Mitochondrial Reactive Oxygen Species and Nephrolithiasis
Minu Sharma, Amarjit S.Naura, SK Singla*
Department of Biochemistry, Panjab University, Chandigarh, India
*Corresponding Author: SK Singla, Department of Biochemistry, Panjab University, Chandigarh, India
Received: 03 December 2019; Accepted: 09 December 2019; Published: 12 December 2019
Article Information
Citation:
Minu Sharma, Amarjit S.Naura, SK Singla. Mitochondrial Reactive Oxygen Species and Nephrolithiasis. Arch Biochem Mol Biol 10 (2019): 071-084.
View / Download Pdf Share at FacebookAbstract
Mitochondrial redox/oxidative balance are vital for cellular life and death. Mitochondria are considered as the most important sub cellular site of reactive oxygen species production in mammalian organs. Reactive oxygen species produced by mitochondria can cause damage to mitochondrial components and initiate degradative processes. The kidney requires ample amount of mitochondria to remove waste from the blood and regulate fluid and electrolyte balance. Adverse conditions of organelle stress such as decreased altered energy metabolism in mitochondria contribute in the progression and development of kidney diseases. Nephrolithiasis is a kidney disease in which solid urinary components form crystals, precipitated out of the urine and shaped into stones. Studies have suggested that oxidative stress and associated renal injury paved the way for crystal deposition in the renal tissue. Mitochondria are anticipated as the foremost source of intracellular reactive oxygen species under oxalate induced nephrolithiasis. Persistent mitochondrial dysfunction results in the progression of nephrolithiasis. Although different approaches to minimize mitochondrial dysfunction through regulation of mitochondrial ROS production using antioxidants have been accomplished yet mitochondria specific antioxidants are the prerequisite. This review offers a glimpse into the role of mitochondrial reactive oxygen species in nephrolithiasis and the future perspective of potential antioxidant therapies.
Keywords
<p>Mitochondria; Kidney; Hyperoxaluria; Nephrolithiasis; Reactive oxygen species; Antioxidants</p>
Article Details
Abbreviations
ATP adenosine triphosphate; CaOx calcium oxalate; COM calcium oxalate monohydrate; IMM inner mitochondrial membrane; MitoQ mitoquinone; mtDNA mitochondrial DNA; mETC mitochondrial electron transport chain; mPTP mitochondrial permeability transition pore; NAG N-acetyl-beta-glucosaminidase; NAC N-Acetyl cysteine; OMM outer mitochondrial membrane; OXPHOS oxidative phosphorylation; 4-PBA 4-phenylbutyrate; RNS reactive nitrogen species; ROS reactive oxygen species; SOD superoxide dismutase; SkQ1 plastoquinonyl-decyl-triphenylphosphonium; SkQR1 plastoquinonyl-decyl-rhodamine19
Introduction
Mitochondria are ubiquitous, intracellular organelles known as powerhouses of the cell [1]. Inside all eukaryotic cells, the mitochondria are involved in essential metabolic processes for production of the adenosine triphosphate (ATP), the energy-rich compound that drives important cell functions. Mitochondria participate in numerous cellular functions including ion homeostasis, heme and steroid synthesis, calcium signalling, apoptosis [2]. Besides this organelle comprises major pathways for apoptosis and accumulates DNA mutations that may be linked to augmented rates of oxidants production and a number of degenerative diseases [3]. Over the last few years, it has been demonstrated that perturbation in mitochondrial activities is one of the major culprit behind renal injury associated with kidney stone formation. This suggests the hypothesis that renal cells use intracellular stress responses to initiate crystallisation. In this regard, mitochondrial reactive oxygen species (ROS) are considered as the perfect connection. Calcium oxalate (CaOx) stones account for the vast majority of calculi in kidney stone formers wherein hyperoxaluria is the major cause. Recently a study with Metabolic syndrome (MS) showed that hyperoxaluria in rats causes severe morphological alterations with a significant impairment of renal function and kidney stone formation [4]. Also, autophagy antagonist chloroquine significantly attenuate oxalate-induced autophagy activation, oxidative injury and mitochondrial damage of renal tubular cells in vitro and in vivo, as well as hyperoxaluria-induced CaOx crystals depositions in rat kidney [5]. Due to high reoccurrence rate of nephrolithiasis, it is one of the most irksome diseases of the kidney, which may lead to chronic kidney disease or permanent renal failure. Its prevalence is rising globally with the increasing incidence in women and children [6]. Around the globe only few groups are engaged in studies to comprehend new roles for the mitochondria, particularly in nephrolithiasis. The aim of the present review is to discuss the role of the mitochondrial reactive oxygen species in the progression of nephrolithiasis and the future perspective of potential antioxidant therapies.
Mitochondria - a unique sub cellular organelle
Mitochondria are remarkable organelles with a specialized double-membrane structure which forms separate regions, termed as outer mitochondrial membrane (OMM), intermembrane space, cristae formed by inner mitochondrial membrane (IMM), and matrix. The OMM has pores that permit passive diffusion of molecules up to 5 kDa. Larger molecules enter the mitochondrion through the translocases present in OMM [7]. The permeability of the OMM increases due to irreversible harm to cells, and proteins located in the intermembrane space, such as cytochrome c, flow out and initiate the apoptosis program. Owing to the numerous folds of cristae with oxysomes, the area of the IMM is about five times greater than that of the OMM. The IMM is embedded with abundant proteins that perform redox reactions, synthesize adenosine triphosphate (ATP), block ionic diffusion, and regulate mitochondrial dynamics. The IMM also encloses the matrix, where the oxidative phosphorylation (OXPHOS) enzyme and mitochondrial genetic material reside [8]. Mammalian mitochondria emerge and function through the co-ordinated action of two genomes: (a) Multiple copies of bacterial-like mitochondrial DNA (mtDNA) that encode for 22 transfer RNAs, 2 ribosomal RNAs, and 13 proteins implicated in oxidative phosphorylation, and (b) the nuclear genome (nDNA) that encodes for about another 1,500 structural and functional proteins [9].
Mitochondria play a vital role in providing the great amount of energy required for several different cellular functions. Substrates obtained from other intracellular processes, such as glycolysis or fatty acid metabolism, are converted to acetyl-CoA, which enters the tricarboxylic acid cycle (TCA), and its complete degradation is coupled with the production of NADH and FADH2, which are the effective electron donors for the mitochondrial electron transfort chain (mETC) [10]. The energy is stored as an electrochemical gradient across the IMM, which explains the presence of the negative mitochondrial membrane potential (ΔΨ). F1Fo-ATP synthase allows H+ to cross the IMM and re enter the matrix, coupling the energy derived from the proton gradient with the phosphorylation of ADP to produce ATP [11]. The new ATP molecules are then ready to leave the mitochondria.
Mitochondrial associated reactive oxygen species (ROS)
It was proposed that during the respiration, 0.4–4% of the total consumed oxygen is converted into superoxide radicals via electron leakage from the respiratory chain, which also acts as second messengers in cellular signaling pathways [12]. Further the released superoxide radicals are dismutated into hydrogen peroxide (H2O2) and O2 by superoxide dismutase (SOD) in the matrix or intermembrane space. H2O2 combines with superoxide radicals to produce Hydroxyl radicals. Superoxide radicals, H2O2, and hydroxyl radicals are the major forms of ROS [13]. In normal conditions, mitochondria are equipped with antioxidant system such as the thioredoxin reductase/thioredoxin/ peroxiredoxin-3,5 system, glutathione peroxidase (GPx), and glutathione (GSH) [14]. Reactive oxygen species (ROS) can lead to mild uncoupling of mitochondria, and the consequential increase in proton conductance can have a negative feedback effect on ROS production [15]. Additionally, to reduce the electrochemical gradient, and to accelerate oxygen consumption, the mitochondrial permeability transition pore (mPTP) is opened which decreases ROS production. Conversely, upsurge ROS has deleterious effects on mitochondrial DNA (mtDNA) and potentially leads to mitochondrial dysfunction, cell injury, and even apoptosis [16]. Mitochondria were considered as the main source of intracellular ROS in both physiology and pathology [17]. The pioneering work of Chance and colleagues showed that isolated mitochondria produce H2O2 [18]. Several different sites of ROS production have been identified in mammalian mitochondria, including complex I and complex III of the mETC and the dihydrolipoamide dehydrogenase enzyme [19-22]. Complex I produces superoxide either by a reduced flavin mononucleotide (FMN) site on complex I (a high ratio of NADH/NAD+) or by reverse electron transfer from the coenzyme Q (CoQ) pool back to complex I [23]. Normally, ROS production by complex III is much lower than ROS production by complex I. But, the role of complex III in superoxide production is significant when it is inhibited. Contribution of Complex II (succinate dehydrogenase) to ROS formation is related to reverse electron transfer, the process by which electrons are transferred from succinate to ubiquinone via complex II and then back to complex I, where ROS are produced [24,25]. Other than the respiratory chain complexes, mitochondrial enzymes also participate in ROS production, such as acyl- CoA dehydrogenase and glycerol α-phosphate dehydrogenase during the oxidation of lipid-derived substrates [26,27]. Monoamine oxidase, Dihydroorotate dehydrogenase, Pyruvate and α-ketoglutarate dehydrogenase enzymes are additional mitochondrial ROS sources [28,29]. The reactive nitrogen species (RNS) which are produced by reaction of anion superoxide with nitric oxide (NO) interact with mitochondrial components and results in alterations of mitochondrial respiration [30,31]. p66Shc protein acts as a redox-enzyme, generating H2O2 by binding cytochrome c [32].
The Kidney
Kidney has the second highest mitochondrial content and oxygen consumption after the heart thus it is considered as one of the most energy consuming organ in the human body [33]. Results from a study measuring the resting energy expenditure of different organs in healthy individuals found that the kidney and heart have the highest resting metabolic rates [34]. This high resting metabolic rate for the kidney is due to the requirement of plenty of mitochondria to supply adequate energy to perform various important tasks like removal of waste from the blood, reabsorbtion of nutrients, maintenance of acid–base homeostasis, blood pressure regulation, and maintaining the balance of electrolytes and fluid. Mitochondria are the exclusive source of energy to the sodium–potassium pump to produce ion gradients across the cellular membrane. The proximal tubule, the loop of Henle, the distal tubule and the collecting duct of the kidney require active transport to reabsorb ions [35]. On the other hand, glomerular filtration is a passive process which is dependent on the hydrostatic pressure in the glomeruli. Proximal tubules reabsorb 80% of the filtrate that passes through the glomerulus, hence require added active transport mechanisms in comparison to other renal cell types [36]. So, they have more mitochondria than any other structure in the kidney. The proper functioning of the proximal tubule depends upon the ability of mitochondria to sense and respond to changes in nutrient availability and energy demand by maintaining mitochondrial homeostasis [33]. Therefore mitochondrial dysfunction plays a crucial role in the pathogenesis of kidney diseases. Evidence is emerging that defects in mitochondrial function may lead to kidney chronic diseases [37-39].
Nephrolithiasis and mitochondria
Nephrolithiasis, a medical condition in which kidney stones are formed from crystals precipitating from the urine, and grow within the urinary tract, has been considered as a common, painful condition. Nephrolithiasis is not simply an isolated urologic disease, but instead a disorder with systemic complications, including an increased risk for chronic kidney disease [40]. Nephrolithiasis has become a rising problem and the third prevalent disorder affecting the urinary tract with high recurrence [41]. This disorder involves a complex of events, such as supersaturation, crystal nucleation, growth, aggregation, retention within renal tubules and migration to the renal papillary surfaces. The majority (up to 80%) of all stones are mainly composed of calcium oxalate (CaOx). Most research on the etiology and prevention of urinary tract stone disease has been directed toward the role of elevated urinary levels of oxalate, calcium, and uric acid in stone formation [42]. An elevated level of urinary oxalate (hyperoxaluria) is considered as an important factor of CaOx nephrolithiasis [43,44].
Oxalate, a by-product of metabolism, is primarily filtered at the glomerulus, where its concentration is usually, 1–5 µM; patients with kidney stone disease may have filtered oxalate loads that are 10–20 times higher [45]. Many laboratories have illustrated that exposure to such high oxalate concentrations can elicit an array of toxic responses at the level of renal cells. Early indication for renal oxalate toxicity was suggested indirectly by studies analyzing the urinary excretion of renal injury marker enzymes like N-acetyl-beta-glucosaminidase (NAG) in stone-forming and healthy individuals; higher than normal urinary enzyme values suggested the presence of tubular damage in patients with stones [46]. Rat models of nephrolithiasis have been used to study such damage in greater detail, revealing renal epithelial membrane disruption, increased incidence of inclusion bodies in tubular cells, discharge of cellular enzymes, epithelial erosion and the formation of plaques at the tips of the renal papillae [47,48]. Cultured renal epithelial cells exposed to elevated oxalate concentrations showed similar toxic effects in vitro: compromised cell integrity, release of cellular enzymes and necrotic and apoptotic cell death [49]. Many researchers have recommended that underlying renal cell injury augments the probability of crystal attachment to renal epithelial cells, both in vivo and in vitro in renal tubular cells [50-52]. Remnants of injured cells may also add to stone formation by forming nidi for crystal nucleation and by promoting crystal agglomeration [53,54].
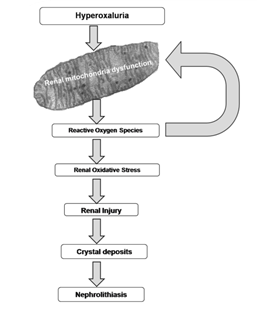
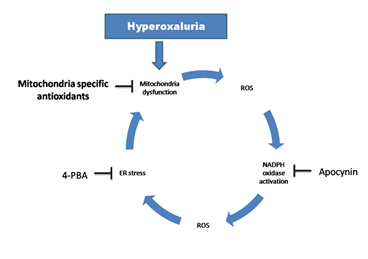
Accumulating data have suggested that high oxalate concentration led to renal oxidative stress which could play a significant and adverse role in the initiation and progression of renal cell injury [55,56]. Reactive oxygen species may be produced in renal cells in response to oxalate exposure by several cellular mechanisms. Augmented free radical generation and related injury to the renal tubules are considered a prelude to the complex sequel of stone formation [57]. Studies have suggested that mitochondria are the key supplier of free radicals in oxalate toxicity, and the role of other non-mitochondrial sources is nominal [58-60] (Figure 1). Another study has shown that calcium oxalate monohydrate (COM) crystals were able to inhibit the mitochondrial respiratory chain in proximal tubular cells [61]. The in vitro and in vivo studies show that oxalate disturbs the electron transport chain in mitochondria and induces the leak of free radicals (Jonassen et al., 2003). Isolated mitochondria reacted to oxalate exposure by the increase of ROS, lipid peroxides and oxidized thiol proteins [62]. Taurine treatment to hyperoxaluric rats has been shown to reverse mitochondrial changes in the kidneys and reduce the crystal deposition [63]. Mitochondrial damage is suggested to be induced by the opening of mitochondrial permeability transition pore (mPTP). Different factors such as calcium overload and oxidative stress results in the opening of the permeability transition pore in the inner mitochondrial membrane. This uncouples oxidative phosphorylation and compromises intracellular ATP levels finally leading to necrotic cell death [64]. Mitochondrial mPTP opening depends on the activation of cyclophilin D in the mitochondrial matrix by ROS produced by NADPH oxidase and is inhibited by cyclosporine A (CSA). CSA averted the depolarization of mitochondrial membrane, a decline in SOD expression, an increase in 4-hydroxy-2-nonenal (4-HNE) and discharge of cytochrome c into the cytosol in NR52E renal epithelial cells exposed to CaOx monohydrate crystals in vitro. In vivo CSA treatment resulted in reduced mitochondrial damage, oxidative stress and CaOx crystal deposition in the kidneys [65]. In view of above reports, mitochondria are not only an originator but also a target of ROS, hence mitochondrial ROS is a key factor in a vicious cycle by which oxidative stress is induced and promoted in nephrolithiasis (Figure 2).
Anti-oxidants targeted to mitochondria
Therapeutic molecules that target reactive oxygen species induced mitochondrial dysfunction hold great promise in diseases that have confirmed involvement of oxidative stress. Nephrolithiasis emerges as one such case. Our research group have been working on kidney stones and its management from last three decades [59,66-72]. The studies in our lab described the critical role of mitochondrial oxidative stress in hyperoxaluria induced nephrolithiasis. N-Acetyl cysteine (NAC), an antioxidant and GSH precursor, proved to be beneficial in combating hyperoxaluria induced nephrolithiasis to some extent (Sharma et al 2015). It has shown an outstanding efficacy in prevention of hyperoxaluria-induced mitochondrial damage. But subsistence of moderate crystalluria in NAC treated hyperoxaluric rats proposed an essential adjuvant therapy along with NAC. Thus a study was designed with NADPH oxidase inhibitor, Apocynin (4-hydroxy-3-methoxy-acetophenone), to evaluate the contribution of a possible feed-forward vicious cycle of ROS between mitochondria and NADPH Oxidase during nephrolithiasis. NAC in combination with Apocynin synergistically inhibited nephrolithiasis progression [59]. To describe systematically how mitochondrial proteins/pathways govern the renal damage and calcium oxalate crystal adhesion in hyperoxaluria large-scale proteomics was conducted. The study suggested that hyperoxaluria modified the levels of several important proteins involved in metabolism and oxidative phosphorylation in renal mitochondria. Also, alterations in mitochondrial molecular chaperones and proteins involved in antioxidant defence were reported [70].
However, the mitochondrion is not a single unit rather mitochondria and the endoplasmic reticulum (ER) are tightly associated. Under conditions of stress, Ca2 + is the most prominent signaling factor that is released from the ER and, at high concentration, mediates the transfer of an apoptosis signal to mitochondria as the executioner organelle for cell death. Therefore, to further explore the causes behind renal stone disease, a study was designed with a small chemical chaperone 4-phenylbutyrate (4-PBA), used as ER stress inhibitor, in hyperoxaluria [68]. Although 4-PBA decreased the hyperoxaluric manifestations yet mitochondria specific antioxidants are the prerequisite (Figure 2).
Some anti-oxidants were designed to amend the function of mitochondria which exploit the unique structural and functional characteristics of this organelle. Some important mitochondrial targets for therapeutic intervention include electron transport chain components, the permeability transition pore, and the mitochondrial membranes. Mitochondria-targeted antioxidants adjust the levels of ROS, thereby reducing mitochondria-driven cell death. At present, a number of compounds that are able to electrophoretically accumulate in mitochondria have been synthesized; they are effective at very low concentrations. The mitochondria-targeted antioxidants include substances like as SkQs and MitoQ. The formation of these chemical compounds was based on the studies by the group of V.P. Skulachev and E.A. Lieberman with lipophilic phosphonium cations [73]. These studies described that lipophilic ions with a delocalized charge shielded by bulky substituents freely penetrate into mitochondria and submitochondrial particles under the action of the electric field of the inner mitochondrial membrane. Results from various studies described that even small concentrations of mitochondria-targeted antioxidants such as MitoQ, SkQ1, SkQR1 were highly efficient in showing antioxidant activity in aqueous solutions, lipid micelles, liposomes, isolated mitochondria, and cell cultures. The protective effect of these substances was demonstrated in various models of ROS-associated diseases, including the models of such pathological states of the kidney as acute kidney injury, kidney ischemia-reperfusion.
MitoQuinone (MitoQ) is a positively charged lipophilic cation, it is accumulated in the negatively charged interior of mitochondria. The antioxidant component of MitoQ is the ubiquinone that is also found in coenzyme Q10. By the action of the enzyme Complex II in the mitochondrial respiratory chain, ubiquinone part of MitoQ is rapidly activated to the active ubiquinol antioxidant. After detoxifying ROS, the ubiquinol part of MitoQ is converted to ubiquinone, which is by complex II be recycled back to active antioxidant ubiquinol. This process makes MitoQ an effective mitochondria-targeted antioxidant. MitoQ reduced intracellular superoxide and inhibited cyst epithelial cell proliferation through extracellular signal-related kinase/MAPK inactivation in Autosomal Dominant Polycystic Kidney Disease [74]. A study investigated the effect of MitoQ in decreasing the severity of renal ischemia-reperfusion injury (IRI) in rats demonstrated that MitoQ can reduce the severity of renal damage in rodent IRI models using T2-weighted imaging and DCE-MRI [75]. Mitoquinone in phase II clinical trials reduced plasma ALT and aspartate aminotransferase in patients with chronic HCV infection [76].
Plastoquinonyl-decyl-triphenylphosphonium (SkQ1)
Several research groups from Russia and other countries synthesized (SkQs) comprising plastoquinone (an antioxidant moiety), a penetrating cation, and a decane or pentane linker. They selected SkQ derivatives with the highest permeability, namely plastoquinonyl-decyl-triphenylphosphonium(SkQ1),plastoquinonyl-decyl-rhodamine19 (SkQR1),and methylplastoquinonyl decyltriphenylphosphonium (SkQ3) to study the anti- and prooxidant properties of these substances [73]. In this compound named SkQ1, ubiquinone was replaced by plastoquinone [77]. SkQ1 manifested a strong therapeutic action on some already pronounced retinopathies, in particular, congenital retinal dysplasia [73]. The effect of SkQ1 on kidney IRI using a culture of kidney epithelial cells has been studied. Preincubation with SkQ1 increased survival of these cells and diminished mitochondrial fission induced by the anoxia/reoxygenation procedure [78].
Plastoquinonyl-decyl-rhodamine19 (SkQR1)
The same research group used a mitochondria-targeted compound containing a charged rhodamine molecule conjugated with plastoquinone named SkQR1 in another experiment [79]. Rats exposed to kidney IRI normalized the ROS level and lipid peroxidized products in kidney mitochondria after an intraperitoneal injection of SkQR1, they also showed significantly decreased BUN and blood creatinine levels. The SKQR1 lowered mortality of experimental rats compared to animals that were subjected to kidney IRI without any given drug. The study concluded that SkQR1 provided linked synergy between antioxidative effects and ischemic tolerance signaling mechanisms owing to the targeted delivery of this compound to mitochondria [80]. Besides SkQR1 reduces the death of kidney epithelium cells and decreases the severity of renal failure caused by gentamycin. SkQR1 also reduced the gentamycin induced hearing loss [81].
Conclusion
The information presented here demonstrated that the disruption in the renal mitochondrial homeostasis due to oxidative stress can result in mitochondrial dysfunction and renal damage. Although much is known about mitochondria functions but the precise role in renal disease remains to be determined. It is apparent; however, that mitochondrial dysfunction occurs early in kidney stone disease. Furthermore, the decrease in the mitochondrial antioxidant defence system after hyperoxaluria might lead to the continued impairment of renal mitochondria function, leading to renal injury and stone formation. Since the repair of renal cell and the recovery of renal function depend upon the ATP production ability of mitochondria, restoring mitochondrial function might reverse cellular injury and restore renal function. In the upcoming time, a more detailed study of the mechanisms of action of various types of mitochondria targeted antioxidants in the nephrolithiasis needs to be conducted, also their therapeutic values and toxicological properties are to be analysed. Collectively, the research outcomes till date necessitate targeting mitochondrial homeostasis in hyperoxaluria induced nephrolithiasis to restore mitochondrial function and initiate renal repair to avoid advance renal dysfunction.
Acknowledgment
The financial assistance provided by the Science and Engineering Research Board (SERB), Government of India, New Delhi is gratefully acknowledged.
Conflict of interest
The authors state no conflict of interest.
References
- Fakouri NB, Hansen TL, Desler C, Anugula S, Rasmussen LJ. From Powerhouse to Perpetrator—Mitochondria in Health and Disease. Biology 8 (2019): 35.
- Sedlackova L, Korolchuk VI. Mitochondrial quality control as a key determinant of cell survival. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1866 (2019): 575-587.
- Vercesi AE, Castilho RF, Kowaltowski AJ, de Oliveira HCF, de Souza-Pinto NC, Figueira TR, Busanello ENB. Mitochondrial calcium transport and the redox nature of the calcium-induced membrane permeability transition. Free Radical Biology and Medicine 129 (2018): 1-24.
- Sáenz-Medina J, Jorge E, Corbacho C, Santos M, Sánchez A, Soblechero P, Virumbrales E, Ramil E, Coronado MJ, Castillón I , et al. Metabolic syndrome contributes to renal injury mediated by hyperoxaluria in a murine model of nephrolithiasis. Urolithiasis 46 (2018): 179-186.
- Duan X, Kong Z, Mai X, Lan Y, Liu Y, Yang Z, Zhao Z, Deng T, Zeng T, Cai C , et al. Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney. Redox biology 16 (2018): 414-425.
- Roudakova K, Monga M. The evolving epidemiology of stone disease. Indian journal of urology : IJU : journal of the Urological Society of India 30 (2014): 44-48.
- Rimessi A, Previati M, Nigro F, Wieckowski MR, Pinton P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. The International Journal of Biochemistry & Cell Biology 81 (2016): 281-293.
- Qin J, Peng ZZ, Li Q, Wen R, Tao LJ. Renal Fibrosis and Mitochondrial Damage. Chin Med J (Engl) 131 (2018): 2769-2772.
- Yan C, Duanmu X, Zeng L, Liu B, Song Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 8 (2019): 379.
- Braschi E, McBride HM. Mitochondria and the culture of the Borg: understanding the integration of mitochondrial function within the reticulum, the cell, and the organism. Bioessays 32 (2010): 958-966.
- Johnson JA, Ogbi M. Targeting the F1Fo ATP Synthase: modulation of the body's powerhouse and its implications for human disease. Curr Med Chem 18 (2011): 4684-4714.
- Granata S, Dalla Gassa A, Tomei P, Lupo A, Zaza G. Mitochondria: a new therapeutic target in chronic kidney disease. Nutr Metab (Lond) 12 (2015): 49.
- Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D , et al. Unraveling the biological roles of reactive oxygen species. Cell Metab 13 (2011): 361-366.
- Rabilloud T, Heller M, Rigobello MP, Bindoli A, Aebersold R, Lunardi J. The mitochondrial antioxidant defence system and its response to oxidative stress. Proteomics 1 (2001): 1105-1110.
- Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382 (2004): 511-517.
- Berry BJ, Trewin AJ, Amitrano AM, Kim M, Wojtovich AP. Use the Protonmotive Force: Mitochondrial Uncoupling and Reactive Oxygen Species. J Mol Biol 430 (2018): 3873-3891.
- [Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative medicine and cellular longevity 2016:1245049-1245049.
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59 (1979): 527-605.
- Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol 1 (2013): 304-12.
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417 (2009): 1-13.
- Mailloux RJ, McBride SL, Harper ME. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem Sci 38 (2013): 592-602.
- Kudin AP, Malinska D, Kunz WS. Sites of generation of reactive oxygen species in homogenates of brain tissue determined with the use of respiratory substrates and inhibitors. Biochim Biophys Acta 1777 (2008): 689-95.
- Hernansanz-Agustin P, Ramos E, Navarro E, Parada E, Sanchez-Lopez N, Pelaez-Aguado L, Cabrera-Garcia JD, Tello D, Buendia I, Marina A , et al. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol 12 (2017): 1040-1051.
- Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299 (2003): 700-704.
- Liu L, Trimarchi JR, Smith PJ, Keefe DL. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 1 (2002): 40-46.
- Seminotti B, Leipnitz G, Karunanidhi A, Kochersperger C, Roginskaya VY, Basu S, Wang Y, Wipf P, Van Houten B, Mohsen AW , et al. Mitochondrial energetics is impaired in very long-chain acyl-CoA dehydrogenase deficiency and can be rescued by treatment with mitochondria-targeted electron scavengers. Hum Mol Genet 2018.
- Lambertucci RH, Hirabara SM, Silveira Ldos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol 216 (2008): 796-804.
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24 (2004): 7779-88.
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29 (2000): 222-230.
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33 (2002): 1451-64.
- Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev 2016:1245049.
- Galimov ER. The Role of p66shc in Oxidative Stress and Apoptosis. Acta naturae 2 (2010): 44-51.
- Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol 13 (2017): 629-646.
- Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Muller MJ. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 92 (2010): 1369-77.
- Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol 48 (1986): 9-31.
- Holechek MJ. Glomerular filtration: an overview. Nephrol Nurs J 30 (2003): 285-90.
- Ishimoto Y, Inagi R. Mitochondria: a therapeutic target in acute kidney injury. Nephrol Dial Transplant 31 (2016): 1062-1069.
- Duann P, Lin PH. Mitochondria Damage and Kidney Disease. Adv Exp Med Biol 982 (2017): 529-551.
- Fontecha-Barriuso M, Martin-Sanchez D, Martinez-Moreno JM, Carrasco S, Ruiz-Andres O, Monsalve M, Sanchez-Ramos C, Gomez MJ, Ruiz-Ortega M, Sanchez-Nino MD , et al. PGC-1alpha deficiency causes spontaneous kidney inflammation and increases the severity of nephrotoxic AKI. J Pathol 2019.
- Mayans L. Nephrolithiasis. Prim Care 46 (2019): 203-212.
- Bayne DB, Chi TL. Assessing Cost-Effectiveness of New Technologies in Stone Management. Urol Clin North Am 46 (2019): 303-313.
- Gao S, Yang R, Peng Z, Lu H, Li N, Ding J, Cui X, Chen W, Dong X. Metabolomics analysis for hydroxy-L-proline-induced calcium oxalate nephrolithiasis in rats based on ultra-high performance liquid chromatography quadrupole time-of-flight mass spectrometry. Sci Rep 6 (2016): 30142.
- Efe O, Verma A, Waikar SS. Oxalate as a potential mediator of kidney disease in diabetes mellitus and obesity. Curr Opin Nephrol Hypertens 2019.
- Mulay SR, Eberhard JN, Desai J, Marschner JA, Kumar SV, Weidenbusch M, Grigorescu M, Lech M, Eltrich N, Muller L , et al. Hyperoxaluria Requires TNF Receptors to Initiate Crystal Adhesion and Kidney Stone Disease. J Am Soc Nephrol 28 (2017): 761-768.
- Asplin JR. The management of patients with enteric hyperoxaluria. Urolithiasis 44 (2016): 33-43.
- Ramaswamy K, Shah O. Metabolic syndrome and nephrolithiasis. Transl Androl Urol 3 (2014): 285-295.
- Liu Y, Liu Q, Wang X, He Z, Li D, Guan X, Tao Z, Deng Y. Inhibition of Autophagy Attenuated Ethylene Glycol Induced Crystals Deposition and Renal Injury in a Rat Model of Nephrolithiasis. Kidney Blood Press Res 43 (2018): 246-255.
- Motin YG, Lepilov AV, Larionov PM. [Renal morphological changes in experimental oxalate nephrolithiasis]. Arkh Patol 79 (2017): 41-47.
- Jonassen JA, Cao LC, Honeyman T, Scheid CR. Mechanisms mediating oxalate-induced alterations in renal cell functions. Crit Rev Eukaryot Gene Expr 13 (2003): 55-72.
- Convento M, Pessoa E, Aragao A, Schor N, Borges F. Oxalate induces type II epithelial to mesenchymal transition (EMT) in inner medullary collecting duct cells (IMCD) in vitro and stimulate the expression of osteogenic and fibrotic markers in kidney medulla in vivo. Oncotarget 10 (2019): 1102-1118.
- Joshi S, Wang W, Khan SR. Transcriptional study of hyperoxaluria and calcium oxalate nephrolithiasis in male rats: Inflammatory changes are mainly associated with crystal deposition. PLoS One 12 (2017): e0185009.
- Joshi S, Clapp WL, Wang W, Khan SR. Osteogenic changes in kidneys of hyperoxaluric rats. Biochim Biophys Acta 1852 (2015): 2000-12.
- Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS. Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int 59 (2001): 637-44.
- Joshi S, Wang W, Khan SR. Transcriptional study of hyperoxaluria and calcium oxalate nephrolithiasis in male rats: Inflammatory changes are mainly associated with crystal deposition. PloS one 12 (2017): e0185009-e0185009.
- Joshi S, Khan SR. Opportunities for future therapeutic interventions for hyperoxaluria: targeting oxidative stress. Expert Opin Ther Targets 23 (2019): 379-391.
- Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, Traxer O, Tiselius HG. Kidney stones. Nat Rev Dis Primers 3 (2017): 17001.
- Maroni PD, Koul S, Chandhoke PS, Meacham RB, Koul HK. Oxalate toxicity in cultured mouse inner medullary collecting duct cells. J Urol 174 (2005): 757-60.
- Khand FD, Gordge MP, Robertson WG, Noronha-Dutra AA, Hothersall JS. Mitochondrial superoxide production during oxalate-mediated oxidative stress in renal epithelial cells. Free Radic Biol Med 32 (2002): 1339-50.
- Sharma M, Kaur T, Singla SK. Role of mitochondria and NADPH oxidase derived reactive oxygen species in hyperoxaluria induced nephrolithiasis: therapeutic intervention with combinatorial therapy of N-acetyl cysteine and Apocynin. Mitochondrion 27 (2016): 15-24.
- Farooq SM, Boppana NB, Devarajan A, Sekaran SD, Shankar EM, Li C, Gopal K, Bakar SA, Karthik HS, Ebrahim AS. C-phycocyanin confers protection against oxalate-mediated oxidative stress and mitochondrial dysfunctions in MDCK cells. PLoS One 9 (2014): e93056.
- McMartin KE, Wallace KB. Calcium oxalate monohydrate, a metabolite of ethylene glycol, is toxic for rat renal mitochondrial function. Toxicol Sci 84 (2005): 195-200.
- Cao LC, Honeyman TW, Cooney R, Kennington L, Scheid CR, Jonassen JA. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int 66 (2004): 1890-900.
- Li CY, Deng YL, Sun BH. Taurine protected kidney from oxidative injury through mitochondrial-linked pathway in a rat model of nephrolithiasis. Urol Res 37 (2009): 211-20.
- Niimi K, Yasui T, Okada A, Hirose Y, Kubota Y, Umemoto Y, Kawai N, Tozawa K, Kohri K. Novel effect of the inhibitor of mitochondrial cyclophilin D activation, N-methyl-4-isoleucine cyclosporin, on renal calcium crystallization. Int J Urol 21 (2014): 707-13.
- Niimi K, Yasui T, Hirose M, Hamamoto S, Itoh Y, Okada A, Kubota Y, Kojima Y, Tozawa K, Sasaki S , et al. Mitochondrial permeability transition pore opening induces the initial process of renal calcium crystallization. Free Radic Biol Med 52 (2012): 1207-17.
- Aggarwal A, Singla SK, Gandhi M, Tandon C. Preventive and curative effects of Achyranthes aspera Linn. extract in experimentally induced nephrolithiasis. Indian journal of experimental biology 50 (2012): 201-8.
- Aggarwal D, Kaushal R, Kaur T, Bijarnia RK, Puri S, Singla SK. The most potent antilithiatic agent ameliorating renal dysfunction and oxidative stress from Bergenia ligulata rhizome. Journal of ethnopharmacology 158 (2014): 85-93.
- Sharma M, Naura AS, Singla SK. Modulatory effect of 4-phenyl butyric acid on hyperoxaluria-induced renal injury and inflammation. Mol Cell Biochem 451 (2019): 185-196.
- Sharma M, Kaur T, Singla SK. Protective effects of N-acetylcysteine against hyperoxaluria induced mitochondrial dysfunction in male wistar rats. Mol Cell Biochem 405 (2015): 105-14.
- Sharma M, Sud A, Kaur T, Tandon C, Singla SK. N-acetylcysteine with apocynin prevents hyperoxaluria-induced mitochondrial protein perturbations in nephrolithiasis. Free Radic Res 50 (2016): 1032-44.
- Chhiber N, Kaur T, Singla S. Rottlerin, a polyphenolic compound from the fruits of Mallotus phillipensis (Lam.) Mull.Arg., impedes oxalate/calcium oxalate induced pathways of oxidative stress in male wistar rats. Phytomedicine 23 (2016): 989-97.
- Aggarwal D, Gautam D, Sharma M, Singla SK. Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. Eur J Pharmacol 791 (2016): 611-621.
- Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG , et al. An attempt to prevent senescence: A mitochondrial approach. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1787 (2009): 437-461.
- Ishimoto Y, Inagi R, Yoshihara D, Kugita M, Nagao S, Shimizu A, Takeda N, Wake M, Honda K, Zhou J , et al. Mitochondrial Abnormality Facilitates Cyst Formation in Autosomal Dominant Polycystic Kidney Disease. Mol Cell Biol 37 (2017): 24.
- Liu X, Murphy MP, Xing W, Wu H, Zhang R, Sun H. Mitochondria-targeted antioxidant MitoQ reduced renal damage caused by ischemia-reperfusion injury in rodent kidneys: Longitudinal observations of T(2) -weighted imaging and dynamic contrast-enhanced MRI. Magnetic resonance in medicine 79 (2018) :1559-1567.
- Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 30 (2010): 1019-26.
- Korshunova GA, Shishkina AV, Skulachev MV. Design, synthesis, and some aspects of the biological activity of mitochondria-targeted antioxidants. Biochemistry (Moscow) 82 (2017): 760-777.
- Bakeeva LE, Barskov IV, Egorov MV, Isaev NK, Kapelko VI, Kazachenko AV, Kirpatovsky VI, Kozlovsky SV, Lakomkin VL, Levina SB , et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochemistry (Mosc) 73 (2008): 1288-99.
- Plotnikov EY, Vasileva A, Arkhangelskaya A, Pevzner I, Skulachev V, Zorov D. Interrelations of mitochondrial fragmentation and cell death under ischemia/reoxygenation and UV?irradiation: Protective effects of SkQ1, lithium ions and insulin. FEBS letters 582 (2008): 3117-3124.
- Plotnikov E, Chupyrkina A, Jankauskas S, Pevzner I, Silachev D, Skulachev V, Zorov D. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1812 (2011): 77-86.
- Jankauskas SS, Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Skulachev VP, Zorov DB. Mitochondria-targeted antioxidant SkQR1 ameliorates gentamycin-induced renal failure and hearing loss. Biochemistry (Moscow) 77 (2012): 666-670.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
