From Bits to Atoms: Machine Learning and Nanotechnology for Cancer Therapy
Mawuli Agboklu*, Frederick A Adrah, Prince Mawuli Agbenyo, Hope Nyavor
The Joint School of Nanoscience and Nanoengineering at the University of North Carolina at Greensboro, Nc, USA
*Corresponding author: Mawuli Agboklu, The Joint School of Nanoscience and Nanoengineering at the University of North Carolina at Greensboro, Nc, USA.
Received: 04 March 2024; Accepted: 11 March 2024; Published: 21 March 2024
Article Information
Citation: Mawuli Agboklu, Frederick A Adrah, Prince Mawuli Agbenyo, Hope Nyavor. From Bits to Atoms: Machine Learning and Nanotechnology for Cancer Therapy. Journal of Nanotechnology Research. 6 (2024): 16-26.
DOI: 10.26502/jnr.2688-85210042
View / Download Pdf Share at FacebookAbstract
Cancer therapy has seen significant advancements in recent years, with the integration of machine learning and nanotechnology emerging as a promising new approach to improve treatment outcomes. This paper explores the synergistic potential of machine learning and nanotechnology-based platforms in enhancing cancer therapy. The paper also proposes a conceptual framework for using Gold Nanoparticles (AuNPs) and Data Mining for enhanced Photothermal therapy. Machine learning techniques offer the ability to analyze large datasets of patient information, tumor characteristics and treatment responses to develop personalized treatment plans tailored to patients. By harnessing machine learning algorithms and nanomedicine, clinicians can optimize treatment strategies, predict treatment outcomes and identify novel therapeutic targets. Nanotechnology provides a multipurpose platform for targeted drug delivery, imaging and diagnostics in cancer therapy. Nanoparticle-based drug delivery systems can deliver therapeutic agents directly to tumor sites while minimizing off-target effects and enhancing treatment efficacy. Additionally, nanoscale imaging agents and sensors enable early detection of cancer biomarkers and monitoring of treatment responses. This work also bridges the gap between scientific research and clinical applications. The integration of machine learning and nanotechnology offers several advantages for enhanced cancer therapy, including personalized treatment approaches, enhanced drug delivery efficiency, early detection methods and predictive modeling for treatment responses. This paper highlights recent advancements, challenges, and future directions in leveraging machine learning and nanotechnology to optimize cancer therapy and improve patient outcomes.
Keywords
<p>Cancer therapy; Machine Learning; Nanotechnology; Photothermal therapy</p>
Article Details
Introduction
The application of Machine Learning (ML) and Nanotechnology in various fields and industries has improved the quality of human lives in the last few years. This has been particularly significant in healthcare, which is one of the fastest growing sectors that has witnessed advanced transformations [1,2]. In the battle against pandemics like COVID-19, nanotechnology and ML have collaborated to combat the spread of the virus [3]. Cancer is one of the most severe chronic diseases that has continuously ravaged the world and caused pain to millions. Different forms of cancer exist, including colorectal cancer (CRC) which ranks third among the most frequently diagnosed cancers and is the third leading cause of cancer-related mortality in both men and women in the United States [4]. To prevent adverse outcomes related to chronic kidney disease (CKD), such as cardiovascular disease, end-stage kidney disease and mortality, it is essential for primary care clinicians to conduct timely screening, diagnosis, and management [5]. This is where blending nanotechnology (atoms) with ML (bits) has the potential to transform healthcare and promote research novelty. There is significant potential for the small sciences, especially nanoscience and nanotechnology – involving the study of the property of matter on an ultra-small scale (10-9 m). This includes the synthesis, fabrication and design of materials at the nanoscale [6]. Existing data shows that approximately 86% of industries in the healthcare space use some form of ML applications to augment current technologies, and more that 80% of health organizational leaders have some Artificial Intelligence (AI) plan in place [7]. ML prototypes, mainly categorized as supervised, unsupervised and reinforcement learning models can be designed to solve many healthcare problems using data mining tools [2,3]. These and other aspects of ML will be delved in more subsequently.
ML applications have been extensive in health, especially predicting cardiovascular diseases, nephropathy and discovery of cancer tumors from radiology images [9]. With the promises ML alone offers to improve healthcare, blending it with Nanotechnology can create an unlimited synergy that will continuously propel scientific research upwards. Due to the unique magnetic, optical, electrical and chemical properties of nanomaterials, nano-based approaches have been critical in the last thirty years [10]. Some of these applications have broken grounds in healthcare management such as dentistry, diagnostic kits, thermal ablation (cancer), sports science and cosmetics, among so many others [5,6]. Merging ML and nanoscience can help hasten the scientific research process for fabricating new nanomaterials and optimizing them for targeted cancer therapeutics. This paper provides a review of the contemporary research work in these two fields and conceptualizes a potential link and framework where ML and nanotechnologies can improve cancer therapy markedly [12].
Overview of Machine Learning in Healthcare
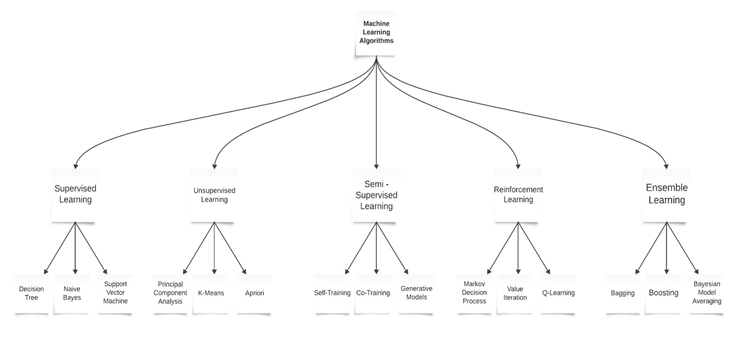
Machine learning is a multi-disciplinary field having a wide-range of research domains reinforcing its existence. The simulation of ML models is significantly related to Computational Statistics whose main aim is to focus on making predictions via computers. It is also co-related to Mathematical Optimization which relates models, applications, and frameworks to the field of statistics. Real world problems have high complexity which make them excellent candidates for application of ML [13]. A major focus of machine learning research is to automatically induce models, such as rules and patterns, from the training data it analyzes. The abundance of machine learning algorithms can be divided into two main classes: supervised and unsupervised learning, based on whether the training data instances are labeled. In supervised learning, the learner is supplied with labeled training instances, where both the input and the correct output are given. In unsupervised learning, the correct output is not provided with the input. Instead, the learning program must rely on other sources of feedback to determine whether or not it is learning correctly. A third class of ML techniques, called semi-supervised learning, uses a combination of both labeled and unlabeled data for training [14]. ML relies on different algorithms to solve data problems. Data scientists like to point out that there’s no single one-size-fits-all type of algorithm that is best to solve a problem. The kind of algorithm employed depends on the kind of problem you wish to solve, the number of variables, the kind of model that would suit it best and so on. Here’s a quick look at some of the commonly used algorithms in machine learning (ML) [15].
In cardiovascular medicine today AI/ML has found wide range of applications in cardiovascular drug therapy, pharmacogenomics, heart failure management, cardiovascular imaging, and diagnostics. Artificial Intelligence (AI) can provide tools to apply precision medicine and big data in cardiovascular medicine therefore, augmenting the effectiveness of the cardiologist. AI/ML algorithms can analyze vastly heterogeneous clinical data without any assumptions accurately for prediction and classification. Therefore, cardiovascular medicine can benefit from the incorporation of AI [16]. Automated electrocardiogram (ECG) interpretation, an enterprise initially undertaken in the 1960s with the advent of digital ECG machines, is now almost universal. It was the first instance in which rudimentary AI effectively streamlined hospital-care and cut costs. Modern ML models are now able to identify different wave morphologies high precision; using this information, clinically significant parameters such as heart rate, axis deviation, and interval lengths can then be calculated [17]. Machine learning can be advantageous for early detection of diseases in human beings. It can detect specific patterns of diseases which will then prompt medical doctors on what to focus their lenses on during the diagnosis process [18]. This way, ML applications become the second pair of eyes for the doctor. Improvements in the field of image recognition and analysis because of machine learning efforts also add to the early detection of cancers, tumors and other types of growths in hidden areas of the human body [19].
Machine Learning Applications in Cancer Therapy
Health problems impact human lives. Machine learning has propelled advancements across various sectors such as computer vision, natural language processing (NLP) and automatic speech recognition (ASR). Given machine learning's capacity to derive insights from data and the pivotal role of data in healthcare, research in machine learning for healthcare is deemed essential [20]. Artificial intelligence (AI) approaches have the potential to affect several facets of cancer therapy. These include drug discovery and development and how these drugs are clinically validated and ultimately administered at the point of care, among others [21]. Machine Learning has been used to predict therapeutic responses of cancer patients to drugs and drug combinations during their treatments [22]. Different ML techniques are used to determine the most accurate outcomes of the predictions [18]. Some ML interventions include Support Vector Machine (SVM) models which are built using recursive feature selection methods and various cancer types datasets to predict patient responses to carboplatin; a popular cancer drug[23]. There are SVM Image-based prediction models which interpret Magnetic Resonance Imaging and ultrasonography image data to differentiate between Breast Conserving Surgeries[24]. Deep neural networks are being used to develop drug synergy predictions in understudied tissues such as bones, prostrate and pancreas as a solution to overcome the resistance of targeted drug therapies which results in non-responsive drug behavior [25,26]. Ensemble classification methods such as Random Forest algorithms which combines multiple classifiers instead of a single classifier are equally being exploited to develop predictive values for treatment response and survival in cancer patients [27]. The integration of ML in cancer therapy represents a transformative advancement in the field of cancer management. These innovative tools offer personalized treatment strategies, improved prognosis and enhanced insights into disease mechanisms [28]. As research in this area continues to evolve, the potential for AI/ML to revolutionize cancer therapy remains promising, offering hope for more effective treatments and ultimately better outcomes for patients battling cancer.
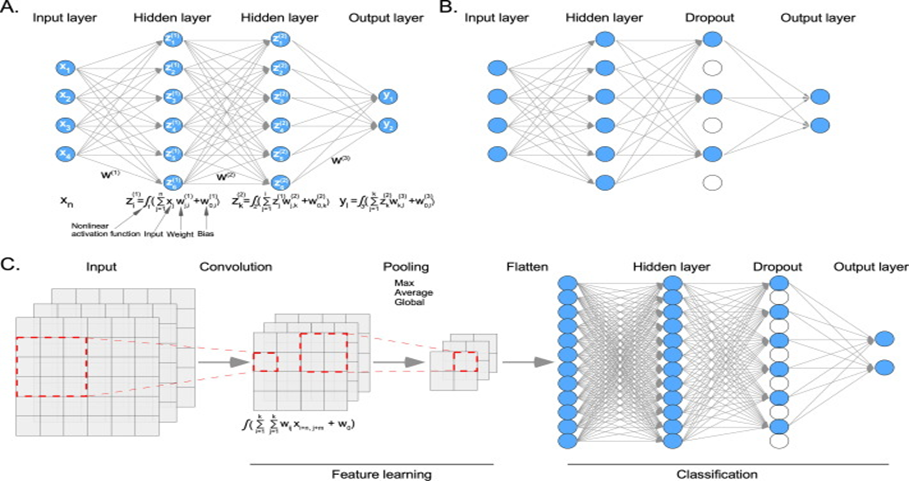
Figure 2: An example of a deep learning model. (Adapted with permission from “Machine learning in the prediction of cancer therapy,” Computational and Structural Biotechnology Journal, vol. 19, pp. 4003–4017, 2021 – An open source journal.
(A) In a deep neural network (DNN) model, each node of the input data layer is fully connected to the hidden layer nodes. The first hidden layer takes input data, multiplies it by weight, and adds a bias before applying a nonlinear activation function. The second hidden layer takes the first hidden layer as input and so on until it reaches the output layer. (B) In a dropout layer, some nodes are randomly removed. (C) During the convolution, the dimension of input data is reduced using a certain kernel size (in this example, 3x3) and the activation function. Then, features are pulled for further reduction. Finally, pulled features are flattened and applied to a DNN.
Nanotechnology in Cancer Therapy
Cancer ranks as the second most common cause of death across all age groups in the United States and is the primary cause of death among individuals under 85 years of age [29]. Cancer is a multifaceted disease marked by intricate cellular changes and a wide array of molecular variations, which collectively pose substantial obstacles to successful treatment [30]. Cancer therapy involves any interventions aimed at the treatment and management of various forms of cancer. Cancer is characterized by its significant heterogeneity and complexity. Treatment strategies are categorized into curative or non-curative therapies, depending on the extent of the disease and the overall clinical condition of the patient [31]. Since its development, nanotechnology in the form of nanomedicine, a nanoscience application for cancer [31], has been instrumental in cancer therapies, ranging from diagnosis to drug delivery and case management. In 1995, Doxil (liposomal doxorubicin) achieved the distinction of being the inaugural nanoparticle-based drug approved for cancer treatment in the United States. Subsequently, numerous other nano-based drugs have emerged on the market for addressing diverse diseases [32]. Nanomaterials display optical, mechanical, magnetic and conductive qualities that are distinct from those of their larger chemical counterparts. The improved properties are as a result of their high surface-to-volume ratio and the quantum confinement effect [33]. These unique properties have made nanomedicine a strong addition, especially for targeting cancer tumors. Nanomedicine has the potential to enhance anticancer therapy. Typically, nanomedicines are employed to regulate the distribution and concentration of chemotherapeutic drugs when administered systemically, thereby enhancing the equilibrium between their effectiveness and adverse effects [34]. Despite this positives, nanomedicine-based mechanisms are costly and mostly hindered by regulatory constraints [35]. On a broader scale, applications of nanoscience in medicine and cancer are becoming increasingly popular. This means the future of health innovation is bright for scientists in these fields. Cancer medicine has seen the benefits of common nanomaterials and devices such as liposomes, dendrimers, Quantum Dots and Carbon nanotubes, among so many others [36]. All cancers are not the same, however, nanomedicine interventions have been widely accepted as a game changer. There are several common hallmarks shared by cancers, such as continuous proliferation and growth, alterations in immune system activity and the initiation of angiogenesis, which involves the formation of new blood vessels [31]. By incorporating nanoscale materials and cutting-edge technologies like nanoparticles and nano-sensors, notable progress has been achieved in enhancing cancer treatment efficacy, reducing adverse effects and improving patient well-being in precision cancer therapy [37]. As ongoing research in this area advances, the promise of nanotechnology to revolutionize cancer treatment continues to inspire optimism among patients and healthcare professionals.
Integrating Machine Learning and Nanotechnology in cancer therapy
Leveraging machine learning algorithms with nanotechnology for cancer treatment in on the rise as this novel treatment methods provide promising optics as a worthy potential alternative to traditional chemotherapy methods [38]. Machine Learning models in conjunction with nanotechnology – based methods contribute to designing and optimizing nanomaterials with specific properties for diverse cancer treatments [38,39]. ML models can predict nanomaterial properties and optimize their fabrication processes to suite treatments. These contributions enhance targeted drug delivery systems, speed up research processes and if they make clinical trials and enter the market, have the potential to reduce treatment costs [40]. The increasing importance of nanoparticles in cancer therapy and cancer nanomedicine because of its ability to encase therapeutic agents such as chemotherapeutic drugs, peptides or nucleic acids and shield them from degradation by enzymes and other factors in the body environment, control the release of these agents’ over-time, and enable targeted delivery of therapeutic agents through surface modification, efficiency in its delivery is critical to any cancer therapeutic process. ML algorithms such as deep learning, linear regression, K-nearest neighbors, and random forest, have been used to predict the delivery efficiency of nanoparticles to tumors in mice. This predictive modeling aids in facilitating nanoparticle-based drug formulation processes to improve preclinical trial decisions and increase focus on promising outcomes [41]. AI/ML enabled nanorobots are also used for targeted drug delivery. To minimize the side effects of traditional chemotherapy, AI enabled nanorobots are alternatives used to perform targeted drug delivery in cancer patients. They are more efficient in in carrying prompt dosage regimens and maintain them in the bloodstream for a longer period[55].
The symbiotic relationship between ML and nanotechnology has been used to elucidate the interaction between microenvironments and nanomedicines to better understand tumor progression, metastases, and treatment responses. Nanoparticle based drug formulations have again proven to have the potential of enhancing therapeutic outcomes through the synergistic combination of active pharmaceutical ingredients as seen in drugs such as Vyxeos and Hensify used in radiotherapy. ML models were deployed in designing these nanoparticles and predicting the characteristics of the drug – loaded nanoparticles[56]. Many cancer treatments are seeking accuracy and efficiency in treatment methods and ML and nanotechnology is providing them a platform which guides their trials and implementation through quantitative prediction analysis.
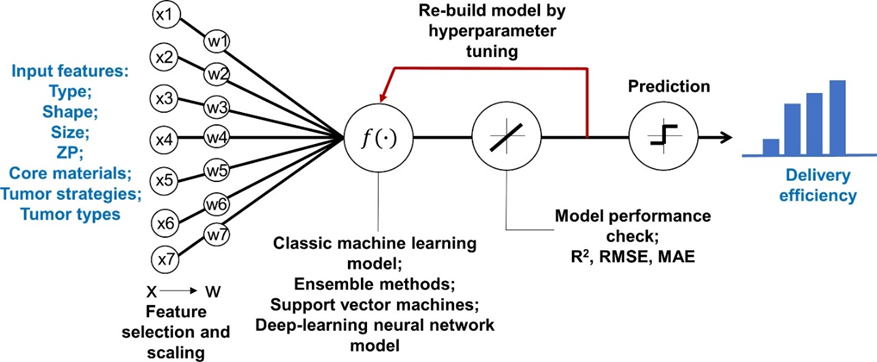
Figure 3: Overview of the study framework to develop machine learning and deep learning models to predict delivery efficiency of nanoparticles to the tumor site in tumor-bearing mice (Adapted with permission from “Predicting Nanoparticle Delivery to Tumors Using Machine Learning and Artificial Intelligence Approaches”, Z. Lin, W.-C. Chou et al). X represents the initial input variables and W represents the variables after feature selections. Abbreviations: R2, adjusted coefficient of determination; RMSE, root mean square error; MAE, mean absolute error.
Machine Learning and Nanotechnology for cancer therapy: A Conceptual framework
Cancer is a leading cause of death across the globe [44]. The prevalence of cancer in developing nations is increasing and poses a significant risk of causing substantial morbidity [45], [46], mortality and economic repercussions within these regions over the next two decades. The evolving global public health challenges presented by the cancer epidemic necessitate a comprehensive and impactful international response. Encouragingly, the majority of cancers in developing countries are preventable, and enhancing the effectiveness of treatment is achievable through early detection [47]. A wide spectrum of cancer therapies have been used over the years including chemotherapy, external beam radiotherapy, surgery and immunotherapy. We propose a conceptual framework for blending Machine Learning and Nanotechnology to enhance cancer therapy.
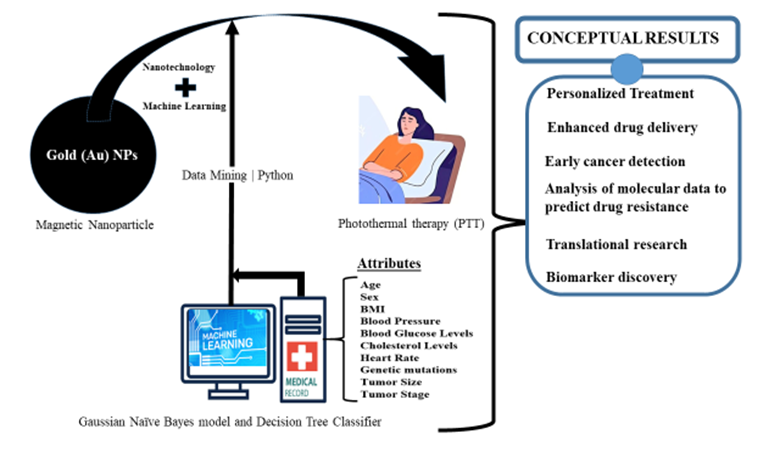
In contemporary times, magnetic nanoparticle (MNP) technologies have become commonplace in their application to biological systems, serving diagnostic and therapeutic objectives with increasing regularity [48]. These technologies, rooted in the utilization of MNPs, have gained widespread adoption for a myriad of biomedical applications [49], including but not limited to diagnostics, therapeutic interventions, and targeted drug delivery within biological systems [50]. Magnetic nanoparticles (MNPs) are tiny particles with a size of less than 100 nanometers, made of magnetic materials such as Iron Oxide (Fe2O3), Cobalt (Co) or Nickel (Ni). MNPs have unique magnetic properties, making them useful in various applications, including cancer diagnosis and treatment [51]. They possess a surface plasmon resonance peak within the visible to infrared region due to their unique size.
Already in active use is the Photo-thermal therapy (PTT) that can use Au coated magnetic nanoparticles under near infrared (NIR) or visible light to target and kill cancer cells through the conversion of light into heat energy [52]. This conceptual framework aims at employing Machine Learning, Python programming and comparative data mining tools to extract hidden patterns in patients who are in cancer remission due to MNPs-based drug delivery. Two Machine learning algorithms (the Gaussian Naïve Bayes model and Decision Tree Classifier) will be utilized to forecast the level of efficacy of these nanomedicine-based interventions. The sources for data collection will include hospitals and machine learning repositories in the United States, aiming to gather data from 200-250 patients. Attributes to be considered are patients’ age, sex, body mass index and other relatable health risk factors. The results obtained will be used to forecast the efficacy of MNPs required to improve cancer treatment based on the attributes from the analyzed datasets.
Current state of Nano-based cancer interventions
Albeit ethical and regulatory drawbacks, cancer therapy has broadly benefitted from nano-based interventions [53], especially over the last couple of years. Currently, metal complexes have played a pivotal role in cancer therapy, offering a wealth of opportunities for drug design through the manipulation of multiple variables, including the metal, ligand, and metal-ligand interaction. This versatile approach has yielded a diverse array of metallodrugs with enhanced functionalities and mechanisms of action compared to purely organic structures. Clinically validated metallodrugs like cisplatin, carboplatin, and oxaliplatin are instrumental in treating various cancer types and are integral components of combination therapies, including immunotherapy [54]. Cancer cases continues to rise by the number globally, and there is the need for more innovative approaches to battle this chronic disease [55]. Chemotherapy has been the most common treatment globally. Their associated side-effects are now being mitigated by anticancer nanomedicines [56], which is a proven positive step in enhanced cancer therapy. It is imperative for continued research and collaboration across cross-disciplinary fields to further advance nano-based cancer interventions, ultimately improving patient prognosis and quality of life in the battle against cancer [57].
Current state of Machine Learning for cancer therapy
With recent advances in AI, machine learning algorithms have been assessed for understanding disease biology and predicting response in cancer management [58]. ML and allied computational methods have become potent instruments in cancer therapy, providing unique chances for personalized treatment plans and enhanced patient results. Machine learning serves as a proven tool for interpreting complex datasets and deriving meaningful insights [59]. In cancer research, the use of data-driven approaches is rapidly expanding, driven by the demand for personalized medical interventions. This growth underscores the potential of machine learning methodologies to transform the landscape of cancer research and treatment [60]. ML has been applied recently to assess biomarker in patients with advanced pancreatic cancer using deep learning techniques [61]. Similarly, deep learning-based methods have also been used recently to conduct surveys on brain tumor, indicating a general progression of AI/ML [62]. Photothermal therapy (PTT), for example, is a minimally invasive process and promisingly effective strategy for thermal ablation of tumors [63]. Currently, Machine learning methods have been deployed to predict PTT conversion efficiency of organic PTT agents [64]. This shows there is big promise for the conceptual framework conceived in this paper. Other applications of ML in cancer have been found in lung cancer management, where integrating AI/ML has helped in analyzing vast datasets and predicting treatment responses [65]. The current state of machine learning in cancer showcases the immense potential of data and artificial intelligence in advancing our understanding of cancer and exploring novel therapeutic approaches.
Challenges
Machine Learning has a lot of positive impact on oncology. From diagnosis to treatment, there has been different ML applications to enhance precision and accuracy and there is potential for more to be done. Despite this progress, there are still many obstacles to be surmounted in the interventions of ML and its cancer related applications. [66]. The success of any machine learning algorithm to predict therapeutic solutions depends on the quality of data fed to the model [67]. Data and data related problems remain the main challenge for machine learning in its cancer therapy interventions [68]. Excessive noise within datasets, heterogeneity of datasets and overfitting of models are challenges being grappled with. The result of this, is interpretation and validation difficulties which makes most predictions black boxes because it is difficult to understand how the predictions were arrived at [69]. Nanotechnology applications in cancer therapy on the other hand also have challenges that can be grouped into biological, technological and study-design. One of the most significant challenges lies in transitioning trials from in vivo and in vitro settings to clinical trials, primarily due to biological factors like the degradation and toxicity of nanoparticles [70]. Technological limitations of scaling up synthesis, optimization and performance predictions also exist. Finally, the centering of nanotechnology – based therapeutic study – designs around cell and animal models also limit the potential of the efficacy of its applications in clinical trials because the complexities of the human biology may not always be present in such environments[71].
Regulatory and Ethical Considerations
Cancer therapies can be both painful and pose – life threatening risks. This underscores the prioritization of safety methods and ethical considerations in deploying therapeutic solutions. Integrating ML and Nanotechnologies in cancer therapies hold significant promise for enhancing treatment and treatment response of patients beyond current standards nonetheless, it is critical that these innovative solutions are established within acceptable regulation and ethical guidelines to ensure their responsible deployment and safeguard the well-being of patients [72].
Regulatory bodies are trying to find balance between developing optimal regulatory frameworks which effectively accommodate the risks, benefits, and unique properties of ML/AI technologies without limiting innovation whiles at the same time ensuring patient safety. The EU and US have varied classification for clinical decision support systems (CDS) including Large Language model (LLM) based CDS used in oncology [73]. Regulation differences of Machine Learning and Artificial Intelligence interventions in healthcare and medical devices in the US, UK and EU burdens manufactures with the difficult hurdle of transitioning approvals from one country to the other[74]. Because of the absence of a universally standardized regulatory framework, the regulatory landscape for the application of nanotechnology in cancer therapies also differ in major markets worldwide. The United States Food and Drugs Administration (USFDA), European Medicines Agency (EMA) and the Medicines and Healthcare Products Regulatory Agency (MHRA) each maintain distinct guidelines and regulations for nanotechnology–based cancer therapeutic products in their jurisdictions however, a shared principle of patient safety is common among them. Safety assessments are made considering factors such as toxicity of the nanomaterials, risk minimization and efficacy of the products [75].
Figure 6: Summary of regulatory and ethical considerations regarding ML and nanotechnology applications for cancer therapy
Ethical considerations are paramount in healthcare delivery, some of the key considerations are patients’ decision autonomy, the principles of beneficence, non-maleficence, justice, and confidentiality. Any therapeutic intervention must inherently incorporate these ethics in them. In integrating ML and nanotechnology to develop cancer therapies, it became evident that ethical considerations extend to areas such as data privacy and security, integrity, confidentiality, and voluntary patient consent. Considerations regarding data privacy and security, such as determining the most efficient computing architectures, selecting machine learning models that prioritize personal identifiable information (PII) security, and deploying encryption architectures that offer robust protection, are crucial for safeguarding patients' data. Ensuring fairness, equity, and unbiasedness in ML therapeutic solutions requires prioritizing data integrity. Although ML algorithms are theoretically neutral mathematical models, they can perpetuate and even amplify existing biases if the training dataset is biased. Thus, achieving a fair balance during data collection and inputting stages is essential to mitigate these biases[76].
Future Perspectives
The convergence of Machine Learning and nanotechnology in advancing cancer therapies offers many benefits to the medical field. This intersection holds promise for a brighter future in cancer treatment, with ongoing research focused on leveraging the integration of multi-omics data through machine learning and deep learning techniques to discover new biomarkers, therapeutic targets and the prediction of patient responses to emerging treatments. Additionally, there is growing interest in utilizing ML algorithms to analyze sophisticated advance imaging technologies like the Magnetic Resonance Imaging (MRI), PET/MRI (positron emission tomography/magnetic resonance imaging) and optical imaging which are combined with nanotechnology-based contrast agents, for early cancer detection and treatment monitoring, which will potentially enhance therapeutic outcomes. As medicine progresses towards the integration of clinical decision support systems to aid physicians in making more precise medical judgments, the development of such systems based-on ML algorithms to harness data from electronic health records, imaging, and molecular profiling holds the potential to reduce diagnosis delays and enhance therapeutic decision-making processes. Notwithstanding the above benefits, it is important that the conversation around regulations is deepened and advanced towards developing global frameworks that will guide the future trajectory of this combined field. Success in this endeavor will ensure that cancer therapy and the broad field of medicine is advanced in a safe and ethical manner without limiting the potential of what could be achieved as well as not leaving room for an abuse of the concept.
Conclusion
The convergence of machine learning and nanotechnology represents a revolutionary advancement in cancer therapy, ushering in a transformative era characterized by tailored and precise treatment approaches. This integration presents unprecedented opportunities to develop personalized and targeted therapies that maximize efficacy while minimizing adverse effects. By harnessing the synergistic potential of these cutting-edge technologies, significant strides have been made in improving cancer drug delivery efficiency, optimizing treatment outcomes, and surmounting obstacles like drug resistance. As ongoing research in this dynamic field continues to unfold, the potential of machine learning and nanotechnology to redefine cancer therapy is poised to reshape the landscape of oncology. This holds immense promise in providing renewed hope for patients and propelling the development of more refined and impactful interventions in the relentless battle against cancer.
References
- R Bhardwaj, AR Nambiar, D Dutta. A study of machine learning in healthcare. presented at the 2017 IEEE 41st annual computer software and applications conference (COMPSAC), IEEE (2017): 236-241.
- M Abdar et al. A review of uncertainty quantification in deep learning: Techniques, applications and challenges. Information fusion 76 (2021): 243-297.
- IN Weerarathna, A Luharia, S Tivaskar, et al. Emerging Applications of Biomedical Science in Pandemic Prevention and Control: A Review. Cureus 15 (2023).
- RL Siegel, NS Wagle, A Cercek, et al. Colorectal cancer statistics, 2023. CA: a cancer journal for clinicians 73 (2023): 233-254.
- TK Chen, DH Knicely, ME Grams. Chronic kidney disease diagnosis and management: a review. Jama 322 (2019): 1294-1304.
- AK Biswas, MR Islam, ZS Choudhury, et al. Nanotechnology based approaches in cancer therapeutics,” Advances in Natural Sciences: Nanoscience and Nanotechnology 5 (2014): 043001.
- A Alanazi, Using machine learning for healthcare challenges and opportunities. Informatics in Medicine Unlocked 30 (2022): 100924.
- E Sullivan, Understanding from machine learning models. The British Journal for the Philosophy of Science (2022).
- A Mustafa, M Rahimi Azghadi. Automated machine learning for healthcare and clinical notes analysis. Computers 10 (2021): 24.
- S Anjum, et al. Emerging applications of nanotechnology in healthcare systems: Grand challenges and perspectives. Pharmaceuticals 14 (2021): 707.
- P Maheshwari, NV Gupta. Advances of nanotechnology in healthcare. Int J Pharm Tech Res 4 (2012): 1221-1227.
- W Liu, Y Wu, Y Hong, et al. Applications of machine learning in computational nanotechnology,” Nanotechnology 33 (2022): 162501.
- J Alzubi, A Nayyar, A Kumar. Machine learning from theory to algorithms: an overview. in Journal of physics: conference series, IOP Publishing (2018): 012012.
- K Kapitanova, S Son. Machine learning basics. Intelligent Sensor Networks (2012): 3-29.
- B Mahesh. Machine learning algorithms-a review. International Journal of Science and Research (IJSR) 9 (2020): 381-386.
- P Mathur, S Srivastava, X Xu, et al. Artificial intelligence, machine learning, and cardiovascular disease,” Clinical Medicine Insights: Cardiology 14 (2020): 1179546820927404.
- SJ Al’Aref, et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. European heart journal 40 (2019): 1975-1986.
- K Kourou, TP Exarchos, KP Exarchos, et al. Machine learning applications in cancer prognosis and prediction,” Computational and structural biotechnology journal 13 (2015): 8-17.
- Z Hu, J Tang, Z Wang, et al. Deep learning for image-based cancer detection and diagnosis-A survey. Pattern Recognition 83 (2018): 134-149.
- M Ghassemi, T Naumann, P Schulam, et al. A review of challenges and opportunities in machine learning for health,” AMIA Summits on Translational Science Proceedings 2020 (2020): 191.
- D Ho. Artificial intelligence in cancer therapy. Science 367 (2020): 982-983.
- R Rafique, SR Islam, JU Kazi. Machine learning in the prediction of cancer therapy. Computational and Structural Biotechnology Journal 19 (2021): 4003-4017.
- C Huang, R Mezencev, JF McDonald, et al. Open source machine-learning algorithms for the prediction of optimal cancer drug therapies. PLoS One 12 (2017): e0186906.
- ME Ozer, PO Sarica, KY Arga. New machine learning applications to accelerate personalized medicine in breast cancer: rise of the support vector machines. Omics: a journal of integrative biology 24 (2020): 241-246.
- A Sharma, R Rani. A systematic review of applications of machine learning in cancer prediction and diagnosis. Archives of Computational Methods in Engineering 28 (2021): 4875-4896.
- Y Kim, S Zheng, J Tang, et al. Anticancer drug synergy prediction in understudied tissues using transfer learning. Journal of the American Medical Informatics Association 28 (2021): 42-51.
- Ö Akar, O Güngör. Classification of multispectral images using Random Forest algorithm. Journal of Geodesy and Geoinformation 1 (2012): 105-112.
- J Liao, et al. Artificial intelligence assists precision medicine in cancer treatment. Frontiers in Oncology 12 (2023): 998222.
- RL Siegel, AN Giaquinto, A Jemal. Cancer statistics, 2024. CA: A Cancer Journal for Clinicians (2024).
- DJ García-Domínguez, et al. Cancer Nano-Immunotherapy: The Novel and Promising Weapon to Fight Cancer. International Journal of Molecular Sciences 25 (2024): 1195.
- M Bjornmalm, KJ Thurecht, M Michael, et al. Bridging bio-nano science and cancer nanomedicine. ACS nano 11 (2017): 9594-9613.
- J Wolfram, M Ferrari. Clinical cancer nanomedicine. Nano today 25 (2019): 85-98.
- V Uskokovic. Entering the era of nanoscience: time to be so small. J Biomed Nanotechnol 9 (2013): 1441-1470.
- R van der Meel, E Sulheim, Y Shi, et al. Smart cancer nanomedicine,” Nature nanotechnology 14 (2019): 1007-1017.
- EKH Chow, D Ho, Cancer nanomedicine: from drug delivery to imaging. Science translational medicine 5 (2013): 216rv4-216rv4.
- F Alam, M Naim, M Aziz, et al. Unique roles of nanotechnology in medicine and cancer-II. Indian Journal of Cancer 52 (2015): 1–9.
- Y Wang, S Sun, Z Zhang, et al. Nanomaterials for cancer precision medicine,” Advanced materials 30 (2018): 1705660.
- O Adir, et al. Integrating artificial intelligence and nanotechnology for precision cancer medicine. Advanced Materials 32 (2020): 1901989.
- C Chen, Z Yaari, E Apfelbaum, et al. Merging data curation and machine learning to improve nanomedicines. Advanced drug delivery reviews 183 (2022): 114172.
- E Champa-Bujaico, P García-Díaz, AM Díez-Pascual. Machine learning for property prediction and optimization of polymeric nanocomposites: a state-of-the-art. International Journal of Molecular Sciences 23 (2022): 10712.
- Z Lin, WC Chou, YH Cheng, et al. Predicting nanoparticle delivery to tumors using machine learning and artificial intelligence approaches. International journal of nanomedicine (2022): 1365-1379.
- M Soltani, et al. Enhancing clinical translation of cancer using nanoinformatics. Cancers 13 (2021): 2481.
- A Naeem, et al. Convergence of artificial intelligence and nanotechnology in the development of novel formulations for cancer treatment. in A Handbook of Artificial Intelligence in Drug Delivery, Elsevier (2023): 499-529.
- S Sheikhi, NA Hosori, S Yari. An Overview of the Application of Nanoscience in Cancer Diagnosis and Treatment. Asian Pacific Journal of Environment and Cancer 6 (2023): 87-91.
- M Xu, et al. Cancer nanomedicine: emerging strategies and therapeutic potentials. Molecules 28 (2023): 5145.
- X Ma, H Yu, Cancer issue: global burden of cancer. The Yale journal of biology and medicine 79 (2006): 85.
- PE Petersen, Oral cancer prevention and control–the approach of the World Health Organization. Oral oncology 45 (2009): 454-460.
- MM Selim, S El-Safty, A Tounsi, et al. A review of magnetic nanoparticles used in nanomedicine. APL Materials 12 (2024).
- L Devi, P Kushwaha, TM Ansari, et al. Recent trends in biologically synthesized metal nanoparticles and their biomedical applications: a review. Biological Trace Element Research (2023): 1-17.
- G Goya, V Grazu, MR Ibarra, Magnetic nanoparticles for cancer therapy. Current nanoscience 4 (2008): 1-16.
- K Wu, D Su, J Liu, et al. Magnetic nanoparticles in nanomedicine: a review of recent advances,” Nanotechnology 30 (2019): 502003.
- MA Dheyab, et al. Gold nanoparticles-based photothermal therapy for breast cancer. Photodiagnosis and photodynamic therapy (2023): 103312.
- X Zhang, et al. Engineered anti-cancer nanomedicine for synergistic ferroptosis-immunotherapy. Chemical Engineering Journal 455 (2023): 140688.
- Q Peña, et al. Metallodrugs in cancer nanomedicine. Chemical Society Reviews 51 (2022): 2544-2582.
- N Alvarez, A Sevilla. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. International Journal of Molecular Sciences 25 (2024): 1023.
- Q Zhou, et al. Tumor abnormality-oriented nanomedicine design. Chemical Reviews 123 (2023): 10920-10989.
- NJ Johansen, CM Saunders. Value-based care in the worldwide battle against cancer. Cureus 9 (2017).
- RM Saliby, E Saad, S Kashima, et al. Update on Biomarkers in Renal Cell Carcinoma. American Society of Clinical Oncology Educational Book 44 (2024): e430734.
- J Vrdoljak, et al. Applying explainable machine learning models for detection of breast cancer lymph node metastasis in patients eligible for neoadjuvant treatment. Cancers 15 (2023): 634.
- M Bhatt, P Shende. Advancement in machine learning: a strategic lookout from cancer identification to treatment. Archives of Computational Methods in Engineering 30 (2023): 2777–2792.
- S Nowak, et al. Deep learning–based assessment of CT markers of sarcopenia and myosteatosis for outcome assessment in patients with advanced pancreatic cancer after high-intensity focused ultrasound treatment,” European Radiology 34 (2024): 279-286.
- B Panduri, OS Rao. A survey on brain tumour segmentation techniques in deep learning. International Journal of Intelligent Systems and Applications in Engineering 12 (2024): 412-425.
- D Liu, et al. Liquid exfoliation of ultrasmall zirconium carbide nanodots as a noninflammatory photothermal agent in the treatment of glioma, Biomaterials 292 (2023): 121917.
- S Wu, et al. Machine learning assisted photothermal conversion efficiency prediction of anticancer photothermal agents. Chemical Engineering Science 273 (2023): 118619.
- S Srivastava, et al. Unveiling the potential of proteomic and genetic signatures for precision therapeutics in lung cancer management. Cellular Signalling 113 (2024): 110932.
- D Bertsimas, H Wiberg. Machine learning in oncology: methods, applications, and challenges,” JCO Clinical Cancer Informatics 4 (2020).
- S Aminizadeh, et al. The applications of machine learning techniques in medical data processing based on distributed computing and the Internet of Things. Computer methods and programs in biomedicine (2023): 107745.
- KY Ngiam, W Khor, Big data and machine learning algorithms for health-care delivery. The Lancet Oncology 20 (2019): e262–e273.
- Z Yudong, H Jin, Challenges of Deep Learning in Cancers. Technology in Cancer Research & Treatment 22 (2023).
- H Huang, W Feng, Y Chen, et al. Inorganic nanoparticles in clinical trials and translations. Nano Today 35 (2020): 100972,.
- S Gavas, S Quazi, TM Karpinski. Nanoparticles for cancer therapy: current progress and challenges,” Nanoscale research letters 16 (2021): 173.
- DD Ignatavicius, ML Workman. Medical-Surgical Nursing-E-Book: Patient-Centered Collaborative Care, Single Volume. Elsevier Health Sciences (2015).
- B Derraz, et al. New regulatory thinking is needed for AI-based personalised drug and cell therapies in precision oncology. NPJ Precision Oncology 8 (2024): 23.
- S Gilbert, S Anderson, M Daumer, et al. Learning from Experience and Finding the Right Balance in the Governance of Artificial Intelligence and Digital Health Technologies,” Journal of medical Internet research 25 (2023): e43682.
- F Ali, K Neha, S Parveen. Current regulatory landscape of nanomaterials and nanomedicines: A global perspective. Journal of Drug Delivery Science and Technology 80 (2023): 104118.
- H Javed, HA Muqeet, T Javed, et al. Ethical Frameworks for Machine Learning in Sensitive Healthcare Applications. IEEE Access (2023).

 Impact Factor: * 2.9
Impact Factor: * 2.9 Acceptance Rate: 78.36%
Acceptance Rate: 78.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 