An Appraisal of Behavioural and Non-Behavioural Factors Influencing the Male Infertility: Narrative Approach
Sudarshan Nagorao kale*, 1, Jibanananda Mishra2
1Scholar at Lovely Professional University, Phagwara, Punjab, India
2Chief Scientist and COO l AAL Biosciences Research Pvt. Ltd
*Corresponding author: Sudarshan Nagorao kale, Scholar at Lovely Professional University, Phagwara, Punjab, India.
Received: 19 April 2023; Accepted: 27 April 2023; Published: 06 June 2023
Article Information
Citation: Sudarshan Nagorao kale, Jibanananda Mishra. An Appraisal of Behavioural and Non- Behavioural Factors Influencing the Male Infertility: Narrative Approach. International Journal of Applied Biology and Pharmaceutical Technology. 14 (2023): 53-58.
View / Download Pdf Share at FacebookAbstract
Male infertility implies a man can't begin a pregnancy with his female accomplice. Male infertility can have many causes. You may not make sufficient sperm or sound sperm. You might have a hereditary issue like cystic fibrosis. You might have a blockage in your genital plot. According to the latest WHO statistics, approximately 50– 80 million people worldwide suffer from infertility, and male factors are responsible for approximately 20– 30% of all infertility cases. The diagnosis of infertility in men is mainly based on semen analysis. the incidence of male infertility has increased worldwide. Infertility is characterized as the failure of couples to have a child following one year of customary unprotected intercourse, influencing 10 to 15% of couples. It is necessary to study the factors that influence male infertility in each area/region for better management. This paper represents various factors based on behavioral and non-behavioral conditions prompting for male infertility augmentation rapidly.
Keywords
<p>male infertility, behavioral and non-behavioral, systematic literature review SLR, spss analysis, etc</p>
Article Details
1. Introduction
is characterized as the failure of couples to have a child following one year of normal unprotected intercourse, influencing 10-15 percent of couples.1-4 As per the most recent WHO measurements, around 50-80 million individuals overall experience the ill effects of infertility.5,6 Huge scope studies have shown that about portion of all instances of barrenness happen because of female elements, 20 to 30 percent male variables, and 20 to 30 percent because of normal reasons for both gender.6-8 Late meta-examination concentrates by scientists show that male's variables are available in 20-70 percent of barrenness cases.7,9 These discoveries are fundamentally more extensive than recently revealed. Nonetheless, the extensive variety of male infertility in meta-examination studies may not mirror the pervasiveness of this complexity in all regions of the planet on account of reasons, for example, the absence of thorough measurable techniques that incorporate predisposition, heterogeneity in information assortment, and social imperatives. Given the huge commitment of male variables to
infertility in couples, as well as elevated degrees of obscure elements in male barrenness, an absence of comprehension of the basic components is by all accounts perhaps of the main test dealing with this issue. In this article, we have assessed the histological investigations of testicular tissue examples, male conceptive design, factors affecting male barrenness, procedures to find qualities engaged with infertility, accessible remedial strategies for male barrenness, sperm recuperation techniques in fruitless men, and helping regenerative strategy.
Infertility is a condition with mental, monetary, clinical ramifications bringing about injury, stress, especially in a social set-up like our own, with a solid accentuation on youngster bearing. As per the Global Board for Checking Helped Conceptive Innovation, World Wellbeing Association (WHO), infertility is an illness of regenerative framework characterized by inability to accomplish the clinical pregnancy following a year or a greater amount of standard unprotected sexual intercourse.[1] It can likewise be characterized as disappointment of couple to consider following a year of customary intercourse without the utilization of contraception in ladies <35 years; and following a half year of normal intercourse without the utilization of contraception in ladies ≥35 years.
According to the WHO, the general pervasiveness of essential barrenness ranges somewhere in the range of 3.9% and 16.8%. [5] Likewise, the evaluations of infertility shift broadly among Indian states from 3.7% in Uttar Pradesh, Himachal Pradesh, and Maharashtra, [13] to 5% in Andhra Pradesh, [14] and 15% in Kashmir. [15] In addition, the predominance of essential infertility has likewise been displayed to change across the clans and positions inside a similar district in India. [13,16]
It was accounted for that 40% of infertility cases were connected with men, 40% of ladies and 20% of both sexes.[17] As per a metacentric review led by WHO from 1982 to 1985, 20% of cases were credited to male elements, 38% to female variables, 27% had causal variables distinguished in the two accomplices, and 15% couldn't be sufficiently ascribed to either partner.[18] In Indian couples looking for treatment, the male component is the reason in roughly 23%.[15] A new report on the situation with barrenness in India, expresses that almost half of barrenness is connected with the conceptive peculiarities or problems in the male.[19] likewise, more than 25% of infertility cases, no recognizable reason can be followed after routine tests, which leaves the case as unexplained barrenness.
1.2 Male Infertility: An Impoertant Factors
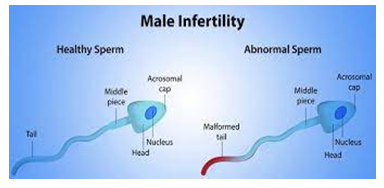
Signs and symptoms you may notice include:Problems with sexual function— for example, difficulty with ejaculation or small volumes of fluid ejaculated, reduced sexual desire, or difficulty maintaining an erection (erectile dysfunction) Pain, swelling or a lump in the testicle area. Recurrent respiratory infections. Infertility affects one in every six couples who are trying to conceive. In at least half of all cases of infertility, a male factor is a major or contributing cause. This means thatabout 10% of all males in the United States who are attempting to conceive suffer from infertility.
The various cultural factors which impact on fertility arethe marriage system, the family system, religious systems, regional subculture, norms concerning desired family size, and other fertility norms such as astrology and breastfeeding. Infertility has significant negative social impacts on the lives of infertile couples and particularly women, who frequently experienceviolence, divorce, social stigma, emotional stress, depression, anxiety and low self-esteem.
2. Literature Review, SLR
Naina KumarandAmit Kant Singh [1] The present study is only a review of various studies conducted all over the world. The exact rates of male infertility from developing countries like ours are difficult to find because of the problem with the definition of male infertility and lack of accurate reporting rather than a true reflection of male infertility. But still in future, we can conduct various research studies to find out the major causes of male infertility and can work in that direction to reduce such factors which can affect the future fertility of males.
Ashok Agarwal [2] This study demonstrates a novel and unique way to calculate the distribution of male infertility around the world. According to our results, at least 30 million men worldwide are infertile with the highest rates in Africa and Eastern Europe. Results indicate further research is needed regarding etiology and treatment, reduce stigma & cultural barriers, and establish a more precise calculation.
Emad Babakhanzadeh [3] male infertility in many cases remains unknown. Therefore, it is necessary to introduce new key factors and diagnostic and noninvasive biomarkers. Over the past few years, the identification and evaluation of small noncoding RNAs in many diseases, including infertility, has helped greatly in understanding the underlying mechanisms of disease. But this alone is not enough, and through increased insight into the complex stages and processes of pregnancy in humans, more key elements must be identified so that infertile couples can enjoy the chance of a natural pregnancy in addition to reducing costs and problems. With the advances in technology and the introduction of new methods and approaches, it is hoped that many of the causes of male infertility will soon be identified and treated.
Christopher L R Barratt [4] the analysis of the literature was primarily provided by WHO Human Reproduction Program (WHO Reproductive Health and Research Department, HRP (the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction)).
Victor Ohenhen[5] The results indicate limited knowledge of the actual causes of male infertility in published studies. The gaps in knowledge that need to be bridged to enable a fuller understanding of the actual causes of male infertility were highlighted.
Hamideh Jafari [6] The present review suggests that the factors affecting male infertility in Iran are similar to those reported from other countries. The results of this study can be used in adopting appropriate strategies for infertility management in Iran.
Karin Hammarberg [7] Men aspire to parenthood as much as women do but have limited knowledge about the factors that influence fertility. The gap between ideal biological and ideal social age for having children appears to be widening, narrowing the time frame in which parenthood can be achieved. This may lead to unfulfilled parenthood aspirations. The findings can inform government policies and public education strategies aimed to support childbearing during the most fertile years, reduce the personal and societal cost of infertility and ART use, and allow people to fulfil their parenthood goals.
Stürup[8] Our results show that food protein deprivation does not affect the sperm viability of the drones. Sperm production in social insect males ceases on eclosion (Hölldobler and Bartz 1985), after which the sperm cells mature and are subsequently stored in the accessory testes until mating. Hence, it might be possible that nutritional manipulations after eclosion have little effect on the sperm cells themselves as they are already produced and are protected inside the accessory testes. However, deterioration in seminal fluid quality as opposed to direct sperm death would also result in reduced sperm viability.
Damayanthi Durairajanayagam [9] The major lifestyle factors discussed in the present review are amongst the multiple potential risk factors that could impair male fertility. However, their negative impact may well be mostly overcome by behaviour modification and better lifestyle choices. Greater awareness and recognition of the possible impact of these lifestyle factors are important amongst couples seeking conception.
3. Suggestion And Findiings
The causes and risk factors of male infertility as identified in the reviewed studies are presented using four broad themes: biological/physiological causes, behavioral/lifestyle risk factors, environmental risk factors, and socio-demographic risk factors.
Behavioral Risk Factors:
Existing evidence points to cigarette or tobacco smoking, alcohol intake, poor health-seeking behavior among men, untreated or poorly sexually transmitted infections, sexual promiscuity, overweight/obesity, medication, and coital frequency as some of the significant behavioral risk factors for male infertility.
NON behavioral factors:
Other risk factors for male infertility included excessive intake of antioxidants, previous exposure to drugs, and the use of native medications, and infections. Illicit drugs such as marijuana, cocaine, anabolic-androgenic steroids, opiates (narcotics), and methamphetamines, psychological stress, caffeine and unhealthy diet were identified as lifestyle risk factors for male infertility in a review conducted by Durairajanayagam.Another significant factor was coital frequency.
4. Risk Factors for Male Infertility
These factors can causemale infertility:
- Enlarged veins (varicocele) in the scrotum, the sac that holds the testicles.
- Genetic disorders, such ascystic fibrosis.
- High heat exposure to testicles from tight clothing or frequent use of hot tubs and saunas.
- Injury to the scrotum or testicles.
- Low sperm count orlow testosterone(hypogonadism).
- Misuse ofanabolic steroids.
- Premature ejaculationor retrograde ejaculation (semen flows back into the bladder).
- Testicular cancerand treatments.
- Undescended testicles.
Male infertility is a worldwide and huge medical condition. A predictable subject is the prerequisite for public and worldwide endeavors with enormous scope, multi-focus concentrates on including different geological areas. Extensive provincial varieties in key records of male conceptive wellbeing have been accounted for (Skakkebaek et al., 2016) yet these are in many cases on a somewhat neighborhood scale. It is basic to figure out possible varieties in sentinel markers of male conceptive wellbeing, in different nations/locales as well as in low and center asset settings universally, to illuminate on additional arrangement and practice.
These tests can help analyze or preclude a male richness issue:
Semen investigation: This test checks for issues with sperm, for example, low sperm count and unfortunate portability. A few men need a needle biopsy to eliminate sperm from the balls and test it. For most men, this is the main necessary test in the workup of fruitlessness. Blood test: A blood test can check testosterone, thyroid and other chemical levels. Hereditary blood tests search for chromosomal irregularities. Scrotal ultrasound: A ultrasound of the scrotum recognizes varicoceles or other testicular issues.
Treatments for male infertility include:
Medications:Medications can raise testosterone or other hormone levels. There are also drugs for erectile dysfunction.
Surgery:Some men need surgery to open blockages in the tubes that store and carry sperm. Varicocele surgery can make sperm healthier and can improve the odds of conception.
5. Conclusion
in this paper we have determine various risk factors based on behavioral and non-behavioral factors for influencing of male infertility. the various studies found that serum problem erection, generic disorder, drugs addiction, and gene problems is man factors for influencing male infertility. The results indicate limited knowledge of the actual causes of male infertility in published studies. The gaps in knowledge that need to be bridged to enable a fuller understanding of the actual causes of male infertility were highlighted. This study demonstrates a novel and unique way to calculate the distribution of male infertility around the world. The exact rates of male infertility from developing countries like ours are difficult to find because of the problem with the definition of male infertility and lack of accurate reporting rather than a true reflection of male infertility. But still in future, we can conduct various research studies to find out the major causes of male infertility and can work in that direction to reduce such factors which can affect the future fertility of males.
References
- ESHRE Capri Workshop Group, Albertini DF, Anderson R, Bhattacharya S, et al. A prognosis-based approach to infertility: understanding the role of time.Hum Reprod 32 (2017): 1556-1559.
- Turchi P. Prevalence, definition, and classification of infertility. In: Cavallini G,Beretta G, editors.Clinical Management of Male Infertility. Springer (2015): 5-11.
- Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology.Clin Biochem 62 (2018): 2-10.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017.Hum Reprod 32 (2017): 1786-1801.
- Briceag I, Costache A, Purcarea V, et al. Fallopian tubes-literature review of anatomy and etiology in female infertility.J Med Life 8 (2015): 129.
- Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature.J Hum Reprod Sci 8 (2015): 191.
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe.Reprod Biol Endocrinol 13 (2015): 37.
- Masoumi SZ, Parsa P, Darvish N, Mokhtari S, Yavangi M, Roshanaei G. An epidemiologic survey on the causes of infertility in patients referred to infertility center in Fatemieh Hospital in Hamadan.Iran J Reprod Med 13 (2015): 513.
- Qi X, Wang K, Zhou G, Xu Z, Yu J, Zhang W. The role of testicular artery in laparoscopic varicocelectomy: a systematic review and meta-analysis.Int Urol Nephrol 48 (2016): 955-965.
- Ampatzidis G, Georgakopoulou D, Kapsi G. Clitoris, the unknown: what do postgraduate students of educational sciences know about reproductive physiology and anatomy?J Biol Educ (2019): 1-10.
- Carson SA, Buster JE, Cesario M, Woodard SP. Recovery and processing of human embryos formed in vivo. Google Patents (2019).
- Li X, Wang Z, Jiang Z, et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes.Proc Natl Acad Sci 113 (2016): 2666-2671.
- Mehrabani D, Hassanshahi MA, Tamadon A, et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats.J Hum Reprod Sci 8 (2015): 103.
- Orman D, Vardi N, Ates B, Taslidere E, Elbe H. Aminoguanidine mitigates apoptosis, testicular seminiferous tubules damage, and oxidative stress in streptozotocin-induced diabetic rats.Tissue Cell 47 (2015): 284-290.
- Chang W-H, Wu M-H, Pan H-A, Guo P-L, Lee C-C. Semen quality and insulin-like factor 3: associations with urinary and seminal levels of phthalate metabolites in adult males.Chemosphere 173 (2017): 594-602.
- Chen G, Zhang S, Jin Y, et al. TPP and TCEP induce oxidative stress and alter steroidogenesis in TM3 Leydig cells.Reprod Toxicol 57 (2015): 100-110.
- Pitia AM, Minagawa I, Uera N, et al. Expression of insulin-like factor 3 hormone-receptor system in the reproductive organs of male goats.Cell Tissue Res 362 (2015): 407-420.
- Ye L, Li X, Li L, Chen H, Ge R-S. Insights into the development of the adult Leydig cell lineage from stem Leydig cells.Front Physiol 8 (2017): 430.
- Bhushan S, Aslani F, Zhang Z, Sebastian T, Elsässer H-P, Klug J. Isolation of Sertoli cells and peritubular cells from rat testes.J Vis Exp 108 (2016): e53389.
- Sahin Z, Ozkaya A, Cuce G, Uckun M, Yologlu E. Investigation of the effect of naringenin on oxidative stress-related alterations in testis of hydrogen peroxide-administered rats.J Biochem Mol Toxicol 31 (2017): e21928.
- Shah S, Saini N, Ashraf S, et al. Comparative expression analysis of gametogenesis-associated genes in foetal and adult bubaline (Bubalus bubalis) ovaries and testes.Reprod Domest Anim 50 (2015): 365-377.
- Duan P, Hu C, Quan C, et al. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways.Toxicology (2016): 341-343: 28-40.
- Yao C, Sun M, Yuan Q, et al. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3.Oncotarget 7 (2016): 2201.
- Zhang L, Chen M, Wen Q, et al. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation.Proc Natl Acad Sci. 2015;112(13):4003-4008. doi:10.1073/pnas.1422371112.
- Cortes D, Clasen-Linde E, Hutson JM, Li R, Thorup J. The Sertoli cell hormones inhibin-B and anti Müllerian hormone have different patterns of secretion in prepubertal cryptorchid boys.J Pediatr Surg 51 (2016): 475-480.
- Dimitriadis F, Tsiampali C, Chaliasos N, Tsounapi P, Takenaka A, Sofikitis N. The Sertoli cell as the orchestra conductor of spermatogenesis: spermatogenic cells dance to the tune of testosterone.Hormones 14 (2015): 479-503.
- Loveland KL, Hedger MP. Activins and inhibins in Sertoli cell biology: implications for testis development and function. In: Griswold MD, editor.Sertoli Cell Biology. Elsevier (2015): 201-232.
- Chaichanathong S, Taya K, Watanabe G, et al. Immunohistochemical localization of inhibin/activin subunits in adult Asian elephant (Elephas maximus) testes.J Vet Med Sci 80 (2018): 549-552.
- Li Y, Fortin J, Ongaro L, et al. Betaglycan (TGFBR3) functions as an inhibin A, but not inhibin B, coreceptor in pituitary gonadotrope cells in mice.Endocrinology 159 (2018): 4077-4091.
- Winters S, Moore JJr, Clark B. Leydig cell insufficiency in hypospermatogenesis: a paracrine effect of activin-inhibin signaling?Andrology 6 (2018): 262-271.
- Gerber J, Heinrich J, Brehm R. Blood-testis barrier and Sertoli cell function: lessons from SCCx43KO mice.Reproduction (Cambridge, England) 151 (2016): R15-R27.
- Li N, Mruk DD, Lee WM, Wong CK, Cheng CY. Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis?Semin Cell Dev Biol 59 (2016): 141-156.
- Cao X-N, Shen L-J, Yan C, et al. Urban fine particulate matter exposure causes male reproductive injury through destroying blood-testis barrier (BTB) integrity.Toxicol Lett 266 (2017): 1-12.
- Fan Y, Liu Y, Xue K, et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier.PLoS One 10 (2015): e0120775.
- Zhang J, Li Z, Qie M, Zheng R, Shetty J, Wang J. Sodium fluoride and sulfur dioxide affected male reproduction by disturbing blood-testis barrier in mice.Food Chem Toxicol 94 (2016): 103-111.
- Goh WSS, Falciatori I, Tam OH, et al. piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis.Genes Dev 29 (2015): 1032-1044.
- Griswold MD. Spermatogenesis: the commitment to meiosis.Physiol Rev 96 (2016): 1-17.
- Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility.Reprod Biomed Online 31 (2015): 309-319.
- O’Hara L, Smith LB. Androgen receptor roles in spermatogenesis and infertility.Best Pract Res Clin Endocrinol Metab 29 (2015): 595-605.
- Galdon G, Atala A, Sadri-Ardekani H. In vitro spermatogenesis: how far from clinical application?Curr Urol Rep 17 (2016): 49.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
