaire (FOSQ) in patients treated with oral appliance therapy [38]. There was a mean difference of 1.8 points between the baseline scores and the scores following treatment with an oral appliance.
Our retrospective study demonstrated a significant improvement in quality of life and functional outcomes using the novel oral appliance O2Vent Optima and ExVent in patients with mild to moderate sleep apnea. O2Vent Optima and ExVent was previously shown to be effective in a first published clinical trial [32]. Relative to the baseline, significant decreases in overall AHI (p<0.001), REM AHI (p=0.001), supine AHI (p=0.007), total hypopneas (p<0.001), total apneas and hypopneas (p<0.001), and arousal index (p<0.001), NREM AHI (p<0.001) and maximum length of hypopnea (p=0.014) were observed after treatment. Relative to the baseline, a significant increase in nadir SpO2 (p<0.001) and mean SpO2 was also observed following treatment (p=0.006). This prospective study demonstrated compelling evidence that O2Vent Optima and ExVent effectively ameliorated sleep disordered breathing and sleep fragmentation. Consequently, it is plausible that therapeutic efficacy of novel combination therapy transformed into improved symptoms and daytime functioning.
The limitations of our study are that a retrospective design is prone to many biases and produces an inferior level of evidence compared to a prospective study. The inherent weaknesses of retrospective studies, such as participants may be recruited by convenience sampling thus not representative of all causing a selection bias, and also the recall bias apply to our study.
Conclusion
MAD therapy often yields significant reductions in OSA severity and are better tolerated by patients with OSA. However, MAD therapy remains less than optimal for greater than 50% of patients (residual apnea–hypopnea index [AHI]>5) and the effects on daytime sleepiness and quality of life have shown conflicting results. Previous studies have demonstrated that combination therapy with novel MAD O2Vent Optima and ExVent, an optional accessory that can be inserted into the O2Vent Optima MAD effectively reduced respiratory events during sleep in patients with mild to moderate OSA. The present retrospective study demonstrated that majority of the patients treated with the combination therapy were compliant, experienced improved snoring, sleep quality, daytime functioning and uninterrupted bed partner’s sleep. Although further prospective clinical studies remain necessary to assess the efficacy of O2Vent Optima and ExVent to assess improvement in daytime functioning and quality of life for OSA patients, the current findings appear promising.
Acknowledgments
None.
Funding details
Funding for this trial was provided by a research grant from the Centre for Sleep and Chronobiology, Toronto, Ontario, Canada
Declaration of interest
The authors report that they have no conflict of interest.
Authors contributions
S.S. conceptualised and prepared the original draft and wrote the manuscript; A.C., H.R., B.W., S.P., B.S., A.T. and B.G. performed data acquisition; S.S., A.C. and H.R. performed data analysis and interpretation; H.R. and I.V. revised the manuscript; S.S. acquired funding. All authors read and agreed to the final version of the manuscript.
Data availability
Data supporting the findings of this study are available upon request from the corresponding author [S.S.].
Ethics declarations
A verbal consent to participate in the survey was obtained and the study was conducted as Quality Improvement Project by the respective clinical facilities.
Consent to publish
The authors consent to the publication of this study, including their data.
References
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177 (2013): 1006-1014.
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328 (1993): 1230-1235.
- S Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep 38 (2015): 677-684.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 182 (2010): 269-277.
- Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med 1 (2013): 329-338.
- Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84 (2015): 1964-1971.
- Lacasse Y, Godbout C, Series F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J 19 (2002): 499-503.
- Marklund M, Stenlund H, Franklin Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest 125 (2004): 1270-1278.
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 383 (2014): 736-747.
- Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 20 (2001): Cd002875.
- Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med 186 (2012): 677-683.
- Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 29 (2006): 244-262.
- Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med 163 (2001): 1457-1461.
- Carvalho B, Hsia J, Capasso R. Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics 9 (2012): 710-716.
- Kushida CA, Morgenthaler TI, Littner MR, et al. American Academy of Sleep. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: an update for 2005. Sleep 29 (2006): 240-243.
- Weaver STE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 5 (2008): 173-178.
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 30 (2007): 711-719.
- Sawyer AM, Gooneratne NS, Marcus CL, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 15 (2011): 343-356.
- Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J 39 (2012): 1241-1247.
- Kushida CA, Morgenthaler TI, Littner MR, et al. Practice Parameters for the Treatment of Snoring and Obstructive Sleep Apnea with Oral Appliances: An Update for 2005. Sleep 29 (2006): 240-243.
- Randerath WJ, Verbraecken J, Andreas S, et al. European Respiratory Society task force on non-CPAP therapies in sleep apnoea. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J 37 (2011):1000-1028.
- Hoekema A, Stegenga B, De Bont LG. Efficacy and comorbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med 15 (2004): 137-155.
- Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 10 (2014): 215-227.
- Aarab G, Lobbezoo F, Hamburger HL, et al. Oral appliance therapy vs nasal continuous positive airway pressure in obstructive sleep apnea: a randomized, placebo-controlled trial. Respiration 81 (2011): 411-419.
- Blanco J, Zamarrón C, Abeleira Pazos MT, et al. Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath 9 (2005): 20-25.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 291 (2004): 2013-2016.
- Hillman D, Mitchell S, Streatfeild J, et al. The economic cost of inadequate sleep. Sleep 41 (2018): 8.
- Tong BK, Tran C, Ricciardiello A, et al. Efficacy of a novel oral appliance and the role of posture on nasal resistance in obstructive sleep apnea. J Clin Sleep Med 16 (2020): 483-492.
- Lavery D, Szollosi I, Czyniewski S, et al. Safety and efficacy of a novel oral appliance in the treatment of obstructive sleep apnea. J Dent Sleep Med 4 (2017): 57-63.
- Dutta R, Tong BK, Eckert DJ. Development of a physiological-based model that uses standard polysomnography and clinical data to predict oral appliance treatment outcomes in obstructive sleep apnea. J Clin Sleep Med 18 (2021): 54
- Lai V, Tong B, Tran C, et al. Combination therapy with mandibular advancement and expiratory positive airway pressure valves reduces obstructive sleep apnea severity. Sleep 42 (2019): 119.
- Sharma S, Conflitti A, Reiter H, et al. Efficacy of the ExVent Accessory for the O2Vent Optima Oral Appliance in the Treatment of Obstructive Sleep Apnea. Adv Bioeng Biomed Sci Res 6 (2023): 131-137.
- Eckert DJ, White DP, Jordan AS, et al. Defining Phenotypic Causes of Obstructive Sleep Apnea. Identification of Novel Therapeutic Targets. American Journal of Respiratory and Critical Care Medicine 188 (2013): 996-1004.
- Marklund M, CarlbergB, Forsgren L, et al. Oral appliance therapy in patients with daytime sleepiness and snoring or mild to moderate sleep apnea: a randomized clinical trial. JAMA Intern Med 175 (2015): 1278-1285.
- Doff MH. A mandibular advancement device did not affect daytime sleepiness and quality of life in obstructive sleep apnoea. Evid Based Med 20 (2015): 215-216.
- Trzepizur W, Cistulli PA, Glos M, et al. Health outcomes of continuous positive airway pressure versus mandibular advancement device for the treatment of severe obstructive sleep apnea: An individual participant data meta-analysis. Sleep 1 (2021): 63.
- Gotsopoulos H, Chen C, Qian J, et al. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med 166 (2002): 743-748.
- Rangarajan H, Padmanabhan S, Ranganathan S, et al. Impact of oral appliance therapy on quality of life (QoL) in patients with obstructive sleep apnea - a systematic review and meta-analysis. Sleep Breath 26 (2022): 983-996.



 Impact Factor: * 3.1
Impact Factor: * 3.1 Acceptance Rate: 76.66%
Acceptance Rate: 76.66%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 