Efficiency of Plasma Activated Water on Planktonic Waterborne Microorganisms Occurring in Water Supply Systems and Dental Unit Waterlines
Nahla C Droste1, Paul Leenders2, Alexander Mellmann1, Karsten Becker3, Thorsten Kuczius1*
1Institute of Hygiene, University and University Hospital Münster, Robert Koch-Straße 41, 48149 Münster, Germany 2VitalFluid BV, High Tech Campus 25-5, 5656AE Eindhoven, The Netherlands 3Friedrich Loeffler-Institute of Medical Microbiology, University Medicine Greifswald, Ferdinand-Sauerbruch-Straße 1, 17475 Greifswald, Germany
*Corresponding Author: T. Kuczius, Institute of Hygiene, University and University Hospital Münster, Robert Koch-Straße 41, 48149 Münster, Germany.
Received: 15 August 2023; Accepted: 25 August 2023; Published: 30 October 2023
Article Information
Citation: Nahla C Droste, Paul Leenders, Alexander Mellmann, Karsten Becker, Thorsten Kuczius. Efficiency of Plasma Activated Water on Planktonic Waterborne Microorganisms Occurring in Water Supply Systems and Dental Unit Waterlines. Dental Research and Oral Health. 6 (2023): 79-87.
View / Download Pdf Share at FacebookAbstract
Since high microbiological loads in water pipes of medical facilities and dental units pose a risk for human health, the establishment of bactericide agents should be advanced to minimize the contamination level. We focused on the efficacy of plasma-activated water (PAW) as a novel disinfectant against waterborne microorganisms being present in medical in-house and dental unit water-lines (DUWL), considering a dilution effect of PAW when flushing the water bearing systems. The efficiency of PAW, activated under defined conditions (90 W for 30 min) in a lab unit, was studied towards eight different waterborne species focusing on Pseudomonas aeruginosa, Acinetobacter spp. and Legionella spp. present in water-lines. PAW, presenting low pH at < 3.2 and high oxidation-reduction potential (763 mV) and conductivity (963 μS/cm) values, was applied in defined units to determine the minimum volume amount added to water for bacterial reduction. Six species failed in growth when exposed to the double PAW volume unit after 30 min incubation. A fivefold volume excess provided sufficient activity to inactivate the waterborne microorganisms while only Acinetobacter baumannii required a tenfold PAW surplus substantially reduction. Microorganisms show a species-specific susceptibility to PAW and cell count reduction strikingly correlates with added PAW volumes. PAW needs to be used in excess to achieve adequate cell reduction in aqueous environments, considering that dilution effects always accompany the disinfection. Our results indicate that PAW is a suitable disinfecting agent in a watery environment applicable for microbiological reduction in DUWLs.
Keywords
<p>Dental chair unit, Plasma-activated water, Oxidant, Disinfection, <em>Legionella</em>, <em>Pseudomonas aeruginosa</em>, <em>Acinetobacter baumannii</em>, Dental unit water lines, Water supply system</p>
Article Details
Introduction
High microbiological loads and contamination of water used in daily medical and dental applications poses a serious risk to human health. Since years, it is known that dental unit waterlines (DUWLs) are often contaminated with bacteria [1]. Most relevant microorganisms are the Gram-negative species Legionella mainly present in warm water lines [2-3], but also in DUWLs [4]. The inhalation or aspiration of Legionella-contaminated aerosols and water can cause Pontiac fever or legionellosis that can be life-threatening [5-8]. Pseudomonas aeruginosa is an opportunistic pathogen occurring mainly in cold water lines and often being present in DUWLs [9]. The organism can cause a variety of life-threatening infections such as pneumonia, bacteraemia, sepsis and wound infection [10-13]. Further potentially human-pathogenic genera were identified in DUWLs such as Sphingomonas spp. and Acinetobacter spp. [14] being partially resistant to antibiotics. Acinetobacter spp. and P. aeruginosa are both intrinsically resistant to many antibiotics, but also capable of acquiring multiple resistance mechanisms, such as b-lactamases or carbapenemases, thus, posing a serious threat of therapeutic failures and necessitating extensive hospital hygiene precautionary measures [15]. Iatrogenic infections caused by DUWL contaminations [16] showed that decontamination deserves special attention. Therefore, it is not uncommon to use additional decontamination steps bases on oxidative active disinfectants [17-18] and quaternary ammonium compounds (QACs) [19-20], which might increase stability and tolerance of the microorganisms to these agents. The application of novel techniques for efficient microbiological decontamination will be considered, whereby the use of oxidatively active substances is appropriate as a sustainable inactivation option. Plasma activated water (PAW) has gained attraction as a novel alternative that provides a biocidal effect on free and in biofilm living bacteria [21-24]. PAW is generated from interaction of atmospheric plasma with water, which leads to the formation of oxidative reactive chemicals, associated with a decrease to an acidic pH value [25-26]. Reactivity based on oxygen reactive agents such as hydrogen peroxide, atomic oxygen, superoxide and ozone [27-28] as well as on nitrogen reactive compounds as nitric oxide and peroxynitrite [29-30]. In this study, we examined the use of PAW for inactivation of various waterborne microorganisms present in DUWLs and watery environments. For this purpose, PAW had to be added to the aqueous milieu with the consequence of dilutions of PAW. We analyzed the susceptibility and tolerance of eight bacterial species out of five genera in two different physiological stages in dilutions of PAW.
Material and Methods
Culturing and identification of bacterial isolates and strains
The reference strains Acinetobacter baumannii, Legionella pneumophila, serogroup 1 and serogroup 5, respectively, L. anisa and Pseudomonas aeruginosa originated from American Type Culture Collection (ATCC) and the German Collection of Microorganisms and Cell Cultures (Leibniz Institute DSMZ, Braunschweig, Germany). The waterborne isolates A. puttii and A. junii, L. anisa, Sphingomonas paucimobilis and Stenotrophomonas maltophilia were obtained from tap water samples in dental units and households by routine analyses performed in Münster, Germany. The isolates, identified as accompanying flora, were sub-cultivated and the genus and species of the isolates were assigned by the MALDI-TOF biotyping technique (Bruker Daltonik, Bremen, Germany) according to the manufacturer´s protocol. Legionella spp. isolates and strains were cultivated resulting in colony forming units (cfu) on buffered charcoal yeast extract agar (BCYE), that was supplemented with glycine, vancomycin, polymyxin B and cycloheximide (GVPC) (Xebios, Düsseldorf, Germany), in a box under moist atmosphere at 36°C after 7 to 10 days. The other bacteria grew on R2A agar plates (Roth, Karlsruhe, Germany) at 36°C within 48h.
Adaption of microorganisms to tap water and survival duration
Though isolated from agar plates, the physiology of waterborne microorganisms that live in water of pipes was adapted to this aqueous environment. To examine this condition regarding the susceptibility to PAW, the microorganisms of Legionella spp. (L. pneumophila SG1 and L. anisa), P. aeruginosa and A. baumannii were adapted to long term survival in tap water of the Institute of Hygiene, Münster, provided by the local tap water supplier. Briefly, single colonies taken from overnight cultures of A. baumannii and P. aeruginosa were suspended in Luria Bertani broth culture medium (LB; Roth, Karlsruhe, Germany) and cultivated at 36°C under constant rotation at 180 rpm for 18-20 h. Sterile tap water was added in a nine-fold volume, and incubation continued at room temperature for 72 h under low shaking conditions (97 rpm). Microorganisms were washed three times and harvested by centrifugation steps (2.000 x g for 20 min each). Finally, cells were suspended in sterilized tap water in half of the original volume and incubation continued for experimental test duration at room temperature with constant low rotation (97 rpm) in order to avoid or reduce biofilm formation. For Legionella species, several well-grown colonies on buffered charcoal yeast extract agar (BCYE; Xebios, Düsseldorf, Germany) were inoculated directly in sterile tap water resulting in 109–1010 cells/ml and were incubated under low shaking (97 rpm) at room temperature for a prolonged time-period. Aliquots of tap water adapted bacteria were periodically analysed for cultivability by dropping the suspension in dilutions on agar plates. Colony forming units (cfus) of A. baumannii and P. aeruginosa were counted on R2A plates after incubation at 36°C for 24 and 48h. Legionella species were dropped on GVPC agar followed by cultivation under moist atmosphere at 36°C for at least 72h. The inactivation assays with PAW were carried out with water-adapted strains at ages of several weeks as indicated.
Generation and usage of plasma activated water (PAW)
Plasma-activated water (PAW) was generated from tap water (Institute of Hygiene, Münster) using a PAW lab unit (VitalFluid, Eindhoven, The Netherlands). According to manufacturer´s specifications, a volume of approximately 500-600 ml tap water was activated at an electric power level of 90 W for 30 min under rigorous stirring. During the activation process, reactive nitrogen and oxygen compounds were formed with the consequence of physical and chemical changes. The pH value and the electric conductivity were determined according to DIN EN 10523 and DIN EN ISO 27888, respectively, using a pH and conductivity meter (Xylem Analytics, Weilheim, Germany). The oxidation-reduction potential (ORP) was measured according to DIN 38404 using a redox potentiometer (Hach, Düsseldorf, Germany) and the oxidizability was detected by means of permanganate index determination according to DIN EN ISO 8467 using a dosimat system (Methrom, Filderstadt, Germany). PAW was prepared freshly for each batch and the pH was checked to a maximum value of 3.2. PAW was stored for a maximum of 24h at room temperature and prior to inactivation studies, it was tested for sufficient activity to inactivate the control reference strain E. coli in each case.
Treatment of bacteria with PAW and survival rate determination through cultivation
Single, freshly cultivated and well-visible colonies on plates of Acinetobacter, Pseudomonas, Stenotrophomonas, Sphingomonas and Legionella spp., as well as aliquots of tap water adapted bacteria were suspended in saline solution (0.85 % (w/v) NaCl; Roth, Germany) followed by adjustment to a density of 107–108 cells ml-1. For the series of tests, suspensions were diluted tenfold in sterile tap water. PAW was added to the dilutions in different volumes, and the mixtures were left to stand at room temperature for 30 min up to 24 hours as indicated. If not directly spotted in 10 µl volumes as triplicates onto plates, bacteria were previously harvested by centrifugation (8000 rpm, 5 min) followed by suspension in sterile tap water in the output volume. Survival was documented by counting the cfus after incubation at 36°C. Samples without PAW application served as controls.
Analysis of the data
All the assays were repeated to multiple times. The data presented resulted from the exemplary test approach with the worst outcome using the mean value from the triplicate plating determination. The total number was determined from the countable dilutions as arithmetic means (± standard deviation).
Results
Species specific susceptibility and stability to PAW treatment
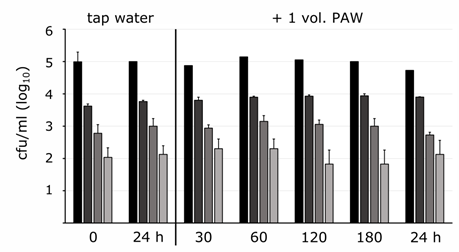
The impact of PAW was studied on waterborne microorganisms in the planktonic state that are able to contaminate dentist´s chair units and water pipes. Bacteria were exposed to oxidative effective PAW (activated at 90 W for 30 min) for a contact time of 30 min, and the survival and the robustness were determined by cultivation, as the cell-counting assay is considered gold standard for the determination of living and active cells. The addition of the equal volume of activated PAW to the initial volume of bacterial suspensions with cell densities of 107 and 106 L. pneumophila cells ml-1 was not sufficient to achieve a cell reduction. Even prolonged incubation with less cells did not result in cell count reductions, neither after 30 min nor after prolonged incubation of up to 24 hours (Figure 1).
Figure 1: Survival kinetics of L. pneumophila in tap water mixed in a 1:1 ratio with PAW. Colony forming unit concentrations (cfu/ml) of L. pneumophila serogroup 5 as tenfold dilutions in tap water, marked by columns from black to light grey, were mixed with an equal PAW volume unit (+ 1 vol. PAW) followed by contact time of 30 min up to 24 hours. Aliquots were spotted on plates and cfus were counted after incubation. Values represent the mean values from one experimental set-up in the triple approach with standard deviation of cells numbers counted and extrapolated in appropriate dilutions.
With increasing PAW volume application the cell count came down. Doubling the PAW volume caused a dominant decrease in the number of survivals (Table 1), as no colonies were detected with S. maltophilia, A. junii, S. paucimobilis and P. aeruginosa in all repeated experimental sets. However, Legionella spp. demonstrated robust properties in most assays and came down by at least 1 up to 3.4 log10 levels under these conditions as well as A. putti (2 log10 levels) and A. baumanii (0.3 log10 levels) (Table 1).
|
Strain/isolate |
Origin |
+ 2 vol. PAW* |
|
Acinetobacter baumannii |
DSM 30007 |
0.3 |
|
Acinetobacter puttii |
Environmental isolate 591 |
2 |
|
Acinetobacter junii |
Environmental isolate 412 |
Ng |
|
Legionella pneumophila SG1 |
ATCC 33152 |
2.6 |
|
Legionella pneumophila SG5 |
ATCC 33737 |
1 |
|
Legionella anisa |
DSM 17627 |
3.1 |
|
Legionella anisa |
Environmental isolate 137 |
3.4 |
|
Pseudomonas aeruginosa |
ATCC 27853 |
Ng |
|
Sphingomonas paucimobilis |
Environmental isolate 649 |
Ng |
|
Stenotrophomonas maltophilia |
Environmental isolate 650 |
Ng |
*Plasma activated water (PAW) was added in the double volume unit (+ 2 vol. PAW) to isolates and strains with an initial concentration of 6 log10 ml-1 for 30 min. The log-level reduction is given after cultivation. Ng = no growth
Table 1: Reduction of cultivatable microorganisms by adding a double PAW volume unit to the bacterial suspension
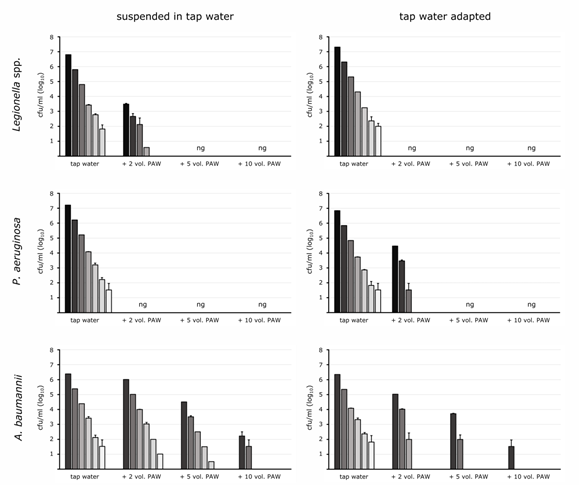
Figure 2: Inactivation of microorganisms by PAW. Tenfold dilutions of microbial species L. spp., P. aeruginosa and A. baumannii, taken from plates and suspended in tap water as well as adapted to tap water for several weeks, were subjected to PAW at double (+2 vol. PAW), fivefold (+5 vol. PAW) and tenfold (+10 vol. PAW) volumes for 30 min. Plate counting, given as cfu/ml, was carried out in triplicates (± standard deviation).
Simulating the situation of water stress, bacteria were adapted to life in tap water for several weeks in order to follow the physiological condition as planktonic microorganism regarding susceptibility and tolerance to PAW. Legionella spp., at an age of 5 weeks in tap water, showed higher susceptibility to PAW than cultured cells because the double PAW surplus was sufficient for total inactivation of both tested strains (Figure 2), proven for both, L. pneumophila and L. anisa. The waterborne pathogen P. aeruginosa was not cultivable at all with double PAW volume while tap water adapted bacteria at an age of 15 weeks showed only reductions of 2-3 log10 levels in high cell concentrations while low densities (<4 log10) were totally inactivated under these conditions (Figure 2). Complete inactivation was achieved with five-fold PAW surplus. P. aeruginosa was thus more robust after adaption to tap water than after short-term water incubation.
Interestingly, A. baumannii turned out to be highly robust to PAW treatment. Since still surviving in saline dilutions following PAW treatment (not shown), the strain showed higher robustness after suspension in tap water. The double PAW volume addition caused minimal reduction in cell numbers, and even the fivefold excess of PAW caused decrease of only 1.9 log10 levels of the individual dilutions (Figure 2). Even with a 10-fold excess of PAW, complete inactivation was not always to be expected. Even though the data of the lowest inactivation rate from the various tests are presented here, it was shown that complete inactivation could not always be expected in the case of massive contamination with A. baumannii in high concentrations under these conditions. The tap water-adapted A. baumannii, incubated in tap water for approximately 10 weeks, was slightly more susceptible to PAW treatment, but again, high excess of PAW was required for unique cell reduction (Figure 2). Cell counts decreased by 1.3 and 2.6 log10 levels after treatment with the double and fivefold PAW volume, respectively, starting from an initial concentration of 106 cells ml-1. Using the tenfold PAW volume, from initial concentrations of ≤5 log10 no bacteria were cultivable after 30 min, and cell counts from the 6 log10 dilution decreased by 4.8 log10 levels.
Physical and chemical property changes of PAW dilutions
Plasma activated water was generated from institute´s tap water whereby physical and chemical properties changed during the process (Table 2). The pH dropped down from pH 8 to acid conditions with an average value of pH 3.2 resulting as means from three water samples. When PAW was added in excess to tap water, which was the basis for the bacterial background environment, the pH values decreased according to the excess volume of PAW. The ORP in PAW of 763 mV remained quite high in the dilutions with five-fold excess to 1:1 mixture (732 to 753 mV, respectively) compared with tap water indicating only 441 mV. The electrical conductivity in PAW was almost twice as high as in tap water and ranged from 600 to 687 µS/cm in the dilutions. Interestingly, the oxidizability (O2 in mg/l) values increased to > 6 mg/l O2 in the dilutions compared with the initial solutions.
|
parameter |
PAW |
tw |
tw |
tw |
tw |
|
+ 1 x PAW |
+ 2 x PAW |
+ 5 x PAW |
|||
|
pH |
3.22 ± 0.23 |
8.03 ± 0.04 |
5.62 ± 1.44 |
4.29 ± 0.92 |
3.87 ± 0.39 |
|
conductivity (µS/cm) |
963 ± 256 |
511 ± 3 |
600 ± 45 |
687 ± 143 |
680 ± 56 |
|
oxidation-reduction potential (mV) |
763 ± 9 |
441 ± 2 |
753 ± 14 |
745 ± 9 |
732 ± 14 |
|
oxidizability (O2 in mg/l) |
4.12 ± 1.1 |
1.87 ± 0.1 |
6.83 ± 1.0 |
6.31 ± 0.6 |
6.21 ± 1.5 |
Plasma activated water (PAW) was mixed with tap water (tw) in excess at given volume ratios
Table 2: Physical and chemical parameters of PAW in dilutions with tap water
Discussion
In medical facilities and dental practices, a good water quality is a prerequisite for health protection of patients and staff, who are frequently exposed to water and aerosols. Generally, water is colonized with microorganisms composed mostly of non-pathogenic but may also have pathogenic potential [31,32]. In this study, we investigated the use of plasma-activated water (PAW) generated from tap water for reduction and inactivation of waterborne bacteria in watery environmental systems. The antimicrobial efficacy of PAW has been evidenced for several microorganisms, yeasts, spores and viruses [33-37], at which the organisms were directly in contact with PAW. Here, the PAW efficacy was analyzed in a watery environment including bacteria by adding defined PAW volume units with the aim to include dilution effects through the matrix. We analysed the minimal addition of PAW to microorganisms, firstly, to achieve a high antimicrobial effectiveness and secondly, to avoid premature damages of the pipes and hoses caused by the oxidative substances.
Physical and chemical parameters in plasma activated water
During the plasma activation process many reactive oxygen and reactive nitrogen species arise accompanied with physicochemical changes [38]. The pH dropped down and at the same time the electrical conductivity, the oxidation-reduction potential (ORP) and the oxidizability rose. The parameter ORP, directly related to the pH value but with inverse correlation [39], correlated positively to inactivation ability. High ORP values are associated with damages of inner and outer membrane integrities of microorganisms [40]. The high conductivity value resulted from the generation of charged species and ions in the water [38]. The compositions and the quantity of reactive substances are not uniform and change over time; however, the pH remained acid [41]. The use of plasma discharges and sources [42], the choice of storage conditions [29] and the water chemistry, especially hardness [35], are parameters that can reduce the antimicrobial effect of PAW. However, a decrease in biological inactivation efficacy was not observed in our hands, even with storage of freshly generated PAW for several hours, which has the advantage of longer usage. However, chemical parameters may be changed and short half-life molecules might disappear.
Plasma activated water has an antimicrobial effect on waterborne microorganisms
The antimicrobial effect of PAW was analyzed on recurrent microorganisms of five different genera, Legionella, Pseudomonas, Acinetobacter, Stenotrophomonas, Sphingomonas, and eight species. These bacteria are primarily colonists of waterlines and able to survive, to grow and to persist in the aqueous environment [43]. Waterborne microorganisms can attach to surfaces and form biofilms [44-46] and are characterized by high tolerances to disinfectants and antibiotics as shown for P. aeruginosa and A. baumannii, which are expressing many efflux pumps [47,48]. We focused on these Gram-negative bacteria because, in general, the majority of well-known waterborne bacteria belong to this group. A double membrane, that is an inner and an outer membrane, provides the microorganisms different properties. Nevertheless, it should be emphazised that in this context, it is known that Gram-positive bacteria generally demonstrate higher tolerance to PAW treatment than Gram-negatives [39]. A 1:1 ratio dilution of the bacterial suspension with PAW resulted in no count reduction, not even after a longer incubation period. However, doubling the excess PAW volume led to total inactivation of several microorganisms as P. aeruginosa, S. paucimobilis and S. maltophilia. Interestingly, species of the genus Acinetobacter varied in susceptibility and stability. A. junii came completely down while A. puttii only reduced by 2 log10 levels and no reduction occurred with A. baumannii. Such a species-specific difference was not recognized for the genus of Legionella with L. anisa and L. pneumophila. The four different strains and isolates showed similar stabilities to double PAW volume. Our results clearly prove various but genus- and species-specific stabilities and sensitivities to PAW treatment, which means that a selection of several and specific bacteria is necessary to analyze an efficient disinfection process. Changes of environmental conditions may lead to altered surface properties with protein and lipid syntheses and expressions due to the adaptation of the bacteriological physiology. Bacteria exposed to water stress for a prolonged period changed their physiological nature compared to sub-cultured microorganisms from plates [49]. Our results gave a differentiated picture of the bacteria with regard to PAW stability. Tap water adapted P. aeruginosa reduced less compared with sub-cultured bacteria, while both Legionella species, L. anisa and L. pneumophila, exposed to tap water for several weeks, came completely down with twice the PAW volume and sub-cultured cells only reduced in numbers. In contrast, A. baumannii demonstrated an impressive stability to this oxidative water dilution. PAW had to be added in a very high excess before a high-marked cell decline occurred, with tap water adapted bacteria being only slightly more susceptible than sub-cultured microorganisms. Thus, one can conclude that Gram-negative genera and their respective environment-adapted physiology differ with respect to susceptibility to PAWs. However, in any case, a surplus of PAW enables adequate cell reduction for disinfection processes under these conditions. We showed that high cell densities led to reduced inactivation of all bacterial genera. Other studies confirmed this impact of high microbial loads to lower inactivation rates by PAW [36,50-51]. This observation may be due to the behavior of bacteria in high densities to PAW. Generally, bacteria tend to clumb and form aggregates, especially in biofilms [52,53]. It is conceivable that planktonic microorganisms, present in high densities, also tend to aggregate which might result in protection and consequently in an increasing tolerance to PAW. On the other hand, the ratio of the reactive nitrogen and oxygen species of PAW to the individual bacterium surface changes. The more bacteria, the fewer reactive species can act on each individual bacterium. Another phenomenon would be a depletion effect of the oxidative substances at high cell densities. Additionally, the presence of inorganic and organic substances in tap water increase the depletion as shown for oxidative disinfectants as chlorine dioxide [54-56]. To obtain an effective reactive species of PAW-to-bacterium ratio at different contamination levels, activation properties can be changed and regulated. Studies showed that prolonged activation times resulted in increased disinfection efficacy with high cell reductions [35,57,58]. Our lab device had a regulation unit that allowed an adjustment of electric power and activation time. With these prerequisites the disinfection procedures could be adjusted to an efficient decontamination under certain conditions using lowly or highly activated water. In addition to the contamination level, several other aspects play an important role for an efficient but environmentally and material friendly application of PAW. Both, the oxidized nitrogen and oxygen species could be reactive and corrosive to surfaces causing material damage, and the plastic and other material surfaces could be able to consume the reactive species of PAW. Furthermore, the PAW oxidation products should have a minimal impact on pollution. In order to be able to initiate the optimal disinfection measures, the optimized PAW activation conditions and respective operating conditions can be determined by monitoring prior to the disinfections.
Conclusion
In conclusion, this study demonstrated that PAW is suitable as a disinfectant to reduce numbers of waterborne microorganisms present in DUWLs. Tap water, plasma-activated at 90 W for 30 min, was characterized by high levels of electrical conductivity, oxidation-reduction potential and oxidizability and by acidic pH. This PAW had an antimicrobial effect after 30 min contact time to microorganisms, especially when added in excess to bacteria in tap water. The higher the PAW surplus, the more efficient was the inhibitory effect. The amount of generated reactive oxygen and nitrogen species, thus the antimicrobial conditions, were sufficient to inhibit growth of seven species out of five different genera such as Sphingomonas paucimobilis, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Legionella pneumophila, L. anisa and Acinetobacter species by addition of surplus volume units of PAW to bacteria in tap water. In contrast, A. baumannii, the third species studied from the genus Acinetobacter besides A. junii and A. puttii, was highly stable to PAW treatment, so that only a tenfold PAW excess in our hands led to growth inhibition. Nonetheless, we conclude that PAW with optimized plasma-activation conditions and inactivation potential is a suitable disinfection agent for inhibition of tolerant and robust microorganisms, and it can also be considered as a suitable disinfectant.
Acknowledgements
The authors gratefully acknowledge Olga Böhler for her technical support and VitalFluid for providing a PAW lab unit.
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that may have an influence on the work reported in this paper.
Funding
The study was supported by the European Regional Development Fund (ERDF) of the European Union in the framework of the MEDUWA Vecht(e) Interreg project (VA-142118) to KB and TK. We thank the Medical Faculty of Münster for the support within the framework of the MedK Program (funding reference 20-0062 (MedK Kohorte 2020_2)).
Contributions
Conceptualization: NCD, KB, TK; Methodology: NCD, PL, TK; Validation and data analyses: NCD, TK; Writing the original draft: NCD; Review: KB, TK, AM; Editing: TK; Project administration: KB, TK; Supervision: AM. All authors have read and agreed to the published version of the manuscript.
References
- Abel LC, Miller RL, Micik RE, et al. Studies on dental aerobiology. IV. Bacterial contamination of water delivered by dental units. J Dent Res 50 (1971): 1567-1569.
- Lück PC, Leupold I, Hlawitschka M, et al. Prevalence of Legionella species, serogroups, and monoclonal subgroups in hot water systems in south-eastern Germany. Zentralblatt für Hygiene und Umweltmedizin - International Journal of Hygiene and Environmental Medicine 193 (1993): 450-460.
- Lück PC, Lau B, Seidel S, et al. Legionellae in dental units-a hygienic risk? Dtsch Zahn- Mund- und Kieferheilkd Zentralbl 80 (1992): 341-346.
- Atlas RM, Williams JF, Huntington MK. Legionella contamination of dental-unit waters. Appl. Environm. Microbiology 61 (1995): 1208-1213.
- McDade JE, Shepard CC, Fraser DW, et al. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. The New England Journal of Medicine 297 (1977): 1197-1203.
- Cordes LG, Fraser DW. Legionellosis: Legionnaires' disease; Pontiac fever. The Medical clinics of North America 64 (1980): 395-416.
- Arnow PM, Chou T, Weil D, et al. Nosocomial Legionnaires' disease caused by aerosolized tap water from respiratory devices. J Inf Dis 146 (1982): 460-467.
- Rousseau C, Ginevra C, Simac L, et al. A Community Outbreak of Legionnaires' Disease with Two Strains of L. pneumophila Serogroup 1 Linked to an Aquatic Therapy Centre. International Journal of Environmental Research and Public Health 19 (2022): 1119.
- Fitzgibbon EJ, Bartzokas CA, Martin MV, et al. The source, frequency and extent of bacterial contamination of dental unit water systems. British Dental Journal 157 (1984): 98-101.
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes and Infection 2 (2000): 1051-1060.
- Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Reviews of Environmental Contamination and Toxicology 201 (2009): 71-115.
- Huber P, Basso P, Reboud E, et al. Pseudomonas aeruginosa renews its virulence factors. Environmental Microbiology Reports 8 (2016): 564-571.
- Crone S, Vives-Flórez M, Kvich L, et al. The environmental occurrence of Pseudomonas aeruginosa. APMIS 128 (2020): 220-231.
- Dang Y, Zhang Q, Wang J, et al. Assessment of microbiota diversity in dental unit waterline contamination. PeerJ 10 (2022): e12723.
- Zavascki AP, Carvalhaes CG, Picao RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther 8 (2010): 71-93.
- Okubo K, Ito T, Okamoto K, et al. Evaluation of the simulator with automatic irrigation control system designed for countermeasures of internal contamination in dental unit water lines. Heliyon 6 (2020): e04132.
- Karpay RI, Plamondon TJ, Mills SE, et al. Combining periodic and continuous sodium hypochlorite treatment to control biofilms in dental unit water systems. J Am Dent Assoc 130 (1999): 957-965.
- Tuvo B, Totaro M, Cristina ML, et al. Prevention and Control of Legionella and Pseudomonas spp. Colonization in Dental Units. Pathogens (Basel, Switzerland) 9 (2020): 305.
- Ramalingam K, Frohlich NC, Lee VA. Effect of nanoemulsion on dental unit waterline biofilm. Journal of Dental Sciences 8 (2013): 333-336.
- Tezel U, Pavlostathis SG. Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Current Opinion in Biotechnology 33 (2015): 296-304.
- Kamgang-Youbi G, Herry JM, Brisset JL, et al. Impact on disinfection efficiency of cell load and of planktonic/adherent/detached state: Case of Hafnia alvei inactivation by plasma activated water. Applied Microbiology and Biotechnology 81 (2008): 449-457.
- Kamgang-Youbi G, Herry JM, Meylheuc T, et al. Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Letters in Applied Microbiology 48 (2009): 13-18.
- Ercan UK, Wang H, Ji H, et al. Nonequilibrium Plasma-Activated Antimicrobial Solutions are Broad-Spectrum and Retain their Efficacies for Extended Period of Time. Plasma Processes Polym 10 (2013): 544-555.
- Zhao YM, Ojha S, Burgess CM, et al. Inactivation efficacy and mechanisms of plasma activated water on bacteria in planktonic state. Journal of Applied Microbiology 129 (2020): 1248-1260.
- Lu P, Boehm D, Bourke P, et al. Achieving reactive species specificity within plasma-activated water through selective generation using air spark and glow discharges. Plasma Processes and Polymers 14 (2017): 1600207.
- Patange A, Lu P, Boehm D, et al. Efficacy of cold plasma functionalised water for improving microbiological safety of fresh produce and wash water recycling. Food Microbiology 84 (2019): 103226.
- Oehmigen K, Hahnel M, Brandenburg R, et al. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes and Polymers 7 (2010): 250-257.
- Naitali M, Kamgang-Youbi G, Herry JM, et al. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma- treated water. Applied and Environmental Microbiology 76 (2010): 7662-7664.
- Shen J, Tian Y, Li Y, et al. Bactericidal effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Scientific Reports 6 (2016): 28505.
- Thirumdas R, Kothakota A, Annapure U, et al. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Science & Technology 77 (2018): 21-31.
- Whitehouse R, Peters E, Lizotte J, et al. Influence of biofilms on microbial contamination in dental unit water, J Dent 19 (1991): 290-295.
- Lipphaus P, Hammes F, Kötzsch S, et al. Microbial tap water profile of a medium-sized building and effect of water stagnation, Environ Technol 35 (2014): 620-628.
- Ma R, Wang G, Tian Y, et al. Non-thermal plasma activated water inactivation of food-borne pathogen on fresh produce. Journal of Hazardous Materials 300 (2015): 643-651.
- Guo L, Xu R, Gou L, et al. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Applied and Environmental Microbiology 84 (2018): e00726-18.
- Lin CM, Chu YC, Hsiao CP, et al. The optimization of plasma-activated water treatments to inactivate Salmonella enteritidis (ATCC 13076) on shell eggs. Foods 8 (2019): 520.
- Bai Y, Idris Muhammad A, Hu Y, et al. Inactivation kinetics of Bacillus cereus spores by plasma activated water (PAW). Food Research International 131 (2020): 109041.
- Machado-Moreira B, Tiwari BK, Richards KG, et al. Application of plasma activated water for decontamination of alfalfa and mung bean seeds. Food Microbiology 96 (2021): 103708.
- Mai-Prochnow A, Zhou R, Zhang T, et al. NPJ Interactions of plasma-activated water with biofilms: inactivation, dispersal effects and mechanisms of action. Biofilms Microbiomes 7 (2021): 11.
- Zhao YM, Patange A, Sun DW, et al. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Comprehensive Reviews in Food Science and Food Safety 19 (2020): 3951-3979.
- McFerson L. Understanding ORP’s role in the disinfection process. Water Engineering and Management 140 (1993): 29-31
- Traylor MJ, Pavlovich MJ, Karim S, et al. Long-term antibacterial efficacy of air plasma-activated water. Journal of Physics D: Applied Physics 44 (2011): 472001.
- Oliveira M, Fernández-Gómez P, Álvarez-Ordóñez A, et al. Plasma-activated water: A cutting-edge technology driving innovation in the food industry. Food research international (Ottawa, Ont.) 156 (2022): 111368.
- Falkinham JO. Living with Legionella and Other Waterborne Pathogens. Microorganisms 8 (2020): 20-26.
- Di Bonaventura G, Stepanovi´c S, Picciani C, et al. Effect of environmental factors on biofilm formation by clinical Stenotrophomonas maltophilia isolates. Folia Microbiol 52 (2007): 86-90.
- Moritz MM, Flemming HC, Wingender J. Integration of Pseudomonas aeruginosa and Legionella pneumophila in drinking water biofilms grown on domestic plumbing materials. International Journal of Hygiene and Environmental Health 213 (2010): 190-197.
- Qi L, Li H, Zhang C, et al. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii. Front Microbiol 7 (2016): 483.
- Karumathil DP, Yin HB, Kollanoor-Johny A, et al. Effect of chlorine exposure on the survival and antibiotic gene expression of multidrug resistant Acinetobacter baumannii in water. International Journal of Environmental Research and Public Health 11 (2014): 1844-1854.
- Botelho J, Grosso F, Peixe L. Antibiotic resistance in Pseudomonas aeruginosa- Mechanisms, epidemiology and evolution. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy 44 (2019): 100640.
- Lewenza S, Abboud J, Poon K, et al. Pseudomonas aeruginosa displays a dormancy phenotype during long-term survival in water. PloS one 13 (2018): e0198384.
- Deng XT, Shi JJ, Shama G, et al. Effects of microbial loading and sporulation temperature on atmospheric plasma inactivation of Bacillus subtilis spores. Applied Physics Letters 87 (2005): 153901-153903.
- Fernandez A, Shearer N, Wilson DR, et al. Effect of microbial loading on the efficiency of cold atmospheric gas plasma inactivation of Salmonella enterica serovar Typhimurium. International Journal of Food Microbiology 152 (2012): 175-180.
- Kragh KN, Hutchison JB, Melaugh G, et al. Role of Multicellular Aggregates in Biofilm Formation. mBio 7 (2016): e00237.
- Melaugh G, Hutchison J, Kragh KN, et al. Shaping the Growth Behaviour of Biofilms Initiated from Bacterial Aggregates. PloS one 11 (2016): e0149683.
- Ofori I, Maddila S, Lin J, et al. Chlorine dioxide oxidation of Escherichia coli in water- A study of the disinfection kinetics and mechanism. J Environ Sci Health Part A 52 (2017): 598-606.
- Ofori I, Maddila S, Lin J, et al. Chlorine dioxide inactivation of Pseudomonas aeruginosa and Staphylococcus aureus in water: The kinetics and mechanism. J Water Process Eng 26 (2018): 46-54.
- Krüger TIM, Herzog S, Mellmann A, et al. Impact of Chlorine Dioxide on Pathogenic Waterborne Microorganisms Occurring in Dental Chair Units Microorganisms 11 (2023) 1123.
- Guo J, Qin D, Li W, et al. Inactivation of Penicillium italicum on kumquat via plasma-activated water and its effects on quality attributes. International Journal of Food Microbiology 343 (2021): 109090.
- Wang J, Han R, Liao X, et al. Application of plasma-activated water (PAW) for mitigating methicillin-resistant Staphylococcus aureus (MRSA) on cooked chicken surface. LWT - Food Science and Technology 137 (2021): 110465.


 Impact Factor: * 3.1
Impact Factor: * 3.1 Acceptance Rate: 76.66%
Acceptance Rate: 76.66%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 