Promising Therapeutic Targets to halt the Global Pandemic of SARS CoV-2
Archana Chaudhary, Rizwanul Haque*
Department of Biotechnology, School of Earth Biological & Environmental Sciences, Central University of South Bihar, Gaya, Bihar, India
*Corresponding Author: Rizwanul Haque, Professor, Department of Biotechnology, School of Earth Biological & Environmental Sciences, Central University of South Bihar, Gaya, Bihar, India
Received: 11 June 2020; Accepted: 23 June 2020; Published: 03 July 2020
Article Information
Citation:
Archana Chaudhary, Rizwanul Haque. Promising Therapeutic Targets to halt the Global Pandemic of SARS CoV-2. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 144-159.
View / Download Pdf Share at FacebookAbstract
Abstract
The global coronavirus pandemic has posed serious challenges to entire world especially to the healthcare community. The widespread distribution of this virus has led to a major public health concern globally. In response to this crisis we have very rare specific tools to control the growing epidemic and treat those who are sick. We rely on quarantine, isolation, and infection-control measures to prevent disease spread and on supportive care for those who become ill. Now the race is to find viable strategies to discover potential candidate drugs to improve the immune system of patients to win the fight against SARS CoV-2, but at present no specific antiviral treatments or vaccines have been confirmed effective. In current situation while the whole mankind is eagerly waiting for a suitable vaccine, drugs presently being used to treat other diseases can be scrutinized as treatment option for COVID-19 pandemic. Although researchers all over the world are working to investigate the key features, pathogenesis and treatment options, it is deemed necessary to focus on existing competitive therapeutic options and cross-resistance of other viral drugs or vaccines. Here we summarized various promising candidates which are currently being used to treat other illnesses but can be a game changer in case of SARS CoV-2 virus to tackle this emergency. This review on recent updates will surely downsize the translational gap between preclinical testing results and clinical outcomes, which is a notable problem in the rapid development of an effective treatment options to fight this global crises.
Keywords
<p>Coronavirus; SARS-CoV-2; COVID-19; Drug repurposing; Vaccine</p>
Article Details
Introduction
Research team around the world are working on potential treatments and vaccines for the new coronavirus disease known as COVID-19. Various efforts for the development of suitable vaccines are underway, but according to WHO, it is estimated that potential vaccine for COVID-19 will take about 18 months to be available. Even so a suitable vaccine is likely to take more than a year prior it can used to tackle the outbreak because there is not a single magic bullet, but it demands various drugs in our ordnance depot and we will be needed to be used in amalgamation with others. So there is an urgent need to move quickly to point out the existing drugs that are effectual in clinical trials so that we can start medicating patients as rapidly as possible to tackle this global pandemic. Moreover the drugs that can be evaluated and perceive about the targets, the more likely we are to acquire something which is worthwhile. According to Rismanbaf 2020, in inclusion to the drugs currently advised to treat COVID-19, repurposing of existing medicines can also be cast off due to their consequences reported in clinical studies [1]. Lei et al. reported that the medicament of 51 COVID-19 patients with traditional Chinese medicine, interferon, Lopinavir, Ritonavir and short-term (3 to 5 days) corticosteroids was fortunate and resulted in betterment and discharge of 50 patients as well [2]. According to rheumatologically point of view, except for respiratory failure, the critical COVID-19 patients have common features like -1) sudden deterioration of disease around one to two weeks after onset; 2) much lower level of lymphocytes, especially natural killer (NK) cells in peripheral blood; 3) extremely high inflammatory parameters, including C reactive protein (CRP) and pro-inflammatory cytokines (IL-6, TNFα, IL-8, et al); 4) destroyed immune system revealed by atrophy of spleen and lymph nodes, along with reduced lymphocytes in lymphoid organs; 5) the majority of infiltrated immune cells in lung lesion are monocytes and macrophages, but minimal lymphocytes infiltration; 6) mimicry of vasculitis, hypercoagulability and multiple organs damage [3], Based on the above characteristics of COVID-19, in this review we compiled the possible drug targets against this emergency. Various targets for direct-acting antivirals are being look over to manage this viral infections [4, 5]. According to Agostini et al. One grade of antiviral compounds, nucleoside analogues, imitates naturally occurring nucleosides to obstruct viral replication [6]. While these compounds have been victorious therapeutics for diverse viral infections, mutagenic nucleoside analogues, such as ribavirin and 5-fluorouracil, have been ineffective in combating SARS CoV-2. Wang et al. revealed that remdesivir in combination with chloroquine which is a cheap and safe drug are highly effective in the control of coronavirus infection in vitro [7]. Chloroquine is extensively assigned in the whole body, together with lung, following oral administration. The EC90 value of chloroquine against SARS CoV-2 in Vero E6 cells was 6.90 μM, which can be clinically achievable as demonstrated in the plasma of rheumatoid arthritis patients who received 500 mg administration; therefore, it is much clinically applicable against the global epidemic. His report also suggested that EC90 value of remdesivir against COVID-19 in Vero E6 cells was 1.76 μM, suggesting its working concentration is likely to be achieved in NHP so it might be a successful candidate to inhibit viral infection efficiently in a human cell line (human liver cancer Huh-7 cells), which is sensitive to SARS CoV-2 viral infection. The drug which is very effective against zika as well ebola virus -Azithromycin (a macrolide antibacterial) and hydroxychloroquine combination were also reported to treat COVID-19 patients [8]. These molecules may decrease the inflammatory responses and production of excessive cytokine accompanying viral infections. The immunomodulatory mechanisms may be due to decreasing chemotaxis of neutrophils to lungs by inhibiting cytokines and formation of reactive oxygen species. According to Wu et al. 2020, the above described drugs and therapeutic agents include antiviral agents like oseltamivir and supporting agents like Ascorbic acid, Azithromycin, Corticosteroids, Nitric oxide, IL-6 antagonists etc might play a vital role in prevention and control of COVID-19 patients until the approval of vaccines and specific drugs targeting SARS-CoV-2 [9]. Apart from this several Ayurveda medicines are also included in the list of possible drugs to combat this epidemic such as Shadanga Paniya, Agastya Harityaki and Samshamani Vati [10]. These drugs were also recommended by Ministry of Ayush, Shadanga Paniya is a herbal formulation that mainly comprises Cyperus rotundus, Fumaria indica, Vetiveria zizanioides, Pterocarpus santalinus, Pavonia odorata, and Zingiber officinale. This herbal combination is mainly recommended for the treatment of symptoms such as high fever, shivering, muscle aches, headache, loss of appetite, dehydration, fatigue, restlessness, excessive thirst, irritability, and burning sensation. Agastya Harityaki is a potent polyherbal Ayurverdic medicine mainly advised for respiratory problems such as asthma, pneumonia, and chronic bronchitis. The medicine is reported to have antiviral, antibacterial, antifungal, antioxidant, anticarcinogenic, antiaging, antidiabetic, antiulcer, cardioprotective, hepatoprotective, and wound healing properties. Samshamani Vati have tremendous role in the treatment of acute to chronic fever and anemia (500 mg twice a day). Tinospora cordifolia is the main ingredient and responsible for anti-inflammatory and antipyretic properties of Samshamani Vati. Including this Pratimarsha Nasya (Anu taila/sesame oil) has immune booster as well as curative aspect for the challange of Nasobronchial diseases and enhances the respiratory immunity. The compositions present in sesame oil are well known for anti-inflammatory, antipyretic, and antibacterial proprieties. Another potential agents which comprises Trikatu (Pippali, Marich, and Shunthi) and Tulasi is also given green flag by the Ministry of Ayush for the prevention and control of coronavirus infection in India [10]. Khalili et al reported that several antiviral drugs like ribavirin may have potential role in confronting the challenge of the outbreak of SARS CoV-2. Ribavirin, that has an existing inventory and reliable supply chain may be a priority recommendation for therapies developed for the COVID-19 pandemic [11].
In this emergency our country also have plenty of options for repurposing most effective drugs along with antibiotics to treat COVID-19 and our health workers desperately need these information (as summarized in table 1) to limit the current mortality rate. This repurposed strategy might be the basic reason for moderate death ratio in India (0.04 deaths/ 1milion people) as compared to other countries like US & Italy (178 deaths/ 1milion people).
Table 1: Possible candidates for covid-19
Other Possible Drugs- Apart from the above mentioned repurpose drugs there are other drugs which gained less access against coronavirus outbreak but may be a possible game changer if applicable for investigation. These includes various antiviral such as tipranavir, fosamprenavir, enzaplatovir, presatovir, abacavir, bortezomib, elvitegravir, maribavir, raltegravir, montelukast, deoxyrhapontin, polydatin, chalcone, disulfiram, carmofur, shikonin, ebselen, tideglusib, PX12, TDZD-8, cyclosporin A, and cinanserin as reported by joint research team of the Shanghai Institute of Materia Medica and Shanghai Tech University. The team also claimed several herbal medicines such as Rhizoma Polygoni Cuspidati and Radix Sophorae Tonkinensis to be effective against SARS-COV-2 [16]. In addition to this Pawar 2020 also demonstrated that other glycopeptide antibiotics like Oritavancin, Dalbavancin, Bafilomycin A1, Ascorbic acid (Vitamin C) and Telavancin may be exploited against SARS-CoV, Ebola virus and MERS-CoV transcription and replication-competent virus-like particles [8].
Vaccines Targets- The coronavirus emergency may be an inevitable fact of mankind, but there are many strategies available which help to protect ourselves from infection and to treat the disease when it has been developed. At present clinical management includes infection prevention, control measures and supportive care including supplementary oxygen and mechanical ventilation when indicated. The FDA has approved the using of blood plasma from patients who have recovered from COVID-19 with a high neutralizing antibody titer and they may be a valuable donor source of convalescent plasma (CP) (chen et al. 2020) [31, 32]. While many countries across the world are working toward a vaccine against SARS-CoV-2, with much successful clinical trial. According to Praveen Duddu’s article (titled- Coronavirus treatment: Vaccines/drugs in the pipeline for COVID-19) various vaccines are under ways and passed many significant trial to counter this epidemic. Here we also cumulated the current status of vaccine development which shed a positive hope to overcome from this standstill situation of mankind and for which the whole life is eagerly waiting.
Table 2: Current status of vaccine development
Discussion
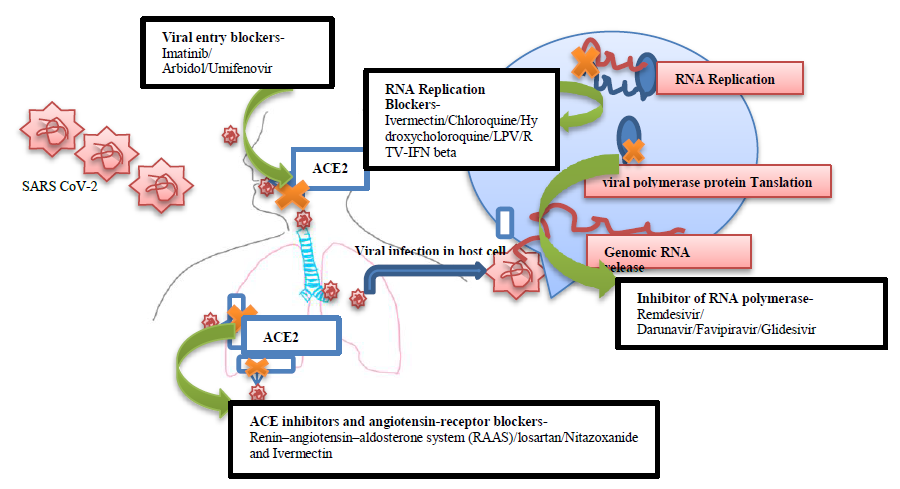
China and rest of the world have faced an outbreak of a novel Corona virus which became a global pandemic with high morbidity and mortality rate. Researchers around the world pushing forward with several attempts to manufacture suitable vaccines and treatments to decelerate the emergency situation and lessen the disease’s damage. An effective and scalable vaccine is likely to take over a year before it can used to tackle the global pandemic. While waiting for suitable vaccine or drugs Repurposing the already existed drugs may create a possible hope to combat COVID-19 emergency. In this emergency situation we need to move quickly to identify existing drugs that are effective in clinical trials so that the world can begin treating patients as rapidly as possible .In this study, we presented a recent updates on all putative repurposable drugs for effective clinical trials and current status of related vaccines stages for potential treatment of 2019-nCoV/SARS-CoV-2. The mysterious coronavirus outbreak comes with a usual symptom of pneumonia and acute respiratory distress syndrome; we have several ways or potential drugs to treat these symptoms with promising approaches to reduce the effect. These promising candidates are summarized above (Table 1) which are currently being used to treat other illnesses but can be investigated as treatments for COVID-19 to halt the emergency situation. The pandemic has rally the development of novel coronavirus vaccines across the world in this report we summarized the current updates of the coronavirus vaccines in various stages of development, across the world (Table-2). Possible target of these drugs were also described in the figure-1. Even after a new vaccine candidate has been shown to offer immunity against the coronavirus in humans, it needs to be tested in larger numbers of people to ensure it is safe to use. Manufacturing and distributing a vaccine at the scale needed to tackle this pandemic will also present significant challenges. This study will surely downsize the translational gap between preclinical testing results and clinical outcomes, which is a notable problem in the rapid development of an effective treatment options to fight this global crises. From a translational perspective, if broadly applied, the above updates will helpful in speed up the potential vaccine development which hopefully provides a greater degree of protection, not just against the COVID-19 virus, but also against the next viral threat. Likewise, it is mandatory to follow the WHO guidelines to limit the spread of COVID-19 until applicable drugs and vaccines have been developed.
Acknowledgements
We would like to thanks Central University of South Bihar, Gaya as well as CSIR-UGC & DST-SERB for financial support.
References
- Rismanbaf A. Potential treatments for COVID-19; a narrative literature review. Archives of Academic Emergency Medicine 8 (2020).
- Lei L, Jian-ya G, Hu W. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. Epub Ahead of Print (2020).
- Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clinical Immunology (2020): 108393.
- De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Review of Anti-Infective Therapy 4 (2006): 291-302.
- Zumla A, Chan JF, Azhar EI, et al. Coronaviruses—drug discovery and therapeutic options. Nature Reviews Drug Discovery 15 (2016): 327-347.
- Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. Journal of Virology 93 (2019).
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research 30 (2020): 269-271.
- Pawar AY. Combating devastating COVID-19 by drug repurposing. International Journal of Antimicrobial Agents (2020).
- Wu R, Wang L, Kuo HC, et al. An update on current therapeutic drugs treating COVID-19. Current Pharmacology Reports 1 (2020).
- Maurya VK, Kumar S, Bhatt ML, et al. Therapeutic Development and Drugs for the Treatment of COVID-19. InCoronavirus Disease 2019 (COVID-19) (2020): 109-126.
- Khalili JS, Zhu H, Mak NS, et al. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID? Journal of Medical Virology (2020).
- Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. New England Journal of Medicine 382 (2020): 2327-2336.
- Jiang F, Deng L, Zhang L, et al. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). Journal of General Internal Medicine (2020): 1-5.
- Faccenda E, Armstrong JF, Harding SD, et al. Coronavirus information. IUPHAR/BPS Guide to Pharmacology. Available online: https://www. guidetopharmacology. org/coronavius. jsp (2020).
- Abd El-Aziz TM, Stockand JD. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infection, Genetics and Evolution (2020): 104327.
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discoveries & Therapeutics 14 (2020): 58-60.
- Haviernik J, Štefánik M, Fojtíková M, et al. Arbidol (Umifenovir): a broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses 10 (2018): 184.
- Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. New England Journal of Medicine (2020)
- Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Research (2020): 104762.
- Vaduganathan M, Vardeny O, Michel T, et al. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. New England Journal of Medicine 382 (2020): 1653-1659.
- Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 11 (2020): 222.
- Hall Jr DC, Ji HF. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Medicine and Infectious Disease (2020): 101646.
- Al Saleh AS, Sher T, Gertz MA. Multiple Myeloma in the Time of COVID-19. Acta Haematologica (2020): 1-7.
- Lythgoe MP, Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends in Pharmacological Sciences (2020).
- Zhou Y, Hou Y, Shen J, et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 6 (2020): 14.
- Lu CC, Chen MY, Chang YL. Potential therapeutic agents against COVID-19: What we know so far. Journal of the Chinese Medical Association (2020).
- Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sciences (2020): 117592.
- Barzegar M, Mirmosayyeb O, Nehzat N, et al. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurology-Neuroimmunology Neuroinflammation 7 (2020).
- Baden LR, Rubin EJ. Covid-19—the search for effective therapy.
- Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turkish Journal of Medical Sciences 50 (2020): 611-619.
- Chen L, Xiong J, Bao L, et al. Convalescent plasma as a potential therapy for COVID-19. The Lancet Infectious Diseases 20 (2020): 398-400.
- Senanayake SL. Drug repurposing strategies for COVID-19.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
