The Constrained Disorder Principle Accounts for The Structure and Function of Water as An Ultimate Biosensor and Bioreactor in Biological Systems
Hillel Lehmann1, David Arkadir2, Yaron Ilan1
1Dearpartment of Medicine and Hebrew University, Faculty of Medicine, Hadassah Medical Center, Ein-Kerem, Jerusalem, Israel
2Department of Neurology, Hebrew University, Faculty of Medicine, and Hadassah Medical Center, Department of Medicine, Jerusalem, Israel
*Corresponding author: Yaron Ilan, Dearpartment of Medicine and Hebrew University, Faculty of Medicine, Hadassah Medical Center, Ein-Kerem, Jerusalem, Israel.
Received: 29 March 2023; Accepted: 04 April 2023; Published: 17 May 2023
Article Information
Citation: Yaron Ilan. The Constrained Disorder Principle Accounts for The Structure and Function of Water as An Ultimate Biosensor and Bioreactor in Biological Systems. International Journal of Applied Biology and Pharmaceutical Technology. 14 (2023): 31-41.
View / Download Pdf Share at FacebookAbstract
The constrained disorder principle (CDP) defines living organisms as systems that comprise an intrinsic disorder bounded by dynamic boundaries. Water plays a substantial role in multiple biological processes affecting nucleic acids' and proteins' structure and function. The paper describes the CDP-accounted water structure dynamicity and variability in water isomers ratio. Per the CDP, the variability in the ratios between water isomers is mandated for the inherent variability of biological systems. This variability underlies water's unique functions and enables the flexibility and adaptability required to cope with internal and external environmental changes. The CDP-dependent water structures also determine energy usage. The paper presents water molecules as ultimate biosensors for stimuli in the environment and as the ultimate bioreactors that respond to perturbations by changing the structure and function of the molecules in their vicinity. Finally, it describes the potential of using water-based signatures of variability to improve artificial intelligence-based algorithms developed for correcting disturbances of biological systems by increasing the degree of disorder in systems or tightening the disorder's boundaries.
Keywords
<p>CDP: constrained disorder principal</p>
Article Details
Abbreviations
BCI: Brain-Computer Interface; AI: artificial intelligence; EEG: electroencephalography; fNIRS: functional near-infrared spectroscopy; EMG: electromyography; EOG: electrooculography; SSVEPs: steady-state visually evoked potentials; SSSEP: steady-state somatosensory evoked potential; MTs: Microtubules; JNK: Jun N-terminal kinase; Con A: concanavalin A; AD: Alzheimer's disease; BSV: Brain signal variability; fMRI: functional magnetic resonance imaging; GAD: generalized anxiety disorder; RSV: Regional signal variability; SMR: sensorimotor rhythm; VR: virtual reality;
Introduction
Brain-computer interface (BCI) has gained much interest over the last few years. BCI is a communication system of brain signals to control malfunctioning organs or external devices [1]. It enables control of an external output device by interpreting the neural activity. The generation of such systems may be independent of the nervous system, such as in Passive BCI, assisting patients with motor disabilities [2]. Motor BCI comprises electrical recordings from the motor cortex of paralyzed humans. The computer decodes the signals and can drive robotic arms or restore movement in a paralyzed hand by stimulating the muscles in the forearm [3]. Cognitive assessment and training can use BCI. Verbal-motor-free BCI-based tests assessed cognitive domains in patients with Amyotrophic Lateral Sclerosis were developed [2]. Integrating a BCI with the sensory cortex can augment dexterity for improved fine control. BCIs can restore vision in people with acquired blindness and control epileptic seizures [3]. BCI-based cognitive training is part of neurofeedback therapy for neurological developmental disorders, including autism, attention-deficit/hyperactivity disorder, stroke patients, and elderly subjects [2]. Brain's plasticity and Hebbian-based motor recovery use BCI for rewarding cortical activity. These are associated with sensory-motor rhythms using self-guided and assistive modalities [4]. Non-invasive BCIs comprise proprioceptive feedback loops, which include numerous sensory variables. It allows the modulation of brain signals to improve the hand's function. Based on BCI, applications are developed for improving learning, communication, social, memory, attention, visuospatial, creative, collaboration, and emotional skills [5].
In the present paper, we describe several of the gaps in clinical implementation and improving the effectiveness of BCI. We present using brain adjuvants and second-generation artificial intelligence (AI) methods to enhance the signals stream and ensure an improved sustainable response.
Current gaps in the development and implementation of BCI
BCIs typically consist of three components: a sensor that records brain neural activity; a decoder that processes the signal input by extracting predictive features and classifying the intended movement based on signal features; and an effector, an external device, typically a robotic limb or a screen cursor, that receives instructions from the decoder to execute an order [6]. BCI's clinical application rests on the premise that activity in at least one cortical region associated with motor or sensory functioning is intact. BCI can bypass brain lesions in the cortex or spine by linking cerebral activity to the effector [6]. The first-in-human implanted, wireless, motor neuroprosthesis used an endovascular stent-electrode. The electrode transmitted signals from the motor cortex for numerous command control digital devices in two patients with flaccid upper limb paralysis [7]. Improvement in the development of BCI-based systems occurred due to progress in understanding neural decoders, systems for neural feature extraction, and brain recording modalities [8]. Nevertheless, there remain multiple challenges to the clinical use of BCIs. Improved engineering and computation overcome some of these gaps. However, there are significant challenges in the clinical implementation of these systems. The low reliability of some techniques used today contributes to the end-users' low adaption rate.
Table 1 summarizes some challenges currently faced in implementing BCI in clinical practice, including the technological and computation barriers BCI systems depend on sensors and associated hardware that acquire brain signals. Improvements in this hardware are critical to the future of BCIs. Traditional BCI platforms involve recording brain signals via Electroencephalography (EEG). These systems comprise a rule-based translation algorithm that produces the control commands [1]. Non-invasive procedures based on EEG lack the spatial resolution to record detailed activity at the neuronal circuit level [9]. Signals travel a distance before being acquired by the EEG machine, and the noise and artifacts are causing fundamental problems. EEG requires good performance in different environments and reliability despite the noise generated by devices. Many BCI targets patients surrounded by many electronic pieces of equipment [10].
Table 1: Several challenges for implementing BCI in clinical practice
|
Hardware / Software |
non-invasive |
a. Low accuracy b. Low reliability c. Isolation of targeted structures d. Lowered accuracy of using non-invasive measures |
|
invasive |
e. Safety - infections, rejection f. Recharged in situ g. Replacement of probes following a failure h. Space limitations i. High cost |
|
|
Decoding |
j. High variability of signal features and continuous change k. Reading and data extraction from recorded data |
|
|
Validation and cost |
l. Low reliability m. Low accuracy of information transfer rate n. Sophisticated o. Small user population p. Delivery of nanomaterials and processing Reading and data extraction from recorded data |
|
|
Ethical |
q. Patient expectations r. Concept of personal identity s. Validity of informed consent |
|
Wireless recording, machine learning analysis, and real-time temporal resolution can improve EEG-based BCI [11]. Placing electrodes on the cortical surface is less invasive but less precise and associated with decreased accuracy [3]. Several challenges include space limitations, replacement of probes following failure, isolation of targeted structures, delivery of nanomaterials, and processing of the recorded data [12]. Functional near-infrared spectroscopy (fNIRS), electromyography (EMG), electrooculography (EOG), and eye tracker combine with EEG [13]. Implementation of multi-sensor data fusion and machine learning-based translation algorithms improves the accuracy of such systems [1]. Artificial intelligence and deep learning algorithms can lead to faster and more accurate sensory input classifications. Neural engineering improved neural recording techniques and clinical translation of neural interfaces.
The electronics used are smaller and faster than neurons. However, challenges of decoding the neural circuits are yet to be overcome [12]. Visual-based BCIs that use P300 or steady-state visually evoked potentials (SSVEPs) improve functionality. The steady-state somatosensory evoked potential (SSSEP) BCIs enhance the visual fatigue that occurs with these BCIs. These are based on selective tactile attention and can overcome motor activity's reduced reliability of motor activity [14]. Invasive BCI systems use implanted electrodes and face a range of complex issues. These systems must be safe and remain intact, functional, and reliable for decades [15]. Long-term safety is of concern as the implant could be associated with infections. These systems must be recharged in situ or have batteries that last for years or decades; they have robust, comfortable, convenient, and discreet external elements that could easily interface with high-performance applications [15].
Motor imagination/movement tasks using functional and reactive tasks combined with cognitive tasks for brain signals increase BCI accuracy [13]. These include using motor imagination with steady-state evoked visual potentials (SSVEP) and motor imagination with P300. SSVEP is most widely combined with P300 to increase the number of commands [13]. Combining more than two modalities is developed for improved brain imaging and prosthesis control. Hybridizing several modalities can augment the number of control commands, improve classification accuracy and reduce the signal detection time. Hybrid BCI systems with multimodal sensory can improve functions [6]. Neurofeedback uses BCI systems to enable the real-time display of subjects' brain activity while performing a task. BCI in this setting allows patients to control their cortical activity. Neurofeedback has improved BCI classification enhancing user control over BCI output [6]. New interface designs may enhance discomfort from daily, long-term use of BCI by ergonomic approaches [16].
High-fidelity connectivity with minor groups of neurons requires the placement of microelectrodes in the cortex [3]. A better understanding of the sensory components is also necessary for improving BCI. A significant challenge for both invasive and non-invasive BCI is decoding the signal. The requirement for months of training, and the marked inter-person differences, make it hard to achieve standardization. Inconsistency of signal features affected by multiple variables, including the users' mental state and circumstances, requires adaptive BCI algorithms and deep learning for proper function [17]. It is unclear why some BCI paradigms or features are effective with some patients and some are not [18]. Establishing systems using BCIs for subjects with disabilities involves validating their value in improving quality of life and cost-effectiveness [19-20].
The validation of BCIs for rehabilitation after strokes or other disorders requires careful comparisons with conventional methods. Current BCIs, with their incomplete abilities, are potentially helpful, mainly for people with very severe disabilities and relatively small populations. Commercial interests have no adequate incentive to produce or promote their widespread dissemination [21]. Ethical issues of using BCI are significant concerns. These include managing patient expectations, the concept of personal identity, and the validity of informed consent [22]. Privacy is a vital issue as the captured neural signals enable access to private data. There are concerns about how BCI data is stored and protected [23]. Current methods for overcoming these gaps focus on improving technology, including computation barriers. Beyond the technological challenges described above, several inherent gaps in the brain function itself remain to improve BCI clinical effectiveness.
Using brain-targeted adjuvants for improving BCI
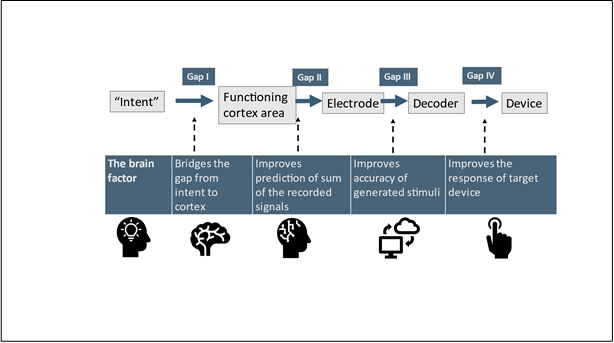
There are multiple engineering and neurological challenges to the clinical application of BCI [12]. Using BCI, targeting brain-relevant mechanisms provides a new venue for improved clinical outcomes. We describe three methods for improving BCI by targeting several brain pathways. Figure 1 presents several gaps that challenge BCI's implementation and improve its efficacy. It shows a schematic presentation of these methods for bridging the current BCI gaps for improving information streaming and the effectiveness of output stimuli.
Microtubules (MTs) are dynamic cytoplasmic tubular polymers that form thecell cytoskeleton [24]. MTs provide the intracellular transport of secretory vesicles, organelles, and intracellular macromolecular assemblies. MTs are involved in cell mitosis and meiosis [25, 26]. MTs are associated with the innate and adaptive arms of the immune system and determine the dynamics of inflammatory cells [27-31]. Polarized CD4 Th1, Th17, CD8 T cells, and NK cells induce MTs destabilization within neurons in multiple sclerosis. Lymphocytes with cytolytic activity drive MTs' axonal destabilization independent of neuronal death [31].
Bidirectional communication between the brain and the intestine occurs in health and disease. Gut-based therapy is a method for affecting systemic systems by generating signals in the intestine and was shown effective in pre-clinical models [32-42]. Preliminary data support the use of this method in humans [33, 36, 43-47]. MTs play a role in gut functions. Jun N-terminal kinase (JNK) is necessary for the elongation of the gut tube and regulates MTs architecture, preserving adhesive contacts between cells in the intestine [48]. Tao-1 destabilizes MTs at the actin-rich cortex, and its loss is associated with the disordered migration of germ cells out of the gut epithelium and subsequent cell death [49].
Methods for altering microglial function by targeting the gut-brain axis carry less toxicity and are easier to control. Targeting the gut MTs using low-dose colchicine exerts a potent anti-inflammatory effect on target organs. Oral administration of non-absorbable low-dose colchicine alleviated immune-mediated hepatitis in mice injected with concanavalin A (Con A). Similarly, it eased the inflammation associated with the metabolic syndrome in the high-fat diet model of type 2 diabetes and fatty liver disease (unpublished). Microglial cells constitute 5-12% of the cells in the brain and are involved in brain homeostasis and its response to triggers [50-52, 53]. Homeostatic dysregulation of the brain's immune system regulated by microglia plays a role in neurodegenerative disorders, including Alzheimer's disease (AD) [54-57]. During disease, the microglia become inflammatory while losing their homeostatic molecular functions [58-62]. In the brain, gut MTs using low-dose colchicine was beneficial in a mouse model of acute neurodegeneration mediated by microglia. The beneficial effect was associated with altering gene expression in genes linked with AD, showing that targeting gut MTs can modulate APOE-regulated genes in microglia in AD (unpublished). This effect may be related to the gut-brain connection by impacting the microbiome [63].
Applying brain-targeted adjuvant to BCI, using non-absorbable low-dose colchicine, which has a high safety profile and targets the gut, can bridge several BCI gaps. The use of a brain adjuvant can improve the intent signals. It may be related to the potential role of MTs in consciousness or other yet-to-be-explored mechanisms [64-66]. The adjuvant can improve the cortex-generated signals and may enhance associations between the relevant brain area with other areas, leading to a better sum of the inputs received from the cortex. At the output stage, adjuvants can improve both the generated stimuli and the closed-loop regulation back to the cortex, thus improving the effectiveness of BCI.
Implementing second-generation artificial intelligence-based variability for improving and sustaining BCI effectiveness
First-generation AI systems analyze large datasets and predict prognoses. At the same time, these systems aid end-users, patients, and healthcare providers; their overall penetration and everyday use are lower than anticipated [67]. Second-generation systems can improve clinical outcomes and adherence by patients and physicians [67]. These platforms can improve organ function and response to therapies while controlling for the dynamic nature of the host and disease. They overcome the "big data" challenges by implementing an n=1 concept, directing the results toward a single subject providing a method for personalizing the therapy in a clinically meaningful way [67]. Second-generation systems provide a platform for generating clinically significant databases, thus enabling better use of the data generated [67].
Variability characterizes multiple functions in nature and can serve as a method for improving biological systems [68-84]. The dynamicity of biological processes determines disease progression, manifestations, and response to stimuli and drugs. Variability characterizes the normal function of many organs, such as the variability in heart rate, gait, breathing, and others [85-88], and is inherent to multiple brain functions [69, 72, 76, 89]. Brain signal variability (BSV) characterizes brain function and reflects the capacity for state transition of neural activities. Prenatal fMRI defines variability patterns of the brain networks and shows the spatial distribution and individual variability in network architecture. Individual variability manifests by decreasing sensorimotor, visual, subcortical, dorsal, and ventral attention networks [90]. Structural brain development covariance may underlie brain variability concerning cognition and disease vulnerability [91]. Genetic variation is associated with altered response to deep brain stimulation in Parkinson's disease, suggesting that variability in brain function is linked to genotypes [92]. The diurnal physiological variability in neuro-metabolite levels suggests a link between chronobiology in brain variability [93].
Patients with a generalized anxiety disorder (GAD) show decreased BSV in widespread regions, including the visual, sensorimotor, frontoparietal, limbic, and thalamus. These systems have decreased BSV associated with an inflexible brain state transfer pattern. A correlation between BSV and trait anxiety score was positive in patients [94]. Differences in cognitive modulation of brain signal variability are associated with subject differences in motor expertise. This process underlies differences in information-processing capacity and information integration during cognitive processing [95]. Regional signal variability (RSV) measures efficiency and modulatory capacity within brain regions and indicates endogenous pain modulatory system responsivity to training following repeated bouts of pain [96]. The second-generation AI system implements personalized variability parameters into treatment algorithms to improve response to chronic interventions in various diseases, including brain disorders [68, 70, 73, 77-82, 97-112]. Implementing second-generation AI in BCI can enhance the accuracy of recording brain inputs. As brain signals are not regular and are dynamic by nature, a system that continuously adapts to changes can improve the accuracy of the information.
The variability in brain networks underlies subject differences in cognition and behaviors [90]. The EEG's sensorimotor rhythms (SMR) used for BCI to rehabilitate motor impairments varies over time and across subjects. The intra- and inter-subject variabilities cause covariate shifts in data distributions that alter the transferability of model parameters among subjects. Machine learning-based methods compensate for inter-and intra-subject variability manifested in EEG-derived feature distributions [113]. Determining the response under a threatening situation showed that both inter-and intra-subject variabilities impacted the performance measured by EEG signals [114].
Second-generation AI includes methods to compensate for inter and intra-subject variabilities [67, 113]. The dynamicity of the system enables it to adapt to constant changes in the host, the disease, the response to intervention, and the environment. As many of these parameters differ between subjects and change over time in the same subject, the system continuously adapts itself to improve the patient's outcome [79, 115]. Variability in brain signals can be quantified and implemented into the second-generation algorithm to improve accuracy. Organ variability is personalized and serves as a basis for subject-tailored platforms, improving information streaming and output stimuli [115]. Second-generation systems can solve the intra-subject and inter-subject variability in the generated signals and the response to stimuli. These systems can improve the response to therapies by implementing signatures of disease-related variabilities into the treatment regimens [68, 70, 73, 77, 80, 82, 98-111]. Implementing a system that controls changes adaptively provides a means for better translation of information into improved personalized stimuli in a dynamic way [115, 116]. The system provides an enhanced platform for a closed loop between brain areas, enabling better summing of the signals between the target organ and the brain.
Examples include systems that follow eye movements that may precede the cortex and quantify and implement into the algorithm eye movement variability [117]. It collects EEG/ECoG data from multiple attempts to quantify personalized variability patterns and add them to cortex-derived data [118, 119]. It quantifies signal variability by adding stimuli to "relevant" and "irrelevant" brain areas and brain autonomic signals, including temperature alterations in different brain areas. It uses intra-brain nano-robots to measure electrical or metabolic activity alteration before cortex-derived signals [120].
Decoy of the effect of stimuli due to compensatory mechanisms and tolerance at different levels of the targets is a significant problem for BCI interventions [79]. Second-generation AI systems overcome these mechanisms, enabling a sustainable long-term response [115]. The use of low-dose colchicine under the control of the second-generation artificial intelligence system can further improve the drug's effectiveness, overcome the loss of response, and reduce side effects [29, 30, 75, 81].
Implementing inherent brain platforms for overcoming gaps in BCI
Like other complex biological systems, the brain carries information but does not necessarily have perfect structure or symmetry. The irregularity that characterizes some of its pathways provides an opportunity to apply notions of physics to this biological system [69, 72, 76, 83 79, 121]. An alliance between classical and correlative brain function improves BCI.
A direct association mediates correlations between components of complex systems between different elements of this system [122]. Direct contact or the transfer of mediators facilitates the effect [123-125]. Recently a system was developed that correlates between two parts of the immune system without direct interaction or a transfer of mediators between them [126]. The associated states may be present within the system and involve a specific correlation between donors and recipients. Learning and memory capabilities are required for such correlations to occur. A "wave type of memory" means continuous feedback from multiple brain areas, and the target organ or device for transmitting and receiving signals are mandatory for efficient correlations to occur [126, 127].
BCI can benefit from indirect correlations between different brain areas, which can implement various energy transfer types by recording or using signals and producing stimuli that are not measured using current technologies. Other kinds of energies may be involved. Recording signals from multiple brain areas that affect or result from these energies can improve the summing of inputs and outputs at various levels. The technology can expand closed-loop inputs and outputs in the brain and target device by recording "unmeasured responses." Implementing methods based on inherent brain factors can bridge the problematic gap from the intent to the cortex by using consciousness-based mechanisms for improved BCI [128-130]. The effects occur at cellular or subcellular levels. These effects propose that some of the principles of quantum physics may apply to biological systems [128, 129, 131, 132].
Translation of the intention of a human subject to stimulate a rat brain motor area responsible for the tail movement supports a non-natural computer-brain interface to induce an out-of-body effect [133]. Combining BCI with virtual reality (VR) is used to rehabilitate neurological diseases. It involves motor imagery, P300, and steady-state visual-evoked potential. Integrating VR scenes into BCI systems improved the recovery process from nervous system injuries, providing better patient feedback and promoting brain function [134].
The transfer of information between different species' brains using non-invasive methods supports the feasibility of a computer-mediated BCI that connects the neural functions between biological entities. Using these technologies is anticipated to expand the prediction of the sum of the recorded signals and the accuracy of generated stimuli at various system levels. Finally, it improves the brain-controlled target organ or device function. It scales up current BCI methods to enhance their beneficial clinical effects. BCI has become an effective solution for multiple brain and spine disorders. It provides a platform for treating numerous non-neurological diseases by improving brain-target organ regulation. Technological challenges involving engineering and computation are being worked on and continuously improved. However, multiple barriers evolve from the inherent brain function. The suggested venues of using brain adjuvants, second-generation AI in enhancing the stream of information and stimuli, and implementing brain-linked methods for improving brain inputs and outputs to targets, set the basis for improving the clinical effectiveness of BCI.
Declaration section:
Ethics approval and consent to participate:
not applicableConsent for publication:
Yes
Availability of data and materials:
Upon request
Competing interests:
YI is the founder of Oberon Sciences; other authors have nothing to disclose
Funding:
not applicable
Authors' contributions:
YI DA and HL wrote the manuscript and approved the final version
Acknowledgements:
not applicable
Authors' information (optional)
Disclosure:
YI is the founder of Oberon Sciences
References
- Chamola V, Vineet A, Nayyar A, et al. Brain-Computer Interface-Based Humanoid Control: A Review. Sensors (Basel) 20 (2020).
- Carelli L, Solca F, Faini A, et al. Brain-Computer Interface for Clinical Purposes: Cognitive Assessment and Rehabilitation. Biomed Res Int (2017): 1695290.
- Rosenfeld JV, Wong YT. Neurobionics and the brain-computer interface: current applications and future horizons. Med J Aust 206 (2017): 363-368.
- Remsik A, Young B, Vermilyea R, et al. A review of the progression and future implications of brain-computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert Rev Med Devices 13 (2016): 445-54.
- Papanastasiou G, Drigas A, Skianis C, et al. Brain computer interface based applications for training and rehabilitation of students with neurodevelopmental disorders. A literature review. Heliyon 6 (2020): e04250.
- Martini ML, Oermann EK, Opie NL, et al. Sensor Modalities for Brain-Computer Interface Technology: A Comprehensive Literature Review. Neurosurgery 86 (2020): E108-E117.
- Oxley TJ, Yoo PE, Rind GS, et al. Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: first in-human experience. J Neurointerv Surg (2020).
- Rabbani Q, Milsap G, Crone NE. The Potential for a Speech Brain-Computer Interface Using Chronic Electrocorticography. Neurotherapeutics 16 (2019): 144-165.
- Nicolas-Alonso LF, Gomez-Gil J. Brain Computer Interfaces, a Review. Sensors 12 (2012): 1211-1279.
- Shih JJ, Krusienski DJ, Wolpaw JR. Brain-Computer Interfaces in Medicine. Mayo Clinic Proceedings 87 (2012): 268-279.
- Abiri R, Borhani S, Sellers EW, et al. A comprehensive review of EEG-based brain-computer interface paradigms. J Neural Eng 16 (2019): 011001.
- Mitrasinovic S, Brown APY, Schaefer AT, et al. Silicon Valley new focus on brain computer interface: hype or hope for new applications? F1000Res 7 (2018): 1327.
- Hong KS, Khan MJ. Hybrid Brain-Computer Interface Techniques for Improved Classification Accuracy and Increased Number of Commands: A Review. Front Neurorobot 11 (2017): 35.
- Ahn S, Kim K, Jun SC. Steady-State Somatosensory Evoked Potential for Brain-Computer Interface-Present and Future. Front Hum Neurosci 9 (2015): 716.
- Waldert S. Invasive vs. Non-Invasive Neuronal Signals for Brain-Machine Interfaces: Will One Prevail? Frontiers in Neuroscience 10 (2016).
- Baek HJ, Chang MH, Heo J, et al. Enhancing the Usability of Brain-Computer Interface Systems. Comput Intell Neurosci (2019): 5427154.
- Galán F, Baker S. Deafferented controllers: a fundamental failure mechanism in cortical neuroprosthetic systems. Frontiers in Behavioral Neuroscience 9 (2015).
- Nakanishi I, Baba S, Li S. Evaluation of Brain Waves as Biometrics for Driver Authentication Using Simplified Driving Simulator (2011).
- Bensmaia SJ, Miller LE. Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nature Reviews Neuroscience 15 (2014): 313-325.
- Bockbrader MA, Francisco G, Lee R, et al. Brain Computer Interfaces in Rehabilitation Medicine. PM&R 10 (2018): S233-S243.
- Olaronke I, Rhoda I, Gambo I, et al. Prospects and Problems of Brain Computer Interface in Healthcare. Current Journal of Applied Science and Technology 23 (2018): 1-7.
- Ienca M, Haselager P. Hacking the brain: brain-computer interfacing technology and the ethics of neurosecurity. Ethics and Information Technology 18 (2016): 117-129.
- Coin A, Mulder M, Dubljevi? V. Ethical Aspects of BCI Technology: What Is the State of the Art? Philosophies 5 (2020): 31.
- Pilhofer M, Ladinsky MS, McDowall AW, et al. Microtubules in bacteria: Ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton. PLoS Biol 9 (2011): e1001213.
- Derivery E, Seum C, Daeden A, et al. Polarized endosome dynamics by spindle asymmetry during asymmetric cell division. Nature 528 (2015): 280-5.
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 7 (2005): 581-90.
- Renkawitz J. Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature (2019).
- Ilan Y. Randomness in microtubule dynamics: an error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol Int (2019).
- Ilan-Ber T, Ilan Y. The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol Immunol 111 (2019): 73-82.
- Ilan Y. Microtubules: From understanding their dynamics to using them as potential therapeutic targets. J Cell Physiol 234 (2019): 7923-7937.
- Miller NM, Shriver LP, Bodiga VL, et al. Lymphocytes with cytotoxic activity induce rapid microtubule axonal destabilization independently and before signs of neuronal death. ASN Neuro 5 (2013): e00105.
- Ilan Y. Oral tolerance: can we make it work? Hum Immunol 70 (2009): 768-76.
- Ilan Y. Oral immune therapy: targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease. Clin Transl Immunology 5 (2016): e60.
- Ilan Y. Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression. Aliment Pharmacol Ther 44 (2016): 1168-1182.
- Ilan Y, Ben Ya'acov A, Shabbat Y, et al. Oral administration of a non-absorbable plant cell-expressed recombinant anti-TNF fusion protein induces immunomodulatory effects and alleviates nonalcoholic steatohepatitis. World J Gastroenterol 22 (2016): 8760-8769.
- Israeli E, Zigmond E, Lalazar G, et al. Oral mixture of autologous colon-extracted proteins for the Crohn's disease: A double-blind trial. World J Gastroenterol 21 (2015): 5685-94.
- Mizrahi M, Shabat Y, Ben Ya'acov A, et al. Alleviation of insulin resistance and liver damage by oral administration of Imm124-E is mediated by increased Tregs and associated with increased serum GLP-1 and adiponectin: results of a phase I/II clinical trial in NASH. J Inflamm Res 5 (2012): 141-50.
- Israeli E, Ilan Y. Oral administration of Alequel, a mixture of autologous colon-extracted proteins for the treatment of Crohn's disease. Therap Adv Gastroenterol 3 (2010): 23-30.
- Shibolet O, Alper R, Zlotogarov L, et al. Suppression of hepatocellular carcinoma growth via oral immune regulation towards tumor-associated antigens is associated with increased NKT and CD8+ lymphocytes. Oncology 66 (2004): 323-30.
- Nagler A, Pines M, Abadi U, et al. Oral tolerization ameliorates liver disorders associated with chronic graft versus host disease in mice. Hepatology 31 (2000): 641-8.
- Ilan Y, Sauter B, Chowdhury NR, et al. Oral tolerization to adenoviral proteins permits repeated adenovirus-mediated gene therapy in rats with pre-existing immunity to adenoviruses. Hepatology 27 (1998): 1368-76.
- Ilan Y, Gingis-Velitski S, Ben Ya'aco A, et al. A plant cell-expressed recombinant anti-TNF fusion protein is biologically active in the gut and alleviates immune-mediated hepatitis and colitis. Immunobiology 222 (2017): 544-551.
- Forkosh E, Ilan Y. The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open Heart 6 (2019): e000993.
- Ilan Y, Shailubhai K, Sanyal A. Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH. Clin Exp Immunol 193 (2018): 275-283.
- Lalazar G, Zigmond E, Weksler-Zangen S, et al. Oral Administration of beta-Glucosylceramide for the Treatment of Insulin Resistance and Nonalcoholic Steatohepatitis: Results of a Double-Blind, Placebo-Controlled Trial. J Med Food 20 (2017): 458-464.
- Lalazar G, Mizrahi M, Turgeman I, et al. Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial. J Clin Immunol 35 (2015): 399-407.
- Halota W, Ferenci P, Kozielewicz D, et al. Oral anti-CD3 immunotherapy for HCV-nonresponders is safe, promotes regulatory T cells and decreases viral load and liver enzyme levels: results of a phase-2a placebo-controlled trial. J Viral Hepat 22 (2015): 651-7.
- Dush MK, Nascone-Yoder NM. Jun N-terminal kinase maintains tissue integrity during cell rearrangement in the gut. Development 140 (2013): 1457-66.
- Pflanz R, Voigt A, Yakulov T, et al. Drosophila gene tao-1 encodes proteins with and without a Ste20 kinase domain that affect cytoskeletal architecture and cell migration differently. Open Biol 5 (2015): 140161.
- Lawson LJ, Perry VH, Dri P, et al. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39 (1990): 151-70.
- El Khoury J. Neurodegeneration and the neuroimmune system. Nat Med 16 (2010): 1369-70.
- Wang Y, Szretter KJ, Vermi W, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13 (2012): 753-60.
- Kierdorf K, Erny D, Goldmann T, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16 (2013): 273-80.
- Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 113 (2016): E1738-46.
- Hickman SE, Kingery ND, Ohsumi TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16 (2013): 1896-905.
- Chiu IM, Morimoto ET, Goodarzi H, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4 (2013): 385-401.
- Satoh J, Kino Y, Asahina N, et al. TMEM119 marks a subset of microglia in the human brain. Neuropathology 36 (2016): 39-49.
- Holtman IR, Raj DD, Miller JA, et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol Commun 3 (2015): 31.
- Moore CS, Ase AR, Kinsara A, et al. P2Y12 expression and function in alternatively activated human microglia. Neurol Neuroimmunol Neuroinflamm 2 (2015): e80.
- Butovsky O, Jedrychowski MP, Cialic R, et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol 77 (2015): 75-99.
- Naj AC, Jun G, Reitz C, et al. Effects of multiple genetic loci on age at onset in late-onset Alzheimer disease: a genome-wide association study. JAMA Neurol 71 (2014): 1394-404.
- Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47 (2017): 566-581 e9.
- Madore C, Yin Z, Leibowitz J, et al. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 52 (2020): 222-240.
- Hameroff SR. Anesthetic Action and "Quantum Consciousness": A Match Made in Olive Oil. Anesthesiology 129 (2018): 228-231.
- Hameroff SR, Craddock TJ, Tuszynski JA. Quantum effects in the understanding of consciousness. J Integr Neurosci 13 (2014): 229-52.
- Hameroff S. Quantum walks in brain microtubules--a biomolecular basis for quantum cognition? Top Cogn Sci 6 (2014): 91-7.
- Ilan Y. Second-Generation Digital Health Platforms: Placing the Patient at the Center and Focusing on Clinically Meaningful Endpoints Title: Second-Generation Artificial Intelligence Algorithms. Frontiers in Digital Health (2020).
- Kenig A, Ilan Y. A Personalized Signature and Chronotherapy-Based Platform for Improving the Efficacy of Sepsis Treatment. Front Physiol 10 (2019): 1542.
- Ilan Y. Overcoming randomness does not rule out the importance of inherent randomness for functionality. J Biosci 44 (2019).
- Khoury T, Ilan Y. Introducing Patterns of Variability for Overcoming Compensatory Adaptation of the Immune System to Immunomodulatory Agents: A Novel Method for Improving Clinical Response to Anti-TNF Therapies. Front Immunol 10 (2019): 2726.
- El-Haj M, Kanovitch D, Ilan Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: a novel platform for designing personalized immunotherapies. Immunol Res 67 (2019): 337-347.
- Ilan Y. Advanced Tailored Randomness: A Novel Approach for Improving the Efficacy of Biological Systems. J Comput Biol 27 (2020): 20-29.
- Ilan Y. Why targeting the microbiome is not so successful: can randomness overcome the adaptation that occurs following gut manipulation? Clin Exp Gastroenterol 12 (2019): 209-217.
- Ilan Y. beta-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: A Manifestation of Randomness in Biological Systems. Front Immunol 10 (2019): 1143.
- Ilan Y. Randomness in microtubule dynamics: an error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol Int 43 (2019): 739-748.
- Ilan Y. Generating randomness: making the most out of disordering a false order into a real one. J Transl Med 17 (2019): 49.
- Kessler A, Weksler-Zangen S, Ilan Y. Role of the Immune System and the Circadian Rhythm in the Pathogenesis of Chronic Pancreatitis: Establishing a Personalized Signature for Improving the Effect of Immunotherapies for Chronic Pancreatitis. Pancreas 49 (2020): 1024-1032.
- Kolben Y, Weksler-Zangen S, Ilan Y. Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: Implementing a personalized signature-based platform for chronotherapy. Obes Rev (2020).
- Ilan Y. Overcoming Compensatory Mechanisms toward Chronic Drug Administration to Ensure Long-Term, Sustainable Beneficial Effects. Mol Ther Methods Clin Dev 18 (2020): 335-344.
- Potruch A, Khoury ST, Ilan Y. The role of chronobiology in drug-resistance epilepsy: The potential use of a variability and chronotherapy-based individualized platform for improving the response to anti-seizure drugs. Seizure 80 (2020): 201-211.
- Forkosh E, Kenig A, Ilan Y. Introducing variability in targeting the microtubules: Review of current mechanisms and future directions in colchicine therapy. Pharmacol Res Perspect 8 (2020): e00616.
- Gelman R, Bayatra A, Kessler A, et al. Targeting SARS-CoV-2 receptors as a means for reducing infectivity and improving antiviral and immune response: an algorithm-based method for overcoming resistance to antiviral agents. Emerg Microbes Infect 9 (2020): 1397-1406.
- Ishay Y, Kessler A, Schwarts A, et al. Antibody response to SARS-Co-V-2, diagnostic and therapeutic implications. Hepatol Commun (2020).
- Shabat Y, Lichtenstein Y, Ilan Y. Short-Term Cohousing of Sick with Healthy or Treated Mice Alleviates the Inflammatory Response and Liver Damage. Inflammation 44 (2021): 518-525.
- Lombardi F. Chaos theory, heart rate variability, and arrhythmic mortality. Circulation 101 (2000): 8-10.
- M HA. Effect of Percutaneous Coronary Intervention on Heart Rate Variability in Coronary Artery Disease Patients. Eur Cardiol 13 (2018): 60-61.
- Konig N, Singh NB, Baumann CR, et al. Can Gait Signatures Provide Quantitative Measures for Aiding Clinical Decision-Making? A Systematic Meta-Analysis of Gait Variability Behavior in Patients with Parkinson's Disease. Front Hum Neurosci 10 (2016): 319.
- Tams C, Stephan PJ, Euliano NR, et al. Breathing variability predicts the suggested need for corrective intervention due to the perceived severity of patient-ventilator asynchrony during NIV. J Clin Monit Comput 34 (2020): 1035-1042.
- Ilan Y. Order Through Disorder: The Characteristic Variability of Systems. Front Cell Dev Biol 8 (2020): 186.
- Xu Y, Cao M, Liao X, et al. Development and Emergence of Individual Variability in the Functional Connectivity Architecture of the Preterm Human Brain. Cereb Cortex 29 (2019): 4208-4222.
- Khundrakpam BS, Lewis JD, Jeon S, et al. Exploring Individual Brain Variability during Development based on Patterns of Maturational Coupling of Cortical Thickness: A Longitudinal MRI Study. Cereb Cortex 29 (2019): 178-188.
- Ligaard J, Sannaes J, Pihlstrom L. Deep brain stimulation and genetic variability in Parkinson's disease: a review of the literature. NPJ Parkinsons Dis 5 (2019): 18.
- Arm J, Al-Iedani O, Lea R, et al. Diurnal variability of cerebral metabolites in healthy human brain with 2D localized correlation spectroscopy (2D L-COSY). J Magn Reson Imaging 50 (2019): 592-601.
- Li L, Wang Y, Ye L, et al. Altered Brain Signal Variability in Patients With Generalized Anxiety Disorder. Front Psychiatry 10 (2019): 84.
- Wang CH, Liang WK, Moreau D. Differential Modulation of Brain Signal Variability During Cognitive Control in Athletes with Different Domains of Expertise. Neuroscience 425 (2020): 267-279.
- Boissoneault J, Sevel L, Stennett B, et al. Regional increases in brain signal variability are associated with pain intensity reductions following repeated eccentric exercise bouts. Eur J Pain 24 (2020): 818-827.
- Hurvitz N, Azmanov H, Kesler A, et al. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur J Hum Genet (2021).
- Azmanov H, Ross EL, Ilan Y. Establishment of an Individualized Chronotherapy, Autonomic Nervous System, and Variability-Based Dynamic Platform for Overcoming the Loss of Response to Analgesics. Pain Physician 24 (2021): 243-252.
- Khoury T, Ilan Y. Platform introducing individually tailored variability in nerve stimulations and dietary regimen to prevent weight regain following weight loss in patients with obesity. Obes Res Clin Pract 15 (2021): 114-123.
- Gelman R, Berg M, Ilan Y. A Subject-Tailored Variability-Based Platform for Overcoming the Plateau Effect in Sports Training: A Narrative Review. Int J Environ Res Public Health 19 (2022).
- Ilan Y. Digital Medical Cannabis as Market Differentiator: Second-Generation Artificial Intelligence Systems to Improve Response. Front Med (Lausanne) 8 (2021): 788777.
- Ishay Y, Kolben Y, Kessler A, et al. Role of circadian rhythm and autonomic nervous system in liver function: a hypothetical basis for improving the management of hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 321 (2021): G400-G412.
- Kolben Y, Weksler-Zangen S, Ilan Y. Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: Implementing a personalized signature-based platform for chronotherapy. Obes Rev 22 (2021): e13108.
- Kenig A, Kolben Y, Asleh R, et al. Improving Diuretic Response in Heart Failure by Implementing a Patient-Tailored Variability and Chronotherapy-Guided Algorithm. Front Cardiovasc Med 8 (2021): 695547.
- Isahy Y, Ilan Y. Improving the long-term response to antidepressants by establishing an individualized platform based on variability and chronotherapy. Int J Clin Pharmacol Ther 59 (2021): 768-774.
- Ishay Y, Potruch A, Schwartz A, et al. A digital health platform for assisting the diagnosis and monitoring of COVID-19 progression: An adjuvant approach for augmenting the antiviral response and mitigating the immune-mediated target organ damage. Biomed Pharmacother 143 (2021): 112228.
- Ilan Y, Spigelman Z. Establishing patient-tailored variability-based paradigms for anti-cancer therapy: Using the inherent trajectories which underlie cancer for overcoming drug resistance. Cancer Treat Res Commun 25 (2020): 100240.
- Hurvitz N, Azmanov H, Kesler A, et al. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur J Hum Genet 29 (2021): 1485-1490.
- Azmanov H, Bayatra A, Ilan Y. Digital Analgesic Comprising a Second-Generation Digital Health System: Increasing Effectiveness by Optimizing the Dosing and Minimizing Side Effects. J Pain Res 15 (2022): 1051-1060.
- Hurvitz N, Elkhateeb N, Sigawi T, et al. Improving the effectiveness of anti-aging modalities by using the constrained disorder principle-based management algorithms. Frontiers in Aging 3 (2022).
- Kolben Y, Azmanov H, Gelman R, et al. Using chronobiology-based second-generation artificial intelligence digital system for overcoming antimicrobial drug resistance in chronic infections. Ann Med 55 (2023): 311-318.
- Gelman R, Hurvitz N, Nesserat R, et al. A second-generation artificial intelligence-based therapeutic regimen improves diuretic resistance in heart failure: Results of a feasibility open-labeled clinical trial. Biomedicine & Pharmacotherapy 161 (2023): 114334.
- Saha S, Baumert M. Intra- and Inter-subject Variability in EEG-Based Sensorimotor Brain Computer Interface: A Review. Front Comput Neurosci 13 (2019): 87.
- Chikara RK, Ko LW. Prediction of Human Inhibition Brain Function with Inter-Subject and Intra-Subject Variability. Brain Sci 10 (2020).
- Ilan Y. Improving Global Healthcare and Reducing Costs Using Second-Generation Artificial Intelligence-Based Digital Pills: A Market Disruptor. Int J Environ Res Public Health 18 (2021).
- Ilan Y. Second-Generation Digital Health Platforms: Placing the Patient at the Center and Focusing on Clinical Outcomes. Front Digit Health 2 (2020): 569178.
- Koba C, Notaro G, Tamm S, et al. Spontaneous eye movements during eyes-open rest reduce resting-state-network modularity by increasing visual-sensorimotor connectivity. Netw Neurosci 5 (2021): 451-476.
- Wang Q, Valdés-Hernández PA, Paz-Linares D, et al. EECoG-Comp: An Open Source Platform for Concurrent EEG/ECoG Comparisons-Applications to Connectivity Studies. Brain Topogr 32 (2019): 550-568.
- Mercier MR, Dubarry A-S, Tadel F, et al. Advances in human intracranial electroencephalography research, guidelines and good practices. NeuroImage 260 (2022): 119438.
- Martins NRB, Angelica A, Chakravarthy K, et al. Human Brain/Cloud Interface. Frontiers in Neuroscience 13 (2019).
- Brizhik L, Foletti A. Nonlinear quantum phenomena and biophysical aspects of complexity related to health and disease. J Biol Regul Homeost Agents 28 (2014): 357-66.
- Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol 8 (2007): 131-5.
- Seijkens T, Kusters P, Chatzigeorgiou A, et al. Immune cell crosstalk in obesity: a key role for costimulation? Diabetes 63 (2014): 3982-91.
- Walsh JT, Watson N, Kipnis J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology 141 (2014): 340-4.
- Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 14 (2013): 668-75.
- Ilan Y. Methods and system for modulating physiological states between biological entities. . Patent US 2018 / 0328917 A1 (2018).
- Shabat Y, Ilan Y. Correlations between components of the immune system [version 1; peer review: awaiting peer review]. F1000Research 10 (2021).
- Hameroff S, Penrose R. Consciousness in the universe: a review of the 'Orch OR' theory. Phys Life Rev 1 (2014): 39-78.
- Penrose R. Uncertainty in quantum mechanics: faith or fantasy? Philos Trans A Math Phys Eng Sci 369 (2011): 4864-90.
- Penrose R. Consciousness, the brain, and spacetime geometry: an addendum. Some new developments on the Orch OR model for consciousness. Ann N Y Acad Sci 929 (2001): 105-10.
- Craddock TJ, Friesen D, Mane J, et al. The feasibility of coherent energy transfer in microtubules. J R Soc Interface 11 (2014): 20140677.
- Buiatti M, Longo G. Randomness and multilevel interactions in biology. Theory Biosci 132 (2013): 139-58.
- Yoo SS, Kim H, Filandrianos E, et al. Non-invasive brain-to-brain interface (BBI): establishing functional links between two brains. PLoS One 8 (2013): e60410.
- Wen D, Fan Y, Hsu SH, et al. Combining brain-computer interface and virtual reality for rehabilitation in neurological diseases: A narrative review. Ann Phys Rehabil Med (2020).


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
