A Step-by-Step Evaluation of the Claim That COVID-19 Vaccines Saved Millions of Lives
Yaakov Ophir*,1, Yaffa Shir-Raz2, Shay Zakov3, Raphael Lataster4, Peter A. McCullough5
1Ariel University, Ariel, Israel and University of Cambridge, Cambridge, UK
2University of Haifa, Haifa, Israel
3The Israeli Public Emergency Council for the COVID-19 Crisis Plenary, Israel
4University of Sydney, Sydney, Australia
5McCullough Foundation, Dallas, Texas, USA
*Corresponding author: Yaakov Ophir, Ariel University, Ariel, Israel and University of Cambridge, Cambridge, UK.
Received: 12 August 2025; Accepted: 18 August 2025; Published: 15 September 2025
Article Information
Citation: Yaakov Ophir, Yaffa Shir-Raz, Shay Zakov, Raphael Lataster, Peter A. McCullough. A Step-by-Step Evaluation of the Claim That COVID-19 Vaccines Saved Millions of Lives. International Journal of Applied Biology and Pharmaceutical Technology. 16 (2025): 35-50.
View / Download Pdf Share at FacebookAbstract
Concerns about potential harms of COVID-19 vaccines are often met with the widespread claim that the vaccines saved millions of lives. A recent U.S. Senate hearing on vaccine safety (May 21, 2025) even opened with the declaration that “there is no scientific question about that fact.” This article offers a structured, step-by-step evaluation of the empirical basis for that claim, building on the authors’ prior comprehensive investigation. Step 1 analyzes the mathematical models behind the ‘millions saved’ claim, including the one cited in the Senate hearing. Step 2 revisits the collapse of the initial narrative concerning vaccine efficacy against infection and transmission, which served as the cornerstone of the mass vaccination campaign. Step 3 examines the revised justification that followed: the claim that vaccines continued to protect against severe illness and death. This step draws on data from randomized trials (3.1), observational studies (3.2), and official public health dashboards (3.3). Taken together, this analysis shows that the ‘millions saved’ narrative lacks empirical support (readers are strongly encouraged to consult the full article and assess the evidence). To understand how such an unsupported narrative could emerge and dominate, Step 4 traces the direct mechanisms behind its rise: methodological flaws (4.1), misrepresentation and misinterpretation of findings (4.2, 4.3), and suppression of dissenting voices (4.4). By focusing on transient signals of success while overlooking concerns about efficacy and safety, a fragile assertion appears to have solidified into a widely accepted belief that shaped global health policy.
Keywords
<p>COVID-19 vaccines; Vaccine efficacy; Vaccine safety; Methodological critique; Scientific censorship</p>
Article Details
Introduction
Two years have passed since the COVID-19 pandemic was officially declared over, yet the topic of vaccines remains highly sensitive in both public and scientific discourse. Efforts to question the legitimacy of the mass vaccination campaign or to raise concerns about potential harms are often met with a perceived moral boundary: the widespread claim that the “COVID-19 vaccines have saved millions and millions of lives.”1 Notably, this assertion was treated as established fact during the recent U.S. Senate PSI hearing (May 21, 2025), which focused on adverse outcomes associated with the vaccines.1 Ranking Member Richard Blumenthal opened the hearing with the following statement:
“As we talk about the side effects of COVID vaccines, I think we need to be clear about the most important fact. For all Americans, COVID-19 vaccines have saved millions and millions of lives. There is no scientific question about that fact…
One study found that 3 million American deaths were averted… in the United States… I would like this study entered into the record.1
This confident assertion brings forth a key question: Is there truly concrete and conclusive scientific evidence to support the claim that the mass vaccination campaign during the COVID-19 pandemic resulted in a net benefit of millions of lives saved? At the heart of this discussion lies a foundational principle of medical practice: every intervention, no matter how promising, must be evaluated by weighing its potential benefits against its potential harms over time. This basic principle is manifested in the recent straightforward justification of the Australian Government’s Department of Health for their recommendation against COVID-19 vaccination for healthy children and adolescents:
“This is because the risk of severe illness was extremely low in this cohort over the course of the pandemic, and benefits of vaccination are not considered to outweigh the potential harms” (bold added).2
While this basic principle is clear, its practical implementation has been far less straightforward. Existing studies on COVID-19 vaccines have yet to provide a robust, long-term comparison between potential benefits and harms. The only datasets with the methodological rigor to address this crucial question at a gold standard are the original randomized controlled trials (RCTs) conducted by vaccine manufacturers prior to the FDA’s Emergency Use Authorization (EUA) of the vaccines. Yet even these trials, in their published form, did not offer a sufficient benefit–harm analysis. In fact, an analysis by the third author of this article revealed that in Pfizer’s pivotal trial, for every case of severe COVID-19 potentially prevented by the vaccine, approximately two to three additional serious adverse events were reported in the vaccine group.3
This concerning ratio joins an expanding body of real-world evidence and peer-reviewed studies (many co-authored by the last author) that document vaccine-related harms, including serious adverse events and deaths.4-13 Together, these findings have reached a point where, as of 2025, it has become increasingly difficult to dismiss or overlook this side of the medical equation. Nonetheless, when concerns about vaccine-related harms are raised, they are often answered by a return to the overarching justification that introduced this article: the widely repeated claim that “COVID-19 vaccines have saved millions and millions of lives,” and that “there is no scientific question about that fact”.1 Building on our prior, more technical and comprehensive review of this topic,14 this article offers a structured, step-by-step analysis of the empirical basis for the widely cited claim that “the vaccines saved millions of lives.”
The analysis begins with a critical examination of the modeling study referenced in the recent U.S. Senate hearing, alongside similar hypothetical projections (Step 1). It then revisits the collapse of the early narrative surrounding vaccine efficacy (VE) against infection and transmission—a narrative that served as the cornerstone of public justification for the global vaccination campaign (Step 2). The core of the article (Step 3) offers an in-depth evaluation of the revised claim that replaced it: that the vaccines continued to prevent severe illness and death despite their failure to prevent infections. This step draws on evidence from the original Randomized Controlled Trials (3.1), large-scale observational studies conducted during the vaccination campaign (3.2), and real-world data from public health dashboards (3.3). This stepwise structure reflects the central aim of the article: to unpack, with precision and transparency, a claim that has shaped public health discourse and policy on an unprecedented scale. Given the magnitude of the discrepancy between this claim and the available evidence (Steps 1-3), an additional question arises: how did such a fragile assertion gain such widespread and lasting traction? To address this, Step 4 explores the direct mechanisms that enabled the emergence and persistence of the ‘millions saved’ narrative. These include methodological flaws (4.1), misrepresentation and misinterpretation of research findings (4.2, 4.3), and suppression of dissenting voices (4.4).
Ultimately, evaluating interventions of this magnitude requires more than headline-level declarations. It calls for a careful, detailed examination of the underlying evidence. We therefore strongly encourage readers to engage with the full analysis presented in this article—beginning with Step 1, which examines the statistical modeling studies that produced the ‘millions saved’ narrative.
Step 1
What Are the Core Assumptions Behind the ‘Millions Saved’ Models?
We begin our inquiry with the statistical modeling studies that helped shape the narrative claiming that COVID-19 vaccines saved millions of lives. To the best of our knowledge, the specific study cited in the Senate hearing’s opening statement was not a formal academic publication, but a brief blog post published by The Commonwealth Fund on December 13, 2022.15 The post was titled “Two Years of U.S. COVID-19 Vaccines Have Prevented Millions of Hospitalizations and Deaths,” and its authors claimed:
“From December 2020 through November 2022, we estimate that the COVID-19 vaccination program in the U.S. prevented more than 18.5 million additional hospitalizations and 3.2 million additional deaths. Without vaccination, there would have been nearly 120 million more COVID-19 infections” (bold added).
But how did the authors arrive at such a precise and decisive conclusion? They explained:
“We therefore used a computer model of disease transmission to estimate hospitalizations and deaths averted through the end of November 2022. The model incorporates the age-stratified demographics, risk factors, and immunological dynamics of infection and vaccination. We simulated this model to compare the observed pandemic trajectory to a counterfactual scenario without a vaccination program” (bold added).
This blog post stands out for its lack of transparency (i.e., readers are expected to accept its dramatic estimates without being shown the underlying assumptions), but it was not alone in promoting such sweeping claims. This blog post was not alone in promoting such sweeping claims. Other studies have used similar hypothetical statistical models, and some were published in formal academic journals.16-18 The most frequently cited among them is the modeling study by Watson and colleagues, published in The Lancet Infectious Diseases.19 Although such models may help inform public health decisions, their conclusions depend entirely on the soundness of a long and complex chain of assumptions.
A careful examination of these assumptions—as undertaken in a recent critique by the fourth author20 —reveals a range of serious flaws, including:
- • Inflated baseline assumptions, such as exaggerated infection rates and case fatality ratios, which skew the projected number of lives saved.
- • Failure to account for real-world factors that independently contributed to the decline in COVID-19 mortality, including:
- • Underestimating the well-documented and widely-accepted waning of vaccine effectiveness over time (see Step 2), which fundamentally alters any long-term projections of benefit.
- • Disregarding the full spectrum of vaccine-related harms, including serious adverse events and vaccine-enhanced disease, which are essential to consider in any valid risk-benefit assessment (as noted in the Introduction).
- • Overreliance on observational data that are vulnerable to serious confounding, including the healthy vaccinee bias, differential testing patterns, and uncontrolled behavioral variables (see Steps 3 and 4).
- • Undeclared or unresolved financial and political conflicts of interest, which may have influenced modeling choices and interpretations.
i. natural immunity acquired through infection,
ii. early ambulatory multidrug treatment protocols, and
iii. the emergence of milder viral variants over time. These factors reduced mortality, regardless of vaccination status.
Taken together, these methodological shortcomings raise serious concerns about the reliability of models that claim to quantify vaccine-attributable lives saved.
Notably, the present article focuses on the central assumption that underpins these hypothetical models: the presumed scientific efficacy of the COVID-19 vaccines. This supposedly well-established fact serves not only as the cornerstone of the statistical projections but also as a foundational pillar in the broader public narrative that the vaccines saved millions of lives. In particular, the authors of the modeling study cited in the Senate hearing’s opening statement explicitly stated:
“Vaccine efficacies against infection, and symptomatic and severe disease for different vaccine types—for each variant and by time since vaccination—were drawn from published estimates” (bold added).15
In other words, the model did not evaluate Vaccine Efficacy (VE) directly; it simply imported efficacy estimates from prior publications (which are not specified in the blog post). Our aim, therefore, is to trace the origins of these “published estimates” and pose a simple yet fundamental question: To what extent do accumulating research findings and real-world data actually support the perceived efficacy of the COVID-19 vaccines? To begin addressing this question, we turn to the early efficacy claims that formed the primary justification for mass vaccination during the pandemic.
Step 2
What Happened to the Cornerstone Narrative on VE Against Infection and Transmission?
At the outset of the global vaccination campaign, public health authorities and media outlets promoted a confident and promising message: the newly developed vaccines were said to be “95% effective in preventing COVID-19.”21 This bold claim served as the moral and scientific foundation for sweeping public health measures, including vaccine passports, intense societal pressure to vaccinate, and even vaccine mandates. Consider the words of Dr. Anthony Fauci, then Chief Medical Advisor to the U.S. President, on May 16, 2021, roughly five months into the campaign:
“When you get vaccinated you not only protect your own health…, but also you contribute to the community health by preventing the spread of the virus throughout the community… you become a dead end to the virus” (bold added).22
In the same vein, Pfizer CEO Albert Bourla declared on May 25, 2022, that the goal of updated vaccines targeting emerging variants was to:
“prevent the sickness… and that will maximize the chances that people that you love, not to get infected. You vaccinate, not only for yourself. You vaccinate also to protect society” (bold added).23
However, this narrative gradually began to unravel. Reports of breakthrough infections emerged as early as April 2021, when the CDC acknowledged that fully vaccinated individuals were still contracting COVID-19.24 Then, in August 2021, a large-scale preprint study from Qatar based on data from more than 900,000 vaccinated individuals reported a rapid decline in VE against infection.25 This study, later published in The New England Journal of Medicine (NEJM), found that VE was negligible during the first two weeks after the initial dose, rose modestly to 36.8% in the third week, and peaked at 77.5% one month after the second dose.26 From that point onward, VE declined steadily and consistently over time. Notably, this disappointing pattern appeared in both symptomatic and asymptomatic infections, suggesting that the two forms of protection followed a similar trajectory.
These emerging findings were gradually acknowledged by leading public health figures. By December 15, 2021, Dr. Anthony Fauci admitted in the same journal (NEJM) that:
“Vaccination has also been unable to prevent ‘breakthrough’ infections, allowing subsequent transmission to other people even when the vaccine prevents severe and fatal disease.”27
Likewise, on January 10, 2022, Pfizer CEO Albert Bourla conceded:
“The three doses with a booster, they offer reasonable protection against hospitalization and deaths. Against deaths, I think very good, and less protection against infection.”28
Eventually, the House Select Subcommittee on the Coronavirus Pandemic concluded, in its December 2024 final report that:
“Not only did COVID-19 vaccine mandates cause many unintended consequences, but they were also not based in science… It was already evident then and is now commonly known that the vaccines do not prevent you from getting infected or transmitting the virus”.29, p. 346
In summary, the early and widely publicized claim that COVID-19 vaccines were highly effective in preventing infection and transmission (often cited as “95% efficacy”) did not withstand empirical scrutiny. Real-world observational studies soon revealed significant waning of protection, and in some cases even suggested negative vaccine effectiveness.30-32 Millions of vaccinated individuals contracted COVID-19, and public trust in the promise of both personal and communal protection began to erode. This collapse of the original rationale for mass vaccination casts serious doubt on a central assumption underlying the hypothetical statistical models discussed in Step 1—namely, that widespread vaccination substantially reduced infections and viral transmission and, as a result, saved millions of lives. As accumulating evidence now makes clear, this assumption is simply false (for additional methodological concerns regarding how the “95% effective in preventing COVID-19” figure was derived, see Section 4.1).
Step 3
What Is the Evidence That the Vaccines Continued to Prevent Severe Illness and Death?
With the collapse of the initial narrative surrounding protection against infection and transmission, a new justification quickly emerged. Although the vaccines could not prevent infection, they were now said to offer robust and sustained protection against severe illness and death. This revised assumption soon became the central premise behind the updated claim that mass vaccination campaigns saved millions of lives. Unlike the earlier narrative, which could be tested against real-world data on breakthrough infections, this new claim was harder to falsify. After all, one cannot measure a severe illness that never occurred. It became easy and reassuring to think: “Good thing I got vaccinated, otherwise it could have been much worse.”
This conceptual separation between short-lived protection against infection and lasting protection against severe outcomes gained broad acceptance, even though no empirical study had proposed or supported such a distinction at the time. So what does the evidence actually show? Is there truly “no scientific question,” as claimed in the opening remarks of the U.S. Senate hearing, about the continued effectiveness of the vaccines against severe illness and death?
To address this critical question, we conducted an in-depth and technically rigorous review in 2022, while the scientific discourse was still evolving (for details on the challenges we faced in publishing this work, see Section 4.4).14 In the current article, we do not aim to summarize all of our findings. Instead, we present several key refutations from that earlier review, along with additional evidence—beginning with Pfizer’s pivotal RCT (Section 3.1), continuing with major observational studies (Section 3.2), and concluding with official public health dashboards that shaped global perceptions (Section 3.3).
3.1 Severe Illness and Mortality in the Pfizer Trial (Pre-EUA)
The most appropriate scientific method to address the critical question regarding vaccine efficacy against severe illness and death is the longitudinal randomized controlled trial (RCT). RCTs are widely regarded as the gold standard in biomedical research, and when they demonstrate strong clinical value, their findings are often published in top-tier academic journals such as The New England Journal of Medicine (NEJM). Accordingly, the two pivotal trials that supported the Emergency Use Authorization (EUA) of the Pfizer and Moderna vaccines were both published in NEJM in December 2020.21,33 Their publication was met with widespread excitement, as it seemed to mark a turning point in the pandemic: a scientific breakthrough that could finally bring the crisis to an end. However, as early as October 2020, two months before the EUA was granted, BMJ senior editor Peter Doshi had already sounded a note of caution: “None of the vaccine trials are designed to detect a significant reduction in hospital admissions, admission to intensive care, or death.”34 This key limitation was compounded by the short duration of follow-up in these studies. For example, Pfizer’s pivotal trial had a median follow-up of only two months after the second dose.21 Such a limited timeframe is insufficient for drawing meaningful conclusions about rare or long-term critical outcomes, whether beneficial or harmful. Correspondingly, a direct answer to the critical question of protection against severe illness and mortality does not appear explicitly in Pfizer’s main publication. Instead, readers seeking a full understanding must consult a supplementary appendix, which reveals the following:
- • After the first dose, 4 cases of severe COVID-19 occurred in the placebo group, and none in the vaccine group. Interpreting this result is challenging, as both immune response development and the onset of severe illness require time.35 According to the clinical protocol, full immunization was not expected until 7 days after the second dose.
- • Beginning 7 days after the second dose, when participants were officially considered fully vaccinated, 4 cases of severe COVID-19 were recorded in the placebo group, and 1 in the vaccine group during the short, two-month follow-up period of the RCT. While this difference could yield an efficacy estimate of 75%, it was not statistically significant (95% CI: –152.6 to 99.5).
In other words, from an empirical and scientific standpoint, the discussion could have ended here: the Pfizer trial did not provide usable evidence of efficacy against severe disease. The FDA, of course, was fully aware of these underwhelming results. Their EUA press release stated:
“Of these 170 COVID-19 cases [observed in the Pfizer trial], one in the vaccine group and three [not four] in the placebo group were classified as severe. At this time, data are not available to make a determination about how long the vaccine will provide protection.”36
In addition, no hospitalization data were reported in the main Pfizer article.37 The only two hospitalizations due to COVID-19 following full vaccination appeared in Pfizer’s technical briefing to the FDA.38 That document also admitted that:
“The total number of severe cases is small, which limits the overall conclusions that can be drawn.”
Conditional probability of severe illness in Pfizer’s RCT
Yet the issue runs even deeper. Even if one accepts these small numbers at face value, a critical question emerges: What happens to the assumed vaccine efficacy (VE) against severe illness once VE against infection wanes (as discussed in Section 2)? To properly address this, a more relevant outcome measure must be considered, namely, the percentage of severe illness among those who became infected. We elaborate on the importance of this conditional-probability metric in Section 3.2, but it is worth noting already here that Pfizer’s results actually invert when this measure is applied: among participants who became infected, 12.5% of those in the vaccine group developed severe disease, compared to just 5.6% in the placebo group.
Mild COVID-like Symptoms in Pfizer’s RCT
Moreover, even when shifting the focus from severe illness to milder, flu-like symptoms, such as fever or sore throat, the Pfizer trial arguably did not demonstrate any clear clinical benefit for vaccinated individuals. According to the supplementary technical report Pfizer submitted to the FDA, 1,594 of the 21,720 participants in the vaccine group (7.37%) and 1,816 of the 21,728 in the placebo group (9.10%) experienced COVID-like symptoms.38 In practical terms, these figures indicate that the vaccine offered no meaningful clinical benefit within the trial—neither in reducing symptoms, as shown here, nor in preventing severe illness, as discussed earlier, nor in reducing mortality, as will be shown below.
The Main Statistically Significant Finding in Pfizer’s RCT
Essentially, the only significant difference between the study groups was the number of positive SARS-CoV-2 tests (via nucleic acid amplification) among symptomatic participants: 8 positive tests were reported in the vaccine group, compared with 162 in the placebo group.21 Notably, this exclusively laboratory-based finding (i.e., not a clinical outcome) was derived from a very small subset of trial participants. Rather than conducting routine COVID-19 testing across the entire sample (as would be expected in a trial of this significance, and as was standard practice during that phase of the pandemic) testing in this study was highly limited. Based on the supplementary report data,38 we calculated that no more than 8.24% of participants were tested for COVID-19. Additional concerns were raised in real time about potential breaches of the blinding protocol39 and the uneven application of exclusion criteria between groups.40 Further critiques, including a series of commentaries published in the Journal of Evaluation in Clinical Practice, pointed to deeper methodological flaws.41-43 These included the problematic classification of “partially vaccinated” individuals (e.g., counting infections that occurred shortly after the first dose as if they belonged to the unvaccinated group) as well as inconsistencies in case counting windows, whereby infections occurring soon after vaccination were excluded from the analysis. Such practices, along with broader design and analytic biases, may have contributed to inflated estimates of vaccine efficacy (see Section 4.1 for further discussion). Taken together, this single, narrowly defined, and weakly measured non-clinical outcome served as the foundation for the study’s headline claim that the vaccine was “95% effective in preventing COVID-19”.
Mortality Outcomes in Pfizer’s RCT
Finally, we turn to the most meaningful outcome of all: mortality. Across the entire sample of 43,448 participants, not a single COVID-19-related death was recorded in the pivotal randomized controlled trial (RCT) that led to the vaccine’s Emergency Use Authorization (EUA). This fact alone raises serious questions about whether the EUA criteria, which require a pressing public health emergency, were genuinely met. More importantly, Pfizer’s subsequent six-month follow-up showed no statistically significant difference in overall mortality between the groups: 15 deaths occurred in the vaccine group (21,720 participants), compared to 14 in the placebo group (21,728 participants).44 These mortality findings should have prompted a serious reassessment of the policy to vaccinate the entire population.45 If no mortality benefit was observed, on what basis was a favorable risk–benefit ratio assumed?
Even more troubling, during the open label phase of the trial, when placebo participants were allowed to receive the actual vaccine, five additional deaths occurred, all among vaccinated individuals (three from the original vaccine group and two from the original placebo group).46 This leads to a key conclusion: At the time of the vaccination campaign, there was no compelling evidence that the Pfizer vaccine provided reliable protection against severe illness or death from COVID-19. Not a single life was demonstrably saved in the foundational trial by Pfizer. In other words, the null hypothesis regarding mortality could not be rejected in Pfizer’s RCT, despite its large sample size of over 40,000 participants. In such a case, where a serious scientific attempt to reject the null hypothesis fails, there is no statistical justification for deviating from the null in hypothetical projection models. Models that do so essentially contradict the findings of the gold-standard trial and generate outcomes ex nihilo.
3.2 Severe Illness and Mortality in Observational Studies
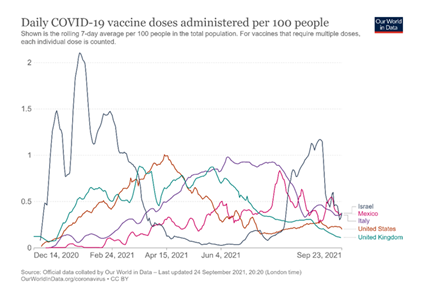
Given the complete lack of empirical evidence from gold-standard RCTs (which are the appropriate method for evaluating whether millions of lives could plausibly be saved), one might ask whether any insights can nevertheless be gleaned from the large-scale observational studies conducted during the mass vaccination rollout. Take, for instance, a major Israeli study on the second booster by Bar-On et al. (2022), also published in the prestigious New England Journal of Medicine.47 The authors reported that “protection against confirmed infection appeared short-lived, whereas protection against severe illness did not wane during the study period.” Perhaps studies of this kind—particularly those conducted in Israel—could be interpreted, in retrospect, as lending empirical support to a conceptual shift in the vaccine narrative. Israel, often referred to as “the world’s laboratory,”48 was the first country to fully vaccinate the majority of its elderly population (Figure 1), and its real-world studies were later used by health authorities, including the FDA, to inform policy decisions (e.g., 49,50). Could these studies retroactively support the notion of a conceptual separation between the two types of vaccine efficacy?
Note: The figure was generated by the first author using data from the Our World in Data website on September 25, 2021. At the onset of the global vaccination campaigns, Israel led all OECD countries in daily vaccine doses administered per 100 people. OECD countries were chosen for comparison based on the assumption that they maintain robust vaccine monitoring systems. For clarity and visual simplicity, only five representative OECD countries are displayed in the figure.
A close examination of key Israeli studies on this topic (e.g., 47,51-53) reveals that the data they report do not support any conclusion of distinct and durable protection against severe illness or mortality. These studies suffer from serious methodological limitations that undermine their conclusions—most notably, their follow-up periods, which were often unequal between vaccinated and unvaccinated participants and, at best, lasted only a few weeks. This stands in stark contrast to the public messaging, which suggested that the vaccine’s protection lasts for six months, and to the clinical protocol itself, which defines full vaccination status only 28 days after the first dose. Detailed technical critiques of key studies, such as Arbel et al. (2022) and Magen et al. (2022), are available in our comprehensive review of this topic.14 Notably, one of the earliest and most influential studies by Dagan et al., published on February 24, 2021, reported “a mean follow-up of 15 days” from the first dose.54 Such a short follow-up period is insufficient for evaluating vaccine efficacy, and the fact that it yielded high efficacy estimates actually raises immediate concerns about the study’s internal validity. In observational, real-world studies, apparent immediate benefits from vaccination are unlikely to reflect a true immunological response (since immunization and severe illness take time to develop as explained above) and are more likely attributable to the well-documented healthy vaccinee bias, which will be discussed further in Section 3.3.
A similar issue appears in an influential Israeli preprint study (Bar-On et al., 2021) from August 31, 2021,53 which served as “real-world data” in the FDA advisory committee’s discussion of Pfizer’s first booster dose.50 While the study officially reported a three-week follow-up period, the actual average follow-up lasted just a few days.55 In other words, even setting aside the inherent limitations of non-randomized observational research, the conclusions drawn from these studies apply to a very narrow timeframe. Thus, there is no valid reason to assume that vaccine-induced protection against severe illness and death would persist long after the short-term protection against infection has already waned.
Conditional Probability as the Key to Evaluating Distinct and Durable VE Against Severe Illness
To credibly support the revised narrative (that protection against severe illness persists long after the vaccine’s short-term protection against infection), it is necessary to demonstrate a genuine separation between these two types of vaccine efficacy. Scientifically, this requires demonstrating that the conditional probability of developing severe illness among those infected is significantly lower in the vaccinated group. Absent such evidence, any apparent reduction in severe illness may merely reflect a transient by-product of the vaccine’s short-term effectiveness in preventing infection. Consider, for example, the aforementioned study by Bar-On et al. (2021).53 According to the summary presented to the FDA advisory committee, the booster dose in this study reduced the risk of severe illness by 15.5-fold (95% CI: 10.5–22.8) compared to individuals who received only the initial two doses.50, p. 44 However, a close look at the underlying data of this preprint tells a different story. Among those who became infected, 330 out of 3,473 individuals in the two-dose group developed severe illness (9.5%), compared to 32 out of 313 in the booster group (10.2%)—a slightly higher rate in the latter. This disappointing pattern was also evident in Pfizer’s original RCT (see Section 3.1), and it continued to appear in subsequent observational studies, as noted by Wohl and Leibowitz.56
A subsequent study by Bar-On et al. (2022) on the second booster,47 for example, reported results that, upon close inspection, suggest only marginal benefit. Setting aside multiple methodological and representational concerns (to be addressed in Step 4), an analysis of the conditional risk among infected individuals between weeks 2 and 6 post-vaccination showed that 0.927% in the vaccinated group developed severe illness, compared to 1.082% in the internal control group. This modest effect (risk ratio ~0.86) falls far short of the strong, independent protection suggested by the study’s broader conclusions. Taken together, this recurring trend challenges the claim that the vaccines provide a distinct, additive protection against severe illness beyond their short-term effect on infection rates.
3.3 Dashboard Data and the Pitfall of Uncontrolled Comparisons
In the absence of empirical evidence from scientific studies, some may turn to public health dashboards in search of compelling evidence of vaccine effectiveness. After all, throughout the pandemic, these dashboards often displayed higher mortality rates among unvaccinated individuals compared to their vaccinated counterparts. While such dashboards cannot be granted scientific credibility, it is nonetheless important to explicitly outline their major limitations. Public dashboards are highly susceptible to a range of well-documented biases and methodological flaws, including:
- • Differential testing policies, whereby unvaccinated individuals were subject to significantly more frequent testing, often mandated by vaccine passport regulations (e.g., 57,58).
- • Misclassification of non-COVID-related illness as COVID-19, particularly in unvaccinated patients who tested positive as a procedural requirement.
- • Misattribution of vaccination status during the early post-vaccination window, a period in which infection risk may actually increase—a pattern documented by Koren et al.32
- • The healthy vaccinee bias mentioned briefly above, especially relevant in older age groups most vulnerable to severe COVID-19 outcomes (see next).
The Healthy Vaccinee Bias
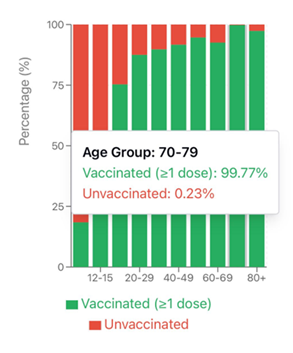
This last bias is especially relevant when interpreting dashboard data (and observational studies). Unlike RCTs, which are specifically designed to eliminate such confounding, observational comparisons remain highly vulnerable. Consider, for example, the Israeli Ministry of Health dashboard: according to its data, nearly all individuals aged 70 and older were vaccinated (see Figure 2). Those few who remained unvaccinated were unlikely to be ideological opponents of vaccination. Rather, they typically belonged to the most vulnerable segments of the population—frail, homebound, seriously ill, or medically ineligible. These are precisely the individuals most likely to experience poor outcomes if infected, regardless of their vaccination status.
Note: This figure was created based on data published on the Israeli Ministry of Health’s public dashboard. The original screenshots (in Hebrew) and the Excel file containing the specific column on unvaccinated population stratified by age were downloaded on January 27, 2023, and are available upon request from the first author.
This real-world distribution of vaccine uptake is a textbook example of the healthy vaccinee bias—the well-documented phenomenon in which healthier individuals are more likely to receive vaccines.59,60 Its presence has been documented in a recent national cohort study from Qatar, which found that vaccinated individuals exhibited significantly lower mortality not only from COVID-19, but also from non-COVID causes.61 Such patterns are implausible unless meaningful baseline health differences existed between vaccinated and unvaccinated groups. In this light, dashboard-based comparisons—especially among older adults—are inherently confounded and cannot be relied upon to infer vaccine effectiveness.
The Sociodemographic Bias
A closely related limitation of public dashboards is their failure to adjust for sociodemographic disparities between vaccinated and unvaccinated groups. For instance, in the large Israeli study on the second booster by Bar-On et al. (discussed earlier), unvaccinated individuals were more likely to belong to minority populations or socioeconomically disadvantaged communities with reduced access to healthcare.47 These underlying disparities are well documented in the literature, yet remain entirely unaddressed in dashboard based comparisons, further undermining their validity as indicators of vaccine effectiveness. Overall, the small and exceptional subgroup of elderly individuals who remained unvaccinated (Figure 2), many of whom were poor, frail, or terminally ill, was often treated as a valid control group in dashboard-based summaries. This misleading framing may have played a key role in shaping the revised narrative that vaccines reliably protected against severe illness and death. Additional discussion of mechanisms that enabled the maintenance of this narrative is provided next (Step 4).
Step 4
What Mechanisms Enabled the Creation and Endurance of the ‘Millions Saved’ Narrative?
Given the lack of solid empirical support for the ‘millions saved’ narrative (Steps 1–3), a crucial question emerges: what enabled the creation and persistence of this unfounded and likely highly exaggerated claim? How were weak and largely irrelevant findings presented as ‘evidence’ of protection against severe illness and death? And how has such a substantial gap between the dominant narrative and the actual data remained invisible to the public, even to this day?. While a comprehensive scientific, psychological, and socioeconomic analysis lies beyond the scope of this paper, we highlight four immediate and concrete factors that, in our view, directly contributed to the emergence of this narrative: flawed research methodologies (Section 4.1), misrepresentation of results in scientific communication (Section 4.2), misinterpretation of findings by public health authorities (Section 4.3), and systematic suppression of dissenting voices (Section 4.4).
4.1 Flawed Research Methodologies
A first explanation for the creation of the narrative concerns the research methodologies that were implemented to generate the promising outcomes of the vaccines. Consider, for example, the foundational RCT that led to the emergency use authorization (EUA) of the Pfizer vaccine.21 Beyond its inability to produce meaningful results regarding efficacy against severe outcomes (Section 3.1), this pivotal RCT also showed signs of methodological irregularities. According to Pfizer’s supplementary technical document submitted to the FDA, 311 participants (1.4%) were excluded from the vaccine group due to “important protocol deviations on or prior to 7 days after Dose 2,” compared to only 60 participants (0.3%) excluded from the placebo group under the same criteria.38 This unexplained and disproportionate exclusion rate raises serious concerns about the trial’s internal consistency and the fairness of its inclusion criteria.
Importantly, by excluding participants after randomization, the trial deviated from the standard intent-to-treat principle, which requires that all randomized participants be included in the final analysis regardless of protocol adherence or outcomes. This principle is fundamental to preserving the validity and generalizability of RCT findings, and its violation may have biased the efficacy estimates in favor of the vaccine. In addition, there is considerable concern that the blinding protocol in this RCT may have been compromised. As Peter Doshi observed, the overlap between common vaccine side effects (such as fever, fatigue, and headache) and COVID-19 symptoms likely enabled both participants and investigators to infer group allocation.39 Participants who suspected they were in the vaccine group (perhaps due to initial adverse effects such as pain and fatigue) may have been less inclined to seek COVID-19 testing, owing to a perceived sense of protection. Similarly, investigators may have exhibited comparable bias when deciding whether to initiate testing. This concern is further exacerbated by the trial’s explicit protocol instructions, which directed investigators to rely on their clinical judgment when determining whether to test symptomatic participants during the first week following vaccination. In practice, this placed investigators in the position of having to decide whether early symptoms were vaccine-related or indicative of SARS-CoV-2 infection. As Doshi noted, “this amounts to asking investigators to make guesses as to which intervention group patients were in.”39 This compromise of proper blinding, combined with the unequal exclusion of participants mentioned above, may have skewed testing behaviors between groups—potentially producing an inflated appearance of vaccine efficacy by overstating COVID-19 rates in the placebo arm.
4.2 Misrepresentation of Results in Scientific Communication
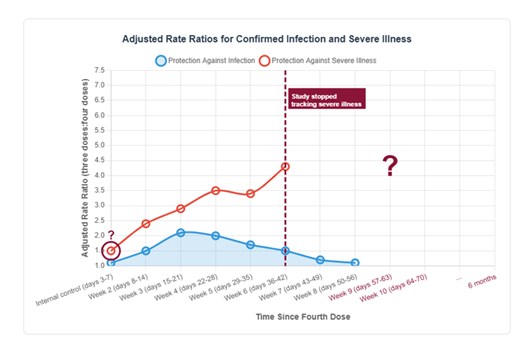
A second explanation for the creation of the ‘millions saved’ narrative concerns the way in which research results were interpreted and presented by scientists. A prominent example is the influential study by Bar-On et al. (2022), discussed above (Section 3.2), which claimed that vaccine efficacy (VE) against severe illness remained high at week 6, even though protection against infection had already declined by that point.47 Earlier, we outlined several major limitations of this study, including its very short follow-up period, failure to account for the healthy vaccinee bias, and the omission of the more accurate outcome measure of conditional probability. Here, we wish to highlight a particularly sophisticated representational choice that, in our view, created a misleading impression of the vaccine’s efficacy. As mentioned above, the study’s follow-up period for severe illness lasted only six weeks—two weeks shorter than the eight-week monitoring period for vaccine effectiveness against infection (and far shorter than the widely publicized claim of six months of protection). As illustrated in the schematic replication below (Figure 3), this narrow focus on week 6 creates something of an illusion: while effectiveness against infection is clearly shown to wane, effectiveness against severe illness appears to remain high. But what happens after week 6 is a mystery. Why did the researchers choose to stop monitoring at precisely that point? Is it not reasonable to expect a similar decline in protection against severe illness and death—albeit with a delay of approximately two weeks, which is the average interval between infectio35and death?35
Another notable red flag in the study by Bar-On et al. (2022)47 concerns the overlooked implications of the anomalous efficacy result observed in the internal control group monitored during days 3 to 7 post-vaccination (adjusted rate ratio = 1.5; see Figure 3). This apparent protective effect is biologically implausible, as the vaccine would not yet have had sufficient time to elicit a meaningful immune response—precisely the rationale for selecting this period as a control. Such an unexpected finding should have raised immediate concern that at least part of the reported vaccine effectiveness against severe illness (during the narrow follow-up window at week 6) may in fact be attributable to biases such as the counting window issues and the healthy vaccinee bias discussed earlier (Section 3.3). In sum, the study’s conclusion of durable protection was not grounded in solid long-term data, but rather rested on a truncated follow-up period, questionable assumptions about control groups, and a representational framing that obscured key limitations. The central clinical question—whether protection against severe illness persists once the vaccine no longer prevents infection—was never adequately addressed.
Note: This figure is a schematic replication of the data presented in Bar-On et al. (2022), illustrating the adjusted rate ratios for confirmed infection (blue line) and severe illness (red line) following the fourth vaccine dose. The dashed line marks the point at which data collection on severe illness was stopped. The red circle highlights the biologically implausible effect observed during the internal control period (days 3–7), and the question mark indicates the absence of follow-up data on severe illness beyond Week 6, when VE against infection was continuing to decline.
4.3 Misinterpretation of Findings by Public Health Officials
A third explanation for the endurance of the narrative concerns the gap between scientific findings and the public health decisions made on their basis. Consider, for example, the FDA’s authorization of the second booster dose for older adults and immunocompromised individuals on March 29, 2022. In its official news release, the FDA confidently asserted that “emerging evidence suggests that a second booster dose… improves protection against severe COVID-19”.49 Yet the only scientific source cited to support this claim was an Israeli study conducted at Sheba Medical Center—a study that did not support the claim.62 Published just two weeks earlier in the New England Journal of Medicine, this small observational study did not examine severe illness, nor did it include older or immunocompromised populations. Instead, it focused on healthy healthcare workers and concluded that the fourth dose “may have only marginal benefits”.62 In other words, the FDA’s unequivocal public claim was backed by a study that neither investigated nor demonstrated what the FDA asserted it had.
This peculiar misinterpretation of a relatively straightforward scientific outcome may have stemmed from a natural human tendency to interpret ambiguous data in ways that validate one’s prior beliefs and decisions. Many of the experts and public health officials who advocated for the vaccines had received them themselves—often publicly, and with considerable personal and professional investment. When they later contracted COVID-19, sometimes with severe symptoms, they likely experienced a form of cognitive dissonance. To preserve internal coherence, some may have experienced an unconscious psychological pressure to ‘update’ their original narrative (Step 2) and embrace a firm, emotionally charged belief in the vaccines’ ability to protect against severe illness and death, despite the absence of convincing empirical evidence to support it (Step 3).
Another example of misinterpretation by public health authorities is the overreliance on uncontrolled, and at times distorted, data from national COVID-19 dashboards. As mentioned in Section 3.3, dashboard data are highly vulnerable to testing disparities, as unvaccinated individuals were often tested at significantly higher rates than vaccinated individuals, due to policy-driven restrictions. To overcome this inherent bias, Koren, Altuvia, and Levi (2021) analyzed dashboard data on incoming passengers at Israel’s international airport during August–October 2021.32 In this unique context, where all travelers—vaccinated and unvaccinated—were required to undergo COVID-19 testing upon entry, the dashboard data indicated a significantly lower vaccine efficacy against infection than the official rates publicly declared by the Israeli Ministry of Health. Regrettably, however, the second author of this article observed that within just 24 hours of these findings being published, the relevant dashboard data were retroactively modified—showing fewer positive cases among vaccinated travelers and more among the unvaccinated.63 Shortly thereafter, Israeli social activist Yariv Hammer reported that the Ministry of Health had removed the entire section of the dashboard that presented this unique dataset from the country’s main entry portal.64 These actions lead us directly to our final explanation, which moves beyond scientific misinterpretations and biases into the realm of more deliberate forms of misconduct.
4.4 Systematic Suppression of Critical Voices
Our fourth and final explanation for the endurance of the ‘millions saved’ narrative concerns the widespread suppression of dissenting voices during the COVID-19 pandemic. We encountered this suppression directly when attempting to publish the comprehensive review that forms the foundation of the current article. In 2022, we submitted that review to approximately ten leading medical journals, including The New England Journal of Medicine (NEJM), which played a central role in shaping COVID-19 discourse and is cited multiple times in this article. In every instance, our manuscript was rejected within days, without undergoing peer review and without substantive explanation. Of course, desk rejections are not in themselves proof of systematic censorship. We are fully aware of the competitive nature of scientific publishing. However, in this case, we made significant efforts to engage the editors, if only to receive a more reasoned decision. Concerning NEJM, we even submitted a formal appeal, emphasizing the urgency and scientific merit of the work. We noted that this was our fourth attempt to engage with the NEJM on this topic; our three prior letters, each offering critical commentary on NEJM-published studies, had likewise been desk-rejected. In our appeal, we warned that the continued suppression of dissenting scientific voices risks undermining public trust in science itself. This appeal was also rejected, again without any substantive explanation.
Another illustrative case is that of the fourth author (Lataster), who argued—based on emerging evidence of myocarditis and UK government data on the number needed to vaccinate—that the risks of vaccination may outweigh the benefits for young, healthy individuals. Despite the rigor of his analysis, he was initially unable to publish it in a high-profile journal, eventually settling for a rapid response in The BMJ.65 Notably, the very concerns he raised are now reflected in evolving public health guidance, as seen in the recent recommendation revision by the Australian Government’s Department of Health.2
These personal accounts are only two examples of a much broader phenomenon.66 Throughout the pandemic, those who dared to question the efficacy or safety of the vaccines were often met with ridicule and scorn. There is also evidence that government agencies and public institutions acted non-transparently, withholding troubling safety information presented to them,67,68 and even pressuring social media platforms to censor expert opinions that contradicted the official public health narrative.69
As U.S. federal judge Terry Doughty wrote in his ruling on Missouri v. Biden:
“During the COVID-19 pandemic, a period perhaps best characterized by widespread doubt and uncertainty, the United States Government seems to have assumed a role similar to an Orwellian ‘Ministry of Truth.’”70
An explicit acknowledgment of this censorship appeared in a September 2024 letter sent by Meta CEO Mark Zuckerberg to the U.S. House Judiciary Committee. In the letter, Zuckerberg admitted that the Biden administration pressured Meta teams to suppress certain content related to the pandemic, including humor and satire. He expressed regret, noting that, in hindsight, the moderation decisions made in 2021 would not have been made under current standards.71 Returning to Australia, a recent Freedom of Information (FOI) request involving the fourth author revealed inconsistencies and a lack of transparency in the reporting of severe COVID-19 outcomes. In data from New South Wales Health on cases, hospitalizations, and deaths by vaccination status, ambiguous terms such as “no effective dose” were used. According to their definition, this included individuals who had either received no vaccine or had received the first dose of a two-dose regimen less than 21 days prior to known exposure—highlighting concerns related to the previously discussed counting window issues (Section 3.1). In their response to the FOI request (dated 17 September 2024, file ref GIPA24/161, doc ref G24/4092), NSW Health acknowledged that this classification was used until February 2022, after which clearer definitions were adopted (e.g., “0 doses” meaning no doses, regardless of timing). However, they declined to release the underlying data needed to verify how these revised terms were applied. This opacity raises legitimate concerns about data reliability. Notably, despite the involvement of a journalist in submitting the request, the issue received no coverage from major news outlets. This censorship was not incidental; it was actively enforced throughout the pandemic. Experts who questioned the dominant narrative were mocked or silenced; physicians who broke ranks were threatened, stripped of their medical licenses, or publicly discredited; and prominent scientists were removed from editorial boards or faced suppression within their own academic communites.66 Such powerful censorship, together with the methodological flaws and representational distortions detailed above, helps explain how a dominant public health narrative lacking solid empirical foundation was able to take hold and endure.
Conclusion: Unpacking the Final COVID Myth
Two years after the official end of the COVID-19 pandemic, it is time to critically reexamine one of its most enduring and widely accepted claims: that “COVID-19 vaccines have saved millions and millions of lives.”1 In this article, we subjected that core narrative to a detailed, step-by-step investigation, building upon our prior, more comprehensive and technically rigorous review of the evidence (Ophir et al., 2023).14 Step 1 analyzed the mathematical models underlying the ‘millions saved’ figure, including the one cited in the U.S. Senate hearing. Step 2 revisited the collapse of the initial narrative regarding vaccine efficacy against infection and transmission, which served as the cornerstone of the mass vaccination campaign and the unprecedented vaccine mandates. Step 3 critically examined the revised justification that followed: the claim that vaccines continued to protect against severe illness and death despite failing to prevent infection. This included a close analysis of the pivotal Randomized Controlled Trial (RCT) that led to the Pfizer vaccine’s Emergency Use Authorization (3.1); the large observational studies that supported continued global rollout (3.2); and the real-world dashboard data used to promote uptake (3.3). Taken together, this comprehensive review leads to a clear conclusion: the available scientific data do not support the claim that the vaccines provided sustained protection against severe illness and death. In other words, to date, there is no empirical foundation for the assertion that “COVID-19 vaccines saved millions and millions of lives.” The declaration made at the opening of the Senate hearing—that “there is no scientific question about that fact”—is simply unfounded. Because meaningful scientific debate depends on careful scrutiny of evidence, we strongly urge readers not to rely solely on this concluding chapter, but to engage directly with the full analysis presented in this paper. Below, we briefly highlight only a few key findings that support the central conclusion:
- • The widely cited claim that ‘millions of lives were saved’ by COVID-19 vaccines is based on hypothetical models that rest on a long sequence of assumptions—many of which are either weak, unvalidated, or demonstrably false. As a result, the outputs of these models are of questionable value and cannot be taken as reliable evidence.
- • A central assumption underlying these models was that COVID-19 vaccines provided strong and durable protection against infection and transmission (i.e., the original and primary justification for the mass vaccination campaign, and vaccine mandates). This assumption was later found to be false, as real-world data revealed that such protection was fragile and short-lived.
- • Despite the collapse of the primary rationale for vaccination, the campaign persisted under a revised claim: that the vaccines continued to offer lasting protection against severe illness and death, even after their short-term effect against infection had waned. This revised claim—premised on a conceptual separation between the two types of efficacy—was never empirically validated, as demonstrated repeatedly throughout this article.
- • In fact, the available data suggest that these two forms of protection are closely linked and follow a similar waning trajectory—albeit with a delay between infection and the onset of severe illness or death.
- • To directly assess the validity of this alleged distinction, we calculated the conditional probability of severe illness in key studies. The results indicated that protection against severe illness was largely a byproduct of the short-lived protection against infection. Crucially, these studies never demonstrated independent or durable protection against severe illness or death.
- • Notably, some studies ceased tracking severe outcomes precisely at the point when such protection would be expected to wane—mirroring the known decline in infection protection and the typical lag between infection and the onset of severe illness or mortality. This timing raises serious concerns regarding misrepresentations of research results.
- • Finally, the pivotal RCT that justified Pfizer’s Emergency Use Authorization (EUA) showed no statistically meaningful difference between vaccine and placebo groups in preventing (1) flu-like symptoms, (2) severe COVID-19, or (3) all-cause mortality. Given the large sample size, the absence of an effect on all-cause mortality should serve as a fundamental reference point for any serious scientific discussion about vaccine impact.
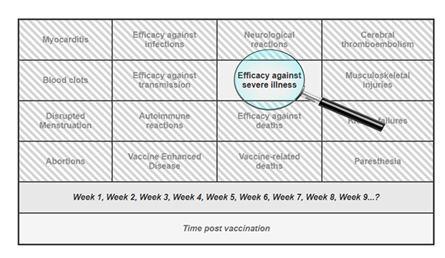
Beyond these specific findings, our investigation also revealed a broader set of methodological issues that further undermine the reliability of the evidence base. These include: (a) follow-up periods that were both too short and inconsistent; (b) implausible efficacy signals emerging immediately after vaccination, before full immunization would have been biologically plausible; and (c) heavy reliance on observational data susceptible to healthy vaccinee bias, differential testing, and multiple other confounders. The central question is not whether some degree of vaccine efficacy was observed at specific moments (e.g., Week 6 in Bar-On et al., 2022), but rather how such fleeting observations came to dominate the broader public narrative. Isolated data points were elevated and decontextualized, while critical considerations—such as (a) waning immunity, (b) the lack of demonstrated mortality benefit, (c) vaccine breakthrough infections leading to hospitalization or death, and (d) an increasingly robust body of evidence on adverse effects—were systematically sidelined (see Figure 4).
This narrowing of focus—peering through the keyhole of one transient success—has allowed a fragile claim to solidify into a powerful myth, reinforced by institutional authority, social conformity, and the systematic suppression of dissenting voices. We therefore urge the scientific and medical communities to take a step back, widen the lens, and return to the foundational principle of medicine that opened the current article: every intervention, however promising, must be evaluated through careful, ongoing assessment of its evidence-based benefits and potential harms over time. To the best of our knowledge, such a balanced and rigorous appraisal has yet to be applied to the COVID-19 vaccines.
Based on the evidence reviewed in this article, we find no solid empirical foundation for the claim that “COVID-19 vaccines saved millions and millions of lives.”1 While these vaccines were widely promoted as safe and effective, accumulating reports of serious adverse events, such as myocarditis, pericarditis, thrombosis, and neurological symptoms, have been documented across multiple studies and pharmacovigilance systems. Moreover, this biologically active intervention was administered repeatedly in the form of boosters, often to healthy individuals with near-zero risk of COVID-related mortality. Taken together with the lack of demonstrable long-term efficacy presented in this article, the available evidence suggests that the risk–benefit balance of the COVID-19 vaccines is, in fact, tilted toward the negative end of this fundamental medical equation.72,73
References
- Homeland Security. The corruption of science and federal health agencies: How health officials downplayed and hid myocarditis and other adverse events associated with the COVID-19 vaccines. Committee on Homeland Security & Governmental Affairs. 2025.
- Australian Government Department of Health and Aged Care. Healthy infants, children and adolescents aged under 18 years are not recommended to receive COVID-19 vaccine. 2025.
- Zakov S. Pfizer's covid-19 vaccine clinical trial review. 2022.
- Rose J. A report on the US vaccine adverse events reporting system (VAERS) of the COVID-1 9 messenger ribonucleic acid (mRNA) biologicals. Science, Public Health Policy, and The Law. 2021;2:59–80.
- Fraiman J, Erviti J, Jones M, et al. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine. 2022;40(40):5798–5805.
- Shir-Raz Y. Breaking: Leaked video reveals serious side-effects related to the pfizer COVID-19 vaccine covered up by the israeli MOH. Real Time Magazine. 2022.
- Witberg G, Barda N, Hoss S, et al. Myocarditis after covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139.
- Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in hong kong adolescents following comirnaty vaccination. Clinical Infectious Diseases. 2021: ciab989.
- Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from december 2020 to august 2021. 2022;327(4):331–340.
- McCullough PA, Hulscher N. Risk stratification for future cardiac arrest after COVID-19 vaccination. World J Cardiol. 2025;17(2):103909.
- Hulscher N, Hodkinson R, Makis W, McCullough PA. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Failure. 2024.
- Takada K, Taguchi K, Samura M, et al. SARS-CoV-2 mRNA vaccine-related myocarditis and pericarditis: An analysis of the japanese adverse drug event report database. Journal of Infection and Chemotherapy. 2025;31(1):102485.
- James A Thorp MD, Claire Rogers M, Kirstin Cosgrove C, et al. Association between COVID-19 vaccination and neuropsychiatric conditions. International Journal of Innovative Research in Medical Science. 2025;10(06):241–248.
- Ophir Y, Shir-Raz Y, Zakov S, McCullough PA. The efficacy of COVID-19 vaccine boosters against severe illness and deaths: Scientific fact or wishful myth? Journal of American Physicians and Surgeons. 2023;28(1).
- Fitzpatrick MC, Moghadas SM, Pandey A, Galvani AP. Two years of U.S. COVID-19 vaccines have prevented millions of hospitalizations and deaths.
- Gupta S, Cantor J, Simon KI, Bento AI, Wing C, Whaley CM. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Affairs. 2021;40(9).
- Agrawal V, Sood N, Whaley C. The impact of the global covid-19 vaccination campaign on all-cause mortality. National Bureau of Economic Research. 2023.
- Meslé MMI, Brown J, Mook P, et al. Estimated number of lives directly saved by COVID-19 vaccination programmes in the WHO european region from december, 2020, to march, 2023: A retrospective surveillance study. The Lancet Respiratory Medicine. 2024;12(9):714–727.
- Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. The Lancet Infectious Diseases. 2022;22(9):1293–1302.
- Lataster R. Metacritique of influential studies purporting COVID-19 vaccine successes: Part 1 - watson et al. Journal of Independent Medicine. 2025;1(2):143–152.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. New England Journal of Medicine. 2020;383(27):2603–2615.
- CBS NEWS. Transcript: Dr. anthony fauci on "face the nation," may 16, 2021. CBS NEWS. 2021.
- World Economic Forum. Davos annual meeting 2022 - conversation with albert bourla, CEO of pfizer - original. 2022.
- COVID-19 vaccine breakthrough infections reported to CDC—United states, january 1–April 30, 2021. morbidity and mortality weekly report. Centers for Disease Control and Prevention (CDC), The Vaccine Breakthrough Case Investigations Team. 2021;70:792.
- Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in qatar. medRxiv. 2021:2021.08.25.21262584.
- Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in qatar. N Engl J Med. 2021;385(24):e83.
- Morens DM, Taubenberger JK, Fauci AS. Universal coronavirus vaccines — an urgent need. N Engl J Med. 2022;386(4):297–299.
- Finance Yahoo. Pfizer CEO: New COVID-19 vaccine that covers omicron ‘will be ready in march'.
- United States House Select Subcommittee on the Coronavirus Pandemic. After action review of the COVID-19 pandemic: The lessons learned and a path forward. committee on oversight and accountability. 2024.
- Lataster R. Should we now discuss possible COVID-19 vaccine negative effectiveness? Australian Journal of General Practice. 2024;53(7).
- Kerr S, Bedston S, Bradley DT, et al. Waning of first- and second-dose ChAdOx1 and BNT162b2 COVID-19 vaccinations: A pooled target trial study of 12.9 million individuals in england, northern ireland, scotland and wales. International Journal of Epidemiology. 2023;52(1):22–31.
- Koren O, Levi R, Altuvia S. Green pass and COVID-19 vaccine booster shots in israel – A more ‘realistic’ empirical assessment analyzing the national airport data. 2021.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416.
- Doshi P. Covid-19 vaccine trial protocols released. BMJ. 2020;371:m4058.
- Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in wuhan, china. J Med Virol. 2020;92(4):441–447.
- FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. 2020.
- Doshi P. Does the FDA think these data justify the first full approval of a covid-19 vaccine? 2021.
- Pfizer-BioNTech. Vaccines and related biological products advisory committee december 10, 2020 meeting briefing document- FDA. 2020.
- Doshi P. Pfizer and moderna’s “95% effective” vaccines—let’s be cautious and first see the full data. 2020.
- Doshi P. Pfizer and moderna’s “95% effective” vaccines—we need more details and the raw data. 2021.
- Fung K, Jones M, Doshi P. Sources of bias in observational studies of covid-19 vaccine effectiveness. J Eval Clin Pract. 2024;30(1):30–36.
- Lataster R. Reply to fung et al. on COVID-19 vaccine case-counting window biases overstating vaccine effectiveness. J Eval Clin Pract. 2024;30(1).
- Doshi P, Fung K. How the case counting window affected vaccine efficacy calculations in randomized trials of COVID-19 vaccines. J Eval Clin Pract. 2024;30(1):105–106.
- Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773.
- Benn CS, Schaltz-Buchholzer F, Nielsen S, Netea MG, Aaby P. Randomised clinical trials of COVID-19 vaccines: Do adenovirus-vector vaccines have beneficial non-specific effects? Iscience. 2023;26(5).
- Canadian Covid Care Alliance. The pfizer inoculations for COVID-19 – more harm than good. 2021.
- Bar-On Y, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in israel. N Engl J Med. 2022.
- Birnhack M. Who controls covid-related medical data? copyright and personal data. IIC - International Review of Intellectual Property and Competition Law. 2021;52(7):821–824.
- Coronavirus (COVID-19) update: FDA authorizes second booster dose of two COVID-19 vaccines for older and immunocompromised individuals. U.S. Food and Drug Administration, News Release. 2022.
- Vaccines and related biological products advisory committee briefing document. U.S. Food and Drug Administration (FDA). 2021.
- Arbel R, Sergienko R, Friger M, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. 2022;28(7):1486–1490.
- Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2022.
- Bar-On Y, Goldberg Y, Mandel M, et al. BNT162b2 vaccine booster dose protection: A nationwide study from israel. medRxiv. 2021:2021.08.27.21262679.
- Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423.
- Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. The Lancet. 2021.
- Wohl A, Leibowitz R. Protection by a fourth dose of BNT162b2 against omicron in israel. N Engl J Med. 2022.
- What covid tests do I still need to travel abroad? The British Broadcasting Corporation (BBC). 2022.
- England NHS. Testing for elective care pre-admission patient pathways. 2022.
- Fürst T, Bazalová A, Fryčák T, Janošek J. Does the healthy vaccinee bias rule them all? association of COVID-19 vaccination status and all-cause mortality from an analysis of data from 2.2 million individual health records. International Journal of Infectious Diseases. 2024;142:106976.
- Furst T, Straka R, Janosek J. Healthy vaccinee effect: A bias not to be forgotten in observational studies on COVID-19 vaccine effectiveness. Polish Archives of Internal Medicine. 2024;134(2):16634.
- Chemaitelly H, Ayoub HH, Coyle P, et al. Assessing healthy vaccinee effect in COVID-19 vaccine effectiveness studies: A national cohort study in qatar. eLife. 2025;14:e103690.
- Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of covid-19 mRNA vaccine against omicron. N Engl J Med. 2022.
- Shir-Raz Y. Has the ministry of health rewritten the history of the dashboard data? (in hebrew). 2021.
- Hammer Y. Prof. retsef levi with airport data analysis: The booster dose is not effective as we are told (in hebrew). Real Time Magazine. 2021.
- Lataster R. Risks outweigh the benefits? myocarditis risk alone appears to exceed the COVID-19 vaccines’ benefits. rapid response to: Risk of myocarditis and pericarditis in mRNA COVID-19-vaccinated and unvaccinated populations: A systematic review and meta-analysis. BMJ Open. 2023;13(6):e065687.
- Shir-Raz Y, Elisha E, Martin B, Ronel N, Guetzkow J. Censorship and suppression of covid-19 heterodoxy: Tactics and counter-tactics. Minerva. 2022;61:407–433.
- Demasi M. FDA urged to publish follow-up studies on covid-19 vaccine safety signals. BMJ. 2022;379:o2527.
- Shir-Raz Y. Adverse effects of the pfizer vaccine covered up by the israeli ministry of health. BROWNSTONE INSTITUTE. 2022.
- Blitzer R. Twitter files expose government influence on suppressing COVID messages that contradicted WH. FOX Business. 2022.
- State of Missouri et al. v. Joseph R. Biden Jr. et al. Case no. 3:22-CV-01213 (W.D. la. filed july 4, 2023) (memorandum ruling on request for preliminary injunction).
- Mark zuckerberg says meta was ‘pressured’ by biden administration to censor covid-related content in 2021. Cable News Network (CNN). 2024.
- Mead MN, Seneff S, Wolfinger R, et al. COVID-19 modified mRNA “vaccines”: Lessons learned from clinical trials, mass vaccination, and the bio-pharmaceutical complex, part 1. International Journal of Vaccine Theory, Practice, and Research. 2024;3(2):1112–1178.
- Mead MN, Seneff S, Rose J, Wolfinger R, Hulscher N, McCullough PA. COVID-19 modified mRNA “vaccines”: Lessons learned from clinical trials, mass vaccination, and the bio-pharmaceutical complex, part 2. International Journal of Vaccine Theory, Practice, and Research. 2024;3(2):1275–1344.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
