A Progressive Approach on Lowering COVID-19 Transmission
G.N. Nirmala*, Sandra Jose
Department of Biotechnology, Vels Institute of Science, Technology and Advanced Studies, Pallavaram, Chennai, India
*Corresponding Author: G.N. Nirmala, Department of Biotechnology, Vels Institute of Science, Technology and Advanced Studies, Pallavaram, Chennai, India
Received: 12 May 2020; Accepted: 03 June 2020; Published: 08 June 2020
Article Information
Citation: G.N. Nirmala, Sandra Jose. A Progressive Approach on Lowering COVID-19 Transmission. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 131-143.
View / Download Pdf Share at FacebookAbstract
Abstract
Coronavirus disease 2019 (COVID-19) is rapidly expanding in the major parts of the world like U.S.A, Spain and Europe and to respond to it, various countries are using national lockdown strategies and uncompromising restrictions on international travels with the intension to prevent the local transmission. Real time RT-PCR was believed to be “gold standard” but proved to be error prone, pointing out the need for an accurate diagnostic tool like ELISA based assay of immune responses. Several drugs have proved efficient against COVID-19 namely, Favipiravir, Remdisivir, Chloroquine and Monoclonal Antibodies. Most of the countries have been very effective in enhancing the survival rates using such diagnostic tools and treatment methods like Convalescent Plasma therapy. This review paper discusses the overall comparison of the severity of cases in the most affected parts of the world, variations in symptoms, effects of medical history, major treatment methods and measures taken in India to control this global pandemic effectively with least mortality rate (3%) when compared with the countries like U.S.A and Spain. Even when a higher recovery rate (22.5%) was reported in India, enhancing self-resistance by maintaining personal hygiene and distancing from public gatherings are necessary to avoid community spread of the epidemic.
Keywords
<p>Convalescent Plasma therapy; ELISA based humoral assay; Indian Strategy; Effect of Past Medical History; Major drugs in use</p>
Article Details
Abbrevations
COVID-19 – Corona Virus Disease – 2019
CrPC- Criminal Procedure Code
ELISA- Enzyme-Linked Immunosorbent Assay
H1N1- Hemagglutinin Type 1 and Neuraminidase Type 1
IgG- Immunoglobulin G
IgM- Immunoglobulin M
IL-6- Interleukin-6
LFIA- lateral-flow immunoassay
mRNA- Messenger RNA
Real time RT-PCR- Real time ReverseTranscription-Polymerase Chain Reaction
rRNA- Ribosomal RNA
SARS-CoV2- Severe Acute Respiratory Syndrome- Corona Virus 2
TNF-α- Tumour Necrosis Factor- α
W.H.O- World Health Organisation
MERS- Middle East respiratory syndrome coronavirus
Introduction
During the past 4 months, a devastating epidemic, coronavirus disease 2019 (COVID-19) has been reported and is rapidly expanding in U.S.A, Spain, France, Italy and Europe with the initial confirmed cases being identified in Wuhan, China. Due to the alarming levels of spread, severity and inaction, on March 11, 2020, the Director-General of W.H.O, categorised COVID-19 as a global pandemic [1, 2]. The onset of the disease was characterised by signs of dry cough, high fever and breathing difficulty while the affected COVID-19 patients with a medical history of cancer, diabetes mellitus, hypertension and cardiovascular disease were in most critical situation [3].
Initially the determination of presence of COVID-19 infection was carried out using real-time RT-PCR kits and it is a technique used to quantify mRNA in the given sample and was believed to be the “gold standard” method which was very sensitive and specific. While in the case of total RNA quantification, the technique determines no variation in rRNA/mRNA ratio [4]. Even when the technique was widely accepted, there were many false positive and false negative results reported in various tests thus pointing out a need for better detection techniques of the target gene of interest [5].
Chloroquine is an anti-malarial drug while it has also been reported as a potential broad-spectrum antiviral drug due to its immune-modulating activity like suppressing the secretion of TNF-α and IL-6 [6-8]. It prevents the viral infection by increasing the pH required for virus fusion and also interferes the glycosylation of cellular receptors. Being used for more than 70 years, chloroquine is a safe and cheap drug and is clinically potent against SARS-CoV2. Hydroxychloroquine, an analogue of chloroquine has high clinical safety profile and it exhibited anti-SARS-CoV activity in vitro studies [9]. In a study conducted on 36 COVID-19 patients, azithromycin added to hydroxychloroquine exhibited significantly a higher rate of virus elimination [10]. Similarly, Chloroquine phosphate is a potent drug against COVID-19 associated pneumonia and the National Health Commission of the People's Republic of China advised it for the treatment [11-15]. Remdisivir is a mono phosphoramidate prodrug and a broad-spectrum antiviral agent which is an adenosine analogue. In a recent study, Remdisivir inhibited SARS-CoV-2 replication in Vero-E6 cells and several other studies showed that it is effective against Bat-CoV, MERS-CoV and human-CoV in primary human lung cells replication [15-17]. Homoharringtonine also called as Omacetaxine mepesuccinate, is a cephalotaxine ester and is known to induce differentiation and apoptosis in some cancer cell types and has established clinical activity against haematological malignancies [18, 19]. Favipiravir is another version of Fujifilm Toyama Chemical’s influenza drug Avigan, and it was used to treat Ebola patients in the African continent where the overall fatality was high in the infected children [20-23]. Monoclonal antibody is known to enhance natural immunity against cancer cells and the therapeutic applications of it for COVID-19 is widely discussed but no monoclonal antibody has been declared for its potency [24-26]. Tocilizumab is a monoclonal antibody currently used to treat rheumatoid arthritis and giant cell arteritis [27-30]. In a study from Tongji Hospital in Wuhan, China, a total of 15 patients with high IL-6 levels, several doses of Tocilizumab was recommended to reduce mortality rate [31]. Similarly fixed dosage of Tocilizumab administered against COVID-19 was successful in a patient who was also suffering from multiple myeloma [32, 33].
This paper discusses the statistical records of the effect of past medical history, percentage of various symptoms manifested in COVID-19 patients based on the publicly available data of the new daily confirmed cases reported from various countries and methods of diagnosis and treatment and a note on Indian strategy of lowering transmission of the infection. The structure, mechanism of action and research update in relation to the epidemic are stated in a tabular form for the top 5 drugs that are used by the countries to treat the patients.
The Effect of Past Medical History in Various Age Groups
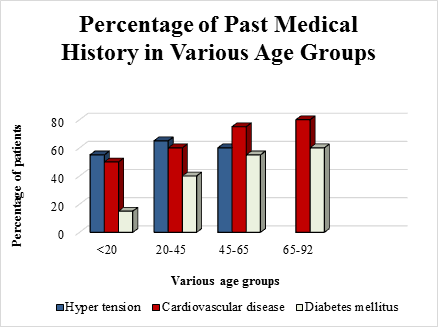
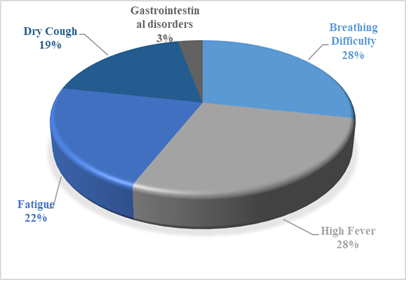
Several reports from China and America state that a majority of affected patients were males [34-36]. The records from Italy show that male sex and age were independent risk factors while all the cases from Germany reported on 30th January 2020 were males with no mentionable medical history [37, 38]. In most of the countries, high fever, dry cough, dizziness, fatigue, shortness of breath and diarrhoea were most common symptoms reported at the time of admission to the hospitals. Gastrointestinal disorders like diarrhoea, nausea etc. were also reported [39, 40]. The percentage of past medical history in various age groups is represented in figure 1 while percentage of various symptoms manifested in COVID-19 patients is depicted in figure 2.
Figure 1: Graphical representation of percentage of past medical history in various age groups like hypertension, cardiovascular disease, and diabetes mellitus. People suffering from cardiovascular diseases and hypertension were highly vulnerable when subjected to COVID-19 and they should take utmost care and precautionary measures against it; based on the reports [34-40].
Detection Methods
Real-time RT-PCR in COVID-19 detection
From all the available tests, the “gold standard” for rapid, sensitive and specific detection is the Polymerase Chain Reaction (PCR) method while for the detection of COVID-19, reverse transcriptase-PCR (RT-PCR) was widely accepted and regarded as an early detection technique but false-negative and false-positive results were reported in several cases [41-44]. Real time RT-PCR uses primers for attachment and polymerisation at the target site and therefore any mutation in the viral RNA sequence might produce false results. Hence, rapid evolution, slow mutations and wide genetic diversity are believed to be the reasons for these false results and targeting multiple genes and performing amplification could solve this ambiguity.
Early Humoral Response to COVID-19
When existing diagnostic methods were highly error prone, the potential of antibody testing for COVID-19 grabbed attention. IgM is the first immunoglobulin secreted during an infection while IgG is produced in maximum and is maintained in the body after an exposure to the antigen. Early Humoral (antibody) Response tests involves testing of IgA, IgM, IgG or the entire sets of antibodies response against the SARS-CoV-2 using ELISA-based assay on the viral nucleocapsid protein [45]. In several studies which were antibody based, it was reported that the positive ratio in IgM antibody test was significantly higher than in RT-PCR detection and that a combined IgG/IgM test seemed to be more effective than testing a single antibody. ELISA and LFIA methods were used and showed 99% sensitivity and with reference to the pandemic, ELISA tests would be safer. In a comparison between IgG and IgM tests, IgG tests were more efficient and accurate [43, 46]. As per W.H.O., ELISA tests are highly specific and sensitive. On April 2nd 2020, the United States of America, approved rapid serological tests made by Cellex© which could give results in 15 minutes’ time, while ELISA kit based on both spike protein and nucleocapsid protein was used for antibody detection in China due to its reduced cost and higher accuracy. Also screening patients with gold standard PCR followed by antibody based tests were also recommended [47-50].
Treatment Methods
As of 12th May 2020, there is no vaccination available but there are certain drugs that are used to reduce the symptoms, there by increasing the chances of recovery and enhances the self-resistance. Convalescent Plasma therapy is preferred by most of the countries and clinical studies showed a higher survival rate. The main way to boost personal immunity is to maintain personal hygiene, a healthy lifestyle and adequate nutritional intake. But the specific mechanism of the virus remains unknown, and no specific drugs have been developed. At present, it is important to control the source of infection, cut off the transmission route, and use the existing drugs and means to control the progress of the disease proactively. Remdisivir, Favipiravir, Hydroxychloroquine, Chloroquine and Homoharringtonine are under clinical studies and these drugs reduce the symptoms of the infection effectively. A drug repurposing model of using a defined combination of these drugs is also in its budding state. Several monoclonal antibodies are also tested for their interaction to COVID-19 membrane proteins which could prevent cell invasion and proliferation of the viral particles. 5 top drugs that are reported to be effective after referring to a total of 16 research papers, their mechanisms of action, structures and latest advancements in relation to COVID-19, are tabulated and represented in table 1.
|
No |
Name |
Structure |
Mechanism |
Research update in relation to COVID-19 |
|
1. |
Remdisivir |
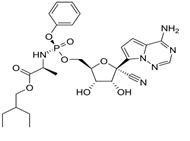
|
Evades exonuclease proofreading activity of RNA [16] |
Faster time to clinical progression and a combination of Remdisivir and chloroquine was recommended [51, 52]. |
|
2. |
Hydroxychloroquine |
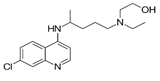 |
Exact mechanisms are not known |
Reduces pneumonia with least toxic effects [53, 54]. |
|
3. |
Favipiravir |
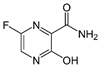 |
Inhibits viral RNA polymerase [55, 58] |
Quicker viral clearance and improvement in chest imaging [56, 57] |
|
3. |
Chloroquine |
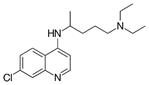 |
Reduces cytokine production [59] |
Suppressing symptoms reducing severity level [60] |
|
4. |
Homoharringtonine |
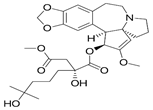 |
Affinity to the A-site cleft in the large ribosomal sub-unit, preventing translation and leads to apoptosis of cells [61] |
From 3 viral inhibitors, hexachlorophene, nitazoxanide and homoharringtonin, homoharringtonin was found to be most effective [62] |
|
5. |
Monoclonal Antibody |
 |
Tocilizumab: Inhibits the binding of Interleukin-6 to its receptor [63] |
The higher lymphocyte count and C-reactive protein levels returned [64, 65] |
|
Table 1: The structure, mechanism of action of the drugs that are used to treat the infection and latest research update of it in relation to COVID-19. Monoclonal antibody is a promising drug for COVID-19 treatment as it prevents cytokine storm which is responsible for sudden death of patients. Hydroxychloroquine effectively cures pneumonia while a combination of effective drugs is also recommended for enhancing survival rates. |
Convalescent Plasma therapy
Several studies reported that convalescent plasma therapy in COVID-19 infected patients has considerably reduced hospital stay and mortality rate. In the case of 2009 severe influenza A H1N1 infection, plasma therapy reduced the mortality rate by 95% and W.H.O. recommended this treatment method for Ebola virus and there were no adverse effects reported [66, 67]. Analysis in 1703 influenza-pneumonia patients who received an infusion of influenza-convalescent human blood products showed a reduction of 21% in the overall mortality rate [68]. For an effective passive antibody therapy, a sufficient amount of the antibody must be administered and when it is given to an infected person, this antibody will be in the circulatory system, reaches tissues and protects against the antigens. In a study of 5 COVID-19 patients, who were treated with convalescent plasma from the donors with IgG and IgM antibodies, it was observed that very high specificity towards the virus was established [69]. On 25th April 2020, reports from India about four COVID-19 patients who received plasma therapy in the capital city showed better chances of recovery and the government decided to conduct it on more patients in the coming days and several hotspots like USA and Italy are looking forward for it.
Side effects reported from various studies
Although several drugs are available none of them are approved for the treatment of COVID-19. The major negative effect of hydroxychloroquine and choloroquine is that they may bind with melanin of retinal epithelium thus damaging the photoreceptor cells and inner layer of retina [70]. While in a study conducted on 1446 consecutive patients there was no specific association between hydroxychloroquine use and mortality rate [71]. Similarly, several fatal adverse effects were reported in 66% of remdisivir treated patients under study, while Favipiravir is an approved drug for influenza in Japan and China and is under investigation process for the use against COVID-19 [72]. Indian Institute of Chemical Technology (IICT), Hyderabad has started the production unit for favipiravir and Drug Controller of India allowed clinical trial of Favipiravir with a maximum treatment duration of 28 days. Homoharringtonine has the major risk of developing myelosuppression, haemorrhage, hyperglycaemia, and fatal harm [73]. Treatment with monoclonal antibodies might induce allergic reactions, diarrhoea, fever, chills etc. while convalescent plasma therapy have not yet reported any potential side effects.
A Note on the Indian Strategy of Lowering Transmission
India has taken necessary steps and precautions to control the spread of the disease by opening control rooms in the same month of the outbreak in Wuhan, China and imposed Section 144 of the CrPC with a complete lockdown till May 3rd. This helped the country to limit the death toll and community spread was avoided in many densely populated states and decided to conduct Convalescent Plasma therapy on more patients in the coming days. In India, multiplex PCR panels were used for diagnostics in the onset of the infection but now if no pathogen was identified the samples were referred for several serological testing. The Indian Council of Medical Research (ICMR) approved 176 labs, to conduct diagnostic tests and the U.S. Food and Drug Administration has approved such kits for diagnostic testing for COVID-19.
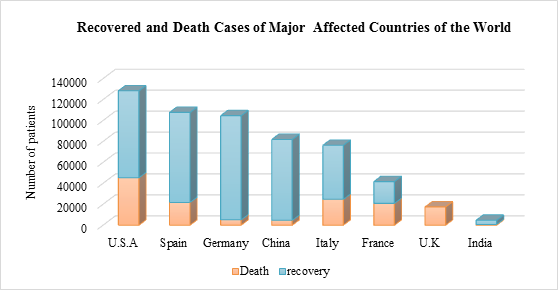
As represented in the figure 3, the overall mortality rate in India is low compared to the other countries, i.e. only 3% and this was achieved in India by unprecedented measures to contain further transmission of COVID-19. In case of various states in India, lockdown that was into action for 21 days which was later extended till May 2nd and from all confirmed cases, 26.24% are in Maharashtra. Union Ministry of Health and Family Welfare and its robust surveillance system is closely monitoring more than 9.5 lakh suspected Covid-19 cases across the country. Also, travel history of all patients with respiratory symptoms and made any international travel or even contacted with any sick person were diagnosed and kept for home quarantine or in isolation in hospitals regardless of a negative test result.
Figure 3: Graphical representation of total number of confirmed deaths and recovered cases from most affected countries of the world and is compared with records from India; based on daily reports of W.H.O. Mortality rate is very low in India when compared to other countries and this is achieved by better diagnostic tools, effective treatment protocols and imposing restrictions on travel and person to person contacts. A huge increase in the number of patients is observed in the United States of America, Spain and Germany while the rate of recovery was lower when compared to other countries.
From the everyday count of reports from various parts of the world, it is clear that India is in a better position than many other countries in case of total number of cases being reported and the recovery of patients as well. However, an aggressive approach and immediate action have to be taken so as to avoid community spread in the states with relatively high population density. Convalescent Plasma therapy should be implemented in more severely affected states of the country to make sure higher survival rate. Also balanced immunity boosting diet, timely medications and even urgent ventillatory support should be made more accessible. Our study has limitations since virus viability was not shown and the sample size was small, due to difficulty to run more tests in a time of emergency. However, the emergency of the outbreak required a fast assessment of the current situation. While the epidemic still shows no sign of ending, further studies are underway to more comprehensively determine the potential of the environment as a transmission medium.
Acknowledgements
Thanks to the Department of Biotechnology, Vels Institute of Science, Technology and Advanced Studies, Chennai.
Disclosure:
All authors have no potential conflicts of interest.
References
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta bio-medica: Atenei Parmensis91 (2020): 157-160.
- Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19—studies needed. New England Journal of Medicine 382 (2020): 1194-1196.
- Jones DS. History in a crisis—lessons for Covid-19. New England Journal of Medicine 382 (2020): 1681-1683.
- Huggett J, Dheda K, Bustin S, et al. Real-time RT-PCR normalisation; strategies and considerations. Genes & Immunity 6 (2005): 279-284.
- Li D, Wang D, Dong J, et al. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep-learning-based CT diagnosis and insights from two cases. Korean journal of radiology 21 (2020): 505-508.
- Krogstad DJ, Gluzman IY, Kyle DE, et al. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238 (1987): 1283-1285.
- Krieg AM. Immune effects and mechanisms of action of CpG motifs. Vaccine 19 (2000): 618-622.
- Shadhu K, Xi C. Inflammation and pancreatic cancer: An updated review. Saudi Journal of Gastroenterology: official journal of the Saudi Gastroenterology Association 25 (2019): 3.
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical Infectious Diseases (2020).
- Hache G, Claude Deharo MD, Brouqui P. Combination of hydroxychloroquine plus azithromycin as potential treatment for COVID 19 patients: pharmacology, safety profile, drug interactions and management of toxicity.
- Saleh M, Gabriels J, Chang D, et al. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circulation: Arrhythmia and Electrophysiology (2020).
- Frie K, Gbinigie K. Chloroquine and hydroxychloroquine: Current evidence for their effectiveness in treating COVID-19. The Centre for Evidence-Based Medicine (2020).
- Bilgin S, Kurtkulagi O, Kahveci GB, et al. Millennium pandemic: a review of coronavirus disease (COVID-19). Experimental Biomedical Research 3 (2020): 117-125.
- Qiu T, Liang S, Dabbous M, et al. Chinese Guidelines Related to Novel Coronavirus Pneumonia (2020).
- Vafaei S, Razmi M, Mansoori M, et al. Spotlight of Remdesivir in Comparison with Ribavirin, Favipiravir, Oseltamivir and Umifenovir in Coronavirus Disease 2019 (COVID-19) Pandemic. Favipiravir, Oseltamivir and Umifenovir in Coronavirus Disease (2019).
- Singh AK, Singh A, Shaikh A, et al. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes & Metabolic Syndrome: Clinical Research & Reviews (2020).
- Chowdhury A, Jahan N, Wang S. One month of the novel coronavirus 2019 outbreak: Is it still a threat?. VirusDisease 1 (2020).
- Quintás-Cardama A, Kantarjian H, Cortes J. Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa 2009. Cancer 115 (2009): 5382-5393.
- Chen R, Plunkett W. Strategy to induce apoptosis and circumvent resistance in chronic lymphocytic leukaemia. Best Practice & Research Clinical Haematology 23 (2010): 155-166.
- Nagata T, Lefor AK, Hasegawa M, Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster medicine and public health preparedness 9 (2015): 79-81.
- de la Torre JC. Extending the antiviral value of favipiravir (2018).
- Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Current treatment options in infectious diseases 8 (2016): 311-328.
- Farrukee R, Hurt AC. Antiviral drugs for the treatment and prevention of influenza. Current Treatment Options in Infectious Diseases 9 (2017): 318-332.
- Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer and Metastasis Reviews 24 (2005): 487-499.
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology 10 (2010): 317-327.
- Melero I, Hervas-Stubbs S, Glennie M, et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nature Reviews Cancer 7 (2007): 95-106.
- Oldfield V, Dhillon S, Plosker GL. Tocilizumab. Drugs 69 (2009): 609-632.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. New England Journal of Medicine 377 (2017): 317-328.
- De Benedetti F, Brunner HI, Ruperto N, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. New England Journal of Medicine 367 (2012): 2385-2395.
- Singh JA, Beg S, Lopez?Olivo MA. Tocilizumab for rheumatoid arthritis. Cochrane Database of Systematic Reviews 7 (2010).
- Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID?19: A single center experience. Journal of Medical Virology (2020).
- Matsuyama Y, Nagashima T, Honne K, et al. Successful treatment of a patient with rheumatoid arthritis and IgA-kappa multiple myeloma with tocilizumab. Internal Medicine 50 (2011): 639-642.
- Zhang X, Song K, Tong F, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Advances 4 (2020): 1307.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395 (2020): 507-513.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395 (2020): 497-506.
- Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chinese Medical Journal (2020).
- Lupia T, Scabini S, Pinna SM, et al. 2019-novel coronavirus outbreak: A new challenge. Journal of Global Antimicrobial Resistance (2020).
- Bastola A, Sah R, Rodriguez-Morales AJ, et al. The first 2019 novel coronavirus case in Nepal. The Lancet Infectious Diseases 20 (2020): 279-280.
- Hormati A, Shahhamzeh A, Afifian M, et al. Can COVID-19 present unusual GI symptoms. J Microbiol Immunol Infect (2020).
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of autoimmunity (2020): 102433.
- Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results (2020).
- Liu R, Liu X, Han H, et al. The comparative superiority of IgM-IgG antibody test to real-time reverse transcriptase PCR detection for SARS-CoV-2 infection diagnosis. medRxiv (2020).
- Lee CY, Lin RT, Renia L, et al. Serological Approaches for COVID-19: Epidemiologic Perspective on Surveillance and Control. Frontiers in Immunology 11 (2020): 879.
- Kontou PI, Braliou GG, Dimou NL, et al. Antibody tests in detecting SARS-CoV-2 infection: a meta-analysis. Diagnostics (2020): 319.
- Patel R, Babady E, Theel ES, et al. Report from the American Society for Microbiology COVID-19 international summit, 23 march 2020: value of diagnostic testing for SARS–CoV-2/COVID-19 (2020).
- Bertolotti P, Deaner B, Deshpande Y, et al. Pooled Sample Testing for SARS-CoV-2 (2020).
- Rando HM, Greene CS, Robson MP, et al. SARS-CoV-2 and COVID-19: An Evolving Review of Diagnostics and Therapeutics. Manubot; (2020).
- World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization (2020).
- Kannan S, Ali PS, Sheeza A, et al. COVID-19 (Novel Coronavirus 2019)-recent trends. Eur. Rev. Med. Pharmacol. Sci 24 (2020): 2006-2011.
- Al-Muharraqi MA. Testing recommendation for COVID-19 (SARS-CoV-2) in patients planned for surgery-continuing the service and ‘suppressing’the pandemic. The British Journal of Oral & Maxillofacial Surgery (2020).
- Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life sciences (2020): 117477.
- Nyarko RO, Boateng E, Kahwa I, et al. A Comparison Analysis on Remdesivir, Favipiravir, Hydroxychloroquine, Chloroquine and Azithromycin in The Treatment of Corona Virus Disease 2019 (Covid-19)-A Review (2020).
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents (2020): 105949.
- Colson P, Rolain JM, Lagier JC, et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 105932 (2020).
- Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (2020).
- Chen C, Huang J, Cheng Z, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv (2020).
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Medical Research 7 (2020): 1-10.
- Yanai H. Favipiravir: A Possible Pharmaceutical Treatment for COVID-19. Journal of Endocrinology and Metabolism 10 (2020): 33-34.
- Noël F, Lima J. Pharmacological aspects and clues for the rational use of Chloroquine/Hydroxychloroquine facing the therapeutic challenges of COVID-19 pandemic. Lat Am J Clin Sci Med Technol [Internet] 2 (2020): 28-34.
- Devaux CA, Rolain JM, Colson P, et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?. International journal of antimicrobial agents (2020): 105938.
- Hassan ST. Shedding Light on the Effect of Natural Anti-Herpesvirus Alkaloids on SARS-CoV-2: A Treatment Option for COVID-19.
- Cao J, Forrest JC, Zhang X. A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antiviral research 114 (2015): 1-10.
- Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. American Journal of Health-System Pharmacy 65 (2008): 1413-1418.
- Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. International Journal of Antimicrobial Agents (2020): 105954.
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proceedings of the National Academy of Sciences 117 (2020): 10970-10975.
- Singh AK, Singh A, Shaikh A, et al. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes & Metabolic Syndrome: Clinical Research & Reviews (2020).
- Van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. New England Journal of Medicine 374 (2016): 33-42.
- Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. Journal of Medical Virology (2020).
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama 323 (2020): 1582-1589.
- Gonasun LM, Potts AM. In vitro inhibition of protein synthesis in the retinal pigment epithelium by chloroquine. Investigative Ophthalmology & Visual Science 13 (1974): 107-115.
- Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. New England Journal of Medicine (2020).
- Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet (2020).
- Kantarjian HM, Talpaz M, Smith TL, et al. Homoharringtonine and low-dose cytarabine in the management of late chronic-phase chronic myelogenous leukemia. Journal of Clinical Oncology 18 (2000): 3513-3521.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
