A New Chromogenic Spray Reagent for Detection and Identification of Indoxacarb in Visceral Material by HPTLC
P . V. More1, V. R. Chandegaonkar1, N. R. Gosavi1, B. P. More1*, K. V. Kulkarni2
1Regional Forensic Science Laboratory, Opposite Vidyut Nagar, Dindori Road, Nasik-422004, M.S., India.
2Directorate of Forensic Science Laboratories, Kalina, Santacruz (East), Mumbai-400098. M.S., India.
*Corresponding Author: Bhausaheb Parashuram More, Regional Forensic Science Laboratory, Opposite Vidyut Nagar, Dindori Road, Nasik-422004, M.S., India
Received: 24 June 2019; Accepted: 08 July 2019; Published: 12 July 2019
Article Information
Citation: P. V. More, V. R. Chandegaonkar, N. R. Gosavi, B. P. More, K. V. Kulkarni. A New Chromogenic Spray Reagent for Detection and Identification of Indoxacarb in Visceral Material by HPTLC. International Journal of Applied Biology and Pharmaceutical Technology 10 (2019): 003-007.
View / Download Pdf Share at FacebookAbstract
There is inherent rise of pesticide poisoning in India. Among all these pesticide indoxacarb is registered for use to control lepidopteran insects on crops, turf grasses, and landscape ornamentals. A novel chromatographic reagent is described for detection and identifications of Indoxacarb by HPTLC by use of furfural reagent. Indoxacarb (I) on acid hydrolysis yield its metabolites namely 4-trifloro-methoxy-phenyl amine (II), Oxidiazine (III) and acetic acid (IV). The 4-trifloro-methoxyphenyl amine (II) under acidic condition reacts with furfural which gives grayish black colored spot on heating. The constituents of viscera (amino, acids, peptides, proteins, etc.) and plant materials do not interfere with the test. The detection limit for Indoxacarb is 0.5μg. The reaction mechanism is discussed. The necessary HPTLC parameter like concentration of analyte also mentioned for the efficient detection of indoxacarb pesticide.
Keywords
<p>Biological materials; Extraction; HPTLC; Oxidiazine; Indoxacarb</p>
Article Details
Introduction
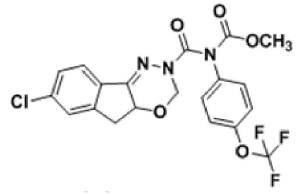
Indoxacarb is an oxidiazine pesticide developed by DuPont that acts against lepidopteran larvae. It is marketed under the names Indoxacarb Technical Insecticide, Steward Insecticide and Avaunt Insecticide. This pesticide is toxic to mammals, birds, fish and aquatic invertebrate. The insecticide belongs to the oxidiazine chemical family hence insecticide that activity occurs via blockage of the sodium channel [1] in the central nervous system and mode of entry via stomach and contact sources. The IUPAC nomenclature of Indoxacarb [2] is (methyl(4aS) -7 - chloro - 2 -{methoxy carbonyl - { 4 (trifluoromethoxy) phenyl } carbamoyl } - 3, 5-dihydroindeno {1, 2-e}{1, 3, 4}oxadiazine-4a-carboxylate). It is widely used in agriculture sector for the protection of the crop [3].
Indoxacarb is easily available in the market. So they are frequently misused in various suicidal attempts, especially in farmer subside cases. Many accidental poisoning cases are also reported due to its easy availability. When such type of cases referred to medical officer for further investigation, it is not possible to say, that which poison was responsible for death. At that time medical officer referred such cases for forensic analysis.
However, from a toxicological point their identification in biological material is very essential. The detection of this insecticide in routine forensic work is achieved by using high performance thin layer chromatography technique, because of its speed and versatility. It is therefore necessary to have a sensitive reagent to detect these insecticides in the presence of fats and proteins. A number of chromomeric reagents, such as diactylmonoxime [4], aniline [5] have been reported for the detection of these insecticides, but furfural [6] was also to be found suitable and sensitive reagent for detection of indoxacarb. Chlorpyrifos in biological samples by hptlc Reported by Pawar R.R et al. [7].
The chemical structure of indoxacarb is shown in Figure 1.
Experimental
Chemical and Reagents
All chemicals and reagents were of analytical grade, distilled water was used throughout the analysis. Technical grade samples of indoxacarb used as standard. Solutions (1mg mL-1) were prepared in acetone. Sulfuric acid (20%) was prepared by dissolving appropriate amount of compound in distilled water; the furfural reagent was prepared by dissolving 2 ml of the compound in 100 ml acetone.
Extraction of insecticide in biological material
Ammonium sulphate (10 gm) was added to samples of biological material (stomach, intestine, liver-spleen and kidney) about 100 gm containing indoxacarb. The sample was then minced individually with water and then extracted in separating funnel with diethyl ether (100ml). The ether extract was transferred to evaporating dish and the aqueous phase was re-extracted with diethyl ether (2 × 50 ml). The extract was combined and the solvent was evaporated at room temp. The residues were dissolved in ethanol (2 ml) and solution was used for further analysis.
Thin layer Chromatography
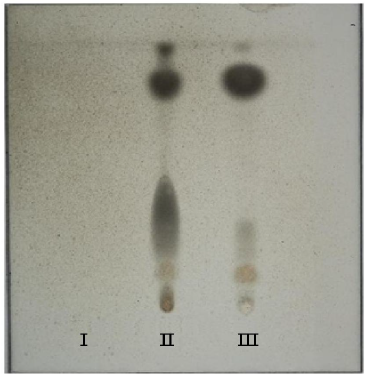
Chromatography was performed on 10 × 10 cm silica gel 60 F254 HPTLC plate (Merck Darmstadt, Germany 1.05628.0001 Desaga (Heidelbarg, Germany). Extract of biological tissue material (10µl) which does contain any poison (Blank sample) along with a standard stock solution of indoxacarb and extract from biological tissue material (10µl) were spotted manually. The plate was developed in previously saturated HPTLC chamber with n-hexane: acetone (8:2) as mobile phase to distance of 9 cm. After development, the plate was dried in air, spray with 20% sulphuric acid followed by (2%) furfural and plate was kept in oven at 90 0c for 10 min. The grayish black spot was observed on HPTLC plate for standard as well as biological tissue (Figure II) at hRf 22 and 90.
Results and Discussion
Indoxacarb on acidic hydrolysis gives three metabolites namely 4-trifloro-methoxy-phenyl amine (II), Oxidiazine (III) and acetic acid (IV) with furfural gives grayish black spot at hRf 22 and 90. This is shown in figure (III). Distinguished grayish black spot from standard Indoxacarb and Indoxacarb from visceral extract were observed at hRf 22 and 90 from recovery experiment the intensity of the spot obtained for the extracts of visceral tissue were compared with those from the standard and found to be most similar to the spot resulting from the 9 mg (10ml) std sol of Indoxacarb, hence the recovery for each insecticide was 90%.
Acknowledgement
Author thanks to Director General, legal and technical, Home Department, Maharashtra Government, Mumbai, Maharashtra for his kind help in carrying out this work.
References
- Bruno lapied, Francose Gerelleav, David B Sattelle. Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels. British Journal of Pharmacology 132 (2001): 587-595.
- Merck Index, Merek and Co. Inc. New Jercy 13 (1997): 893.
- P Glynn Tillman, Glenn G Hammes, Matthew Sacher, Michael Connair E, et al. Toxicity of a formulation of the insecticide indoxacarb to the tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae), and the big?eyed bug, Geocoris punctipes (Hemiptera: Lygaeidae). Pest Management Science 58 (2002): 92-100.
- Pathan, Asif M., Mohammad A. Baseer, and Subhash B. Junne. Application of an organometallic reagent sodium nitroprusside for the detection of organophosphate insecticide monocrotophos. JPC-Journal of Planar Chromatography-Modern TLC 30 (2017): 216-218.
- Vijay R. Chandegaonkar, Deepak C. Joshi Devanand B. Shinde, Dhananjay V. Mane. Thin-Layer Chromatographic Analysis of Boom Flower (Nitrobenzene) In Biological Materials. International journal of plant, animal and Environment sciences 6 (2016): 76-79.
- Fritz Feigl. Spot test in Organic analysis, Elsevier Publisher Ltd., New Delhi- 24 7 (2005): 444-445.
- R.Pawar, N.R. Gosavi, B.P.More and B.B.Daundakar. Chlorpyrifos In Biological Samples by HPTLC. International Journal of Plant, Animal and Environmental Sciences 5 (2015): 300-304.
Citation: P. V. More, V. R. Chandegaonkar, N. R. Gosavi, B. P. More, K. V. Kulkarni. A New Chromogenic Spray Reagent for Detection and Identification of Indoxacarb in Visceral Material by HPTLC. International Journal of Applied Biology and Pharmaceutical Technology 10 (2019): 003-007.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
