Animal Models for the Study of Pulmonary Hypertension: Potential and Limitations
Rita Nogueira-Ferreira1,2*, Gabriel Faria-Costasup2, Rita Ferreira1, Tiago Henriques-Coelho 2*
1QOPNA, Department of Chemistry, University of Aveiro, Portugal
2Department of Physiology and Cardiothoracic Surgery, Faculty of Medicine, University of Porto, Portugal
*Corresponding Author(s): Tiago Henriques-Coelho, Department of Physiology and Cardiothoracic Surgery, Faculty of Medicine, University of Porto, Portugal, Rita Nogueira-Ferreira, QOPNA, Department of Chemistry, University of Aveiro, Portugal.
Received: 12 September 2016; Accepted: 25 September 2016; Published: 28 September 2016
Article Information
View / Download Pdf Share at FacebookAbstract
Pulmonary hypertension (PH) is a multifactorial disease, commonly associated with heart failure. Different experimental models have emerged to help in the understanding of the molecular and cellular mechanisms associated with human PH, providing also a useful approach to test experimental therapies for PH treatment. Although there is no ideal animal model that mimics human PH, animal models have clearly provided valuable insights into the characterization of the cellular and molecular pathways underlying PH onset and progression, and have been successfully applied in the discovery of novel therapeutic approaches. In here we summarize the features of the animal models described within the field of PH research, either the physical, chemical and genetic models, emphasizing its advantages and limitations.
Keywords
Animal models; Chronic hypoxia; Monocrotaline; Pulmonary hypertension
Article Details
Abbreviations
| 5-HTT | Serotonin transporter |
| Ang-1 | Angiopoietin-1 |
| BMPRII | Bone morphogenetic protein receptor type II |
| IL-6 | Interleukin-6 |
| MCT | Monocrotaline |
| PAB | Pulmonary artery banding |
| PAECs | Pulmonary artery endothelial cells |
| PAH | Pulmonary arterial hypertension |
| PAP | Pulmonary artery pressure |
| PASMCs | Pulmonary artery smooth muscle cells |
| PH | Pulmonary hypertension |
| RV | Right ventricle |
| TGF-α | Transforming growth factor-α |
| TNF-α | Tumor necrosis factor-α |
| VEGFR-2 | Vascular endothelial growth factor receptor-2 |
| VIP | Vasoactive intestinal peptide |
Introduction
The World Health Organization classified pulmonary hypertension (PH) into five groups which share a mean, resting, pulmonary artery pressure (PAP) ≥ 25 mmHg. The Group 1 is pulmonary arterial hypertension (PAH), Group 2 is PH associated with left heart disease, Group 3 is PH associated with lung disease and/or hypoxia, Group 4 is PH associated with chronic thromboembolic disease (CTEPH), and Group 5 is PH associated with unclear multifactorial mechanisms (5th World Symposium on PH, Nice, 2013) [1, 2] . Each group reflects specific etiology, pathological and hemodynamic characteristics and therapeutic approaches. However, there are common processes to the pathology of all PH groups. Vasoconstriction, remodeling, thrombosis, and inflammation are the basic mechanisms of pulmonary vascular pathology in PH. Nevertheless, their relevance, origin, and order of appearance may differ depending on the etiology [2-5] . Over the last years, major advances in the understanding of PH pathogenesis allowed a delay in disease progression, reducing the symptoms and increasing the quality of life of PH patients. Unfortunately, PH remains a disease without cure [6]. The fact that the disease is usually diagnosed in advanced stages difficult its study in humans. Animal model studies have allowed the investigation of the various phases of disease progression, being crucial to understand the pathophysiology of PH, and to test experimental therapies. Furthermore, they provide us advantages in terms of economy, control of the experimental conditions, replicability and drug testing envisioning its safety translation to humans [7, 8] .
An ideal PH model should manifest the key clinical, hemodynamic and histopathological features of human PH [7]. Pulmonary hypertension is a complex disease of diverse etiology and so there is no single animal model that accurately reproduces the human disease, even focusing on just one of PH groups [9]. Consequently, a vast list of PH experimental models is currently available (Table 1). Each model has its own characteristics and allows the investigation of specific hypothesis. Some of them are used in the study of different groups of human PH, once they present molecular and pathological features common to those groups [4]. We grouped these models according to the stimuli that result in PH development (physical, chemical, genetic and multiple) and we critically highlight the general advantages and limitations of their use in PH research.
Physical Animal Models
The chronic hypoxia model is one of the most used to study PH pathogenesis and treatment. Its pathological features of pulmonary vasoconstriction and vascular medial hypertrophy mimic the ones observed in human PH [10]. Although being a model of Group 3 PH, it is often used to make conclusions regarding Group 1 PH (PAH) [11]. Chronic hypoxia can be induced by exposing animals to normal air at hypobaric pressure or to oxygen-poor air at normal pressure [12] . This decrease in oxygen pressure causes a strong pulmonary vasoconstrictor response that is characteristic of this model [13]. However, there is little evidence of right ventricle (RV) failure, that is usually the main cause of death in PAH patients [10]. Furthermore, the response to hypoxia varies among animal species, making difficult the translation of findings to human [11, 13] .
Another described animal model of PH resulting from a physical stimulus involves repeated microembolizations with the injection of synthetic microspheres, such as Sephadex® microspheres to induce chronic emboli. Thus, this model is useful to study chronic thromboembolism PH (Group 4 PH). The possibility of target different-sized vessels depending on the diameter size of the microspheres used is an advantage of this approach. However, although this model allows moderate PH development, attention should be taken regarding the microspheres material, since no cellular reaction related with the material type is desired [13, 14] .
The surgical models, on the other hand, are designed to mimic the increased blood flow and pressures imposed on the RV in Group 1 PH. There are two main surgical methods used until today: pulmonary artery banding (PAB) and aorto-caval shunt. The PAB consists in a constriction imposed in the pulmonary artery, which leads to an increased afterload in the RV that drives the hypertrophic response. Thus, it allows separate the cardiac disease from the pulmonary disease, which is not present in this model. Given this, PAB does not replicate the human pathology entirely, but is useful to understand the mechanisms of RV dysfunction, already pointed out as the main determinant of prognosis [11, 15] . The aorto-caval shunt is a volume overload method which displays similar RV hypertrophy when compared with PAB. This model can be combined with the monocrotaline (MCT) model, leading to more severe disease development [4, 11, 16] . The main disadvantages of these surgical methods are related with the fact that they require highly technical skills and are usually associated with a high percentage of animal death [11]. Although not being as used as the chronic hypoxia model, these models are still common. Recently, a novel model of pulmonary artery banding emerged related with an easier method of constricting the pulmonary artery. This new method resulted in a significantly lower surgical mortality and revealed significantly more signs of RV dysfunction [17].
Chemically-Induced Animal Models
Chemically-induced PH models can offer advantage in terms of application simplicity and costs. Amongst these, the MCT animal model is the most broadly used to study PH, in particular the pathophysiology and therapeutic application in the Group 1 PH [8, 11, 18] . Indeed, for more than one decade, most studies on therapy of PAH have employed the MCT model [19]. As recently reviewed [20], the administration of the alkaloid MCT affects both the lungs and the heart, modulating primarily biological processes associated with the vascular remodeling and inflammation, two key pathological features of human PH. However, an important drawback is that the response to MCT is variable among species, strains and even animals [13]. The most common specie used in the MCT model is the rat because it is the one that best develops PAH features after the drug injection [21, 22] . MCT effects require conversion to an active form (MCT pyrrole) in the liver by cytochrome P450, which makes the model dependent on the animals-based metabolic differences [4]. For instance, mice must be injected with the MCT pyrrole active form and not MCT itself. However, the disease development is far less extensive, stagnating in an acute lung injury [22] . Other animals less used are dogs [23] or pigs [24] , which can replicate human PAH more successfully than rodent models. Still, these kinds of animals are more expensive and the disease takes longer to develop [11]. In spite of the limitations of the MCT model, it is largely used once, in comparison with the other models, it is reproducible, less expensive and does not need particular technical skills [25]. Furthermore, it mimics human PH in terms of hemodynamic and histopathological severity, and high mortality [26].
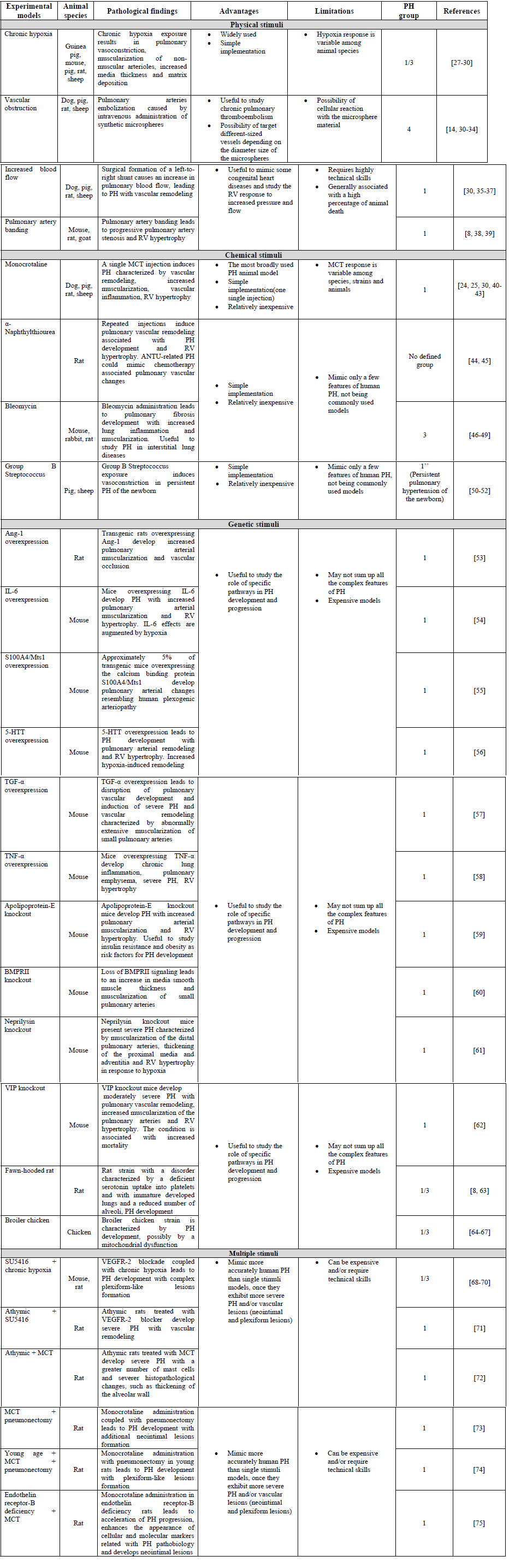
Table 1: Animal models of pulmonary hypertension
5-HTT : Serotonin Transporter; Ang-1 : Angiopoietin-1; BMPRII : Bone Morphogenetic Protein Receptor Type II; IL-6 : Interleukin-6; TGF-α : Transforming Growth Factor-α; TNF-α : Tumor Necrosis Factor-α; VEGFR-2 : Vascular Endothelial Growth Factor Receptor-2; VIP : Vasoactive Intestinal Peptide.
Genetic Animal Models
In the past few years, numerous genetic animal models have emerged in the field of PH research [11, 13, 30]. The transgenic and knockout models allow the evaluation of the effect of overexpressing or downregulating a specific gene in the susceptibility to the development of PH. The high diversity of these models reflects the different molecular pathways underlying PH development [4, 9]. Even though there is not a clear separation, we can group the genetic models by the main processes that they interfere with: vascular tone and inflammation/vascular remodeling. Independently of the group, the most common specie used is the mouse, which is harder to handling in the experimental procedures, such as hemodynamic evaluation [11].
Endothelin (ET)-1 is a potent vasoconstrictor that drives PH development and progression, by acting in its receptors (ETA and ETB). ETA is expressed mainly in pulmonary artery smooth muscle cells (PASMCs) and is related to PASMC proliferation and vasoconstriction. On the other hand, ETB is expressed in pulmonary artery endothelial cells (PAECs) and PASMCs. Activation of ETB in the PAECs causes vasodilatation via the release of nitric oxide and prostaglandin, while stimulation of ETB in the PASMCs causes vasoconstriction [76]. Nevertheless, both heterozygote ET-1 knockouts and ET-1 overexpressers transgenic models fail to alter pulmonary vascular pressures per se [4]. Yet, ETB receptor knockout mice have the vasodilatory effect of this receptor blunted and is linked with enhanced appearance of cellular and molecular markers related with PH pathobiology and development of neointimal lesions when in combination with MCT [75] . Serotonin (5-HT) is also an important regulator of vascular tone associated with PH pathogenesis [3]. Genetic models with 5-HT related alterations greatly contributed to the current knowledge of this mediators’ role. Consistently, tryptophan hydroxylase 1 (involved in 5-HT synthesis) knockout mice [77] , 5-HTT (5-HT transporter) knockout [78] and 5-HT1B (5-HT receptor) knockout [79] attenuate hypoxia-induced PH, while 5-HTT gene overexpressing mice present a more severe form of the disease [56] .
Inflammation is a key feature of PH pathogenesis, being already a therapeutic target [80, 81] . Genetic models are particularly useful for studying the effect of specific cytokines in PH pathophysiology. Among others, TNF-α [58] and TGF-α [57] overexpression is linked to PH. But, by far, the best studied models are the ones that target IL-6 related pathways. In fact, it is documented an increase in serum expression of IL-6 in patients with PAH, which positively correlates with the mortality rate [82, 83] . The mouse model of IL-6 overexpression was first implemented by Steiner et al. [54] . They found RV hypertrophy, an increased muscularization throughout the entire pulmonary vascular bed and the formation of occlusive neointimal angioproliferative lesions composed of endothelial cells and T-lymphocytes. Consistently, Savale et al. [84] found, in an IL-6 knockout model, diminished susceptibility to hypoxia-induced PH. They reported a decrease in media thickening of pulmonary vessels in IL-6 deficient mice and also a role of IL-6 in PASMC migration. Altogether, the IL-6 overexpressing mouse seems to be a model that resembles many of the pathologic features of PH. As a matter of fact, IL-6 has been proposed to regulate several pathways that are implicated in PH. It is believed that IL-6 drives the hyperproliferative state of PASMCs, modulates several pro- and anti-apoptotic factors [54] and the BMP signaling cascade [85] . Since a mutation of the BMPRII gene that encodes for the bone morphogenetic protein receptor II was discovered to be a principal mutation in hereditary PAH [86] , there have been attempts to create BMPRII-deficient mice. However, the complete deletion of the gene is incompatible with life and heterozygotes do not develop adequate disease severity [11]. This problem was overcome by the appearance of smooth muscle-specific transgenic mice expressing a dominant-negative BMPRII under control of a tetracycline gene switch system [60] . There is consistent evidence of the disease development in this model, namely an increase in RV systolic pressure, RV hypertrophy, an increase in muscularization of small pulmonary arteries and some blood flow changes [60, 87] . The BMPRII ligands are also targets of research in the field of PAH. BMP-2 and BMP-4 are the most important factors of this class and act in opposite ways in response to hypoxia: BMP-2 knockout mice have increase severity while the opposite happens with BMP-4 knockout mice [88] .
The protein S100A4/Mst1 is part of a family of calcium-binding proteins whose functions are related with cell proliferation, differentiation, cytoskeletal dynamics and apoptosis [4, 8] . Interestingly, the transgenic mice overexpressing S100A4/Mst1 model was initially developed aiming the study of S100A4/Mst1 role in metastatic cancer [89]. However, it was observed that approximately 5% of the S100A4/Mst1 overexpressing mice develop pulmonary vascular remodeling similar to that observed in PH [55] . Thus, the importance of this model in PH study is related with the presence of pulmonary vascular changes resembling human plexiform lesions, which is a feature absent in the majority of the PH models. Noteworthy, although the majority of the PH models show increased male susceptibility, contrary of what is observed in human PH; two genetic animal models (mice overexpressing the protein S100A4/Mts1 and mice overexpressing the serotonin transporter) showed a PH development female gender specific [90]. The fact that genetic models may not sum up all the complex features of PH, once they focus in the study of particular pathways is a limitation [11]. Furthermore, genetically modified mice are expensive, which may cause a limitation in the number of samples [91].
Animal Models Involving Multiple Stimuli
Animal models that involve chronic hypoxia and MCT models have been developed aiming more severe PH and/or vascular lesions such as neointimal and plexiform lesions [92]. These occlusive lesions, which result from smooth muscle and endothelial cell proliferation, are hallmarks of Group 1 PH, being major contributors for the high pulmonary vascular resistance in PAH [93, 94] . However, they are absent in the most common single stimuli animal models. Those models that combine multiple insults result in more severe PH than single stimuli, suggesting that the pathogenesis of PH requires several insults [11]. SU5416 is a small molecule inhibitor of the vascular endothelial growth factor receptor-2 (VEGFR-2). Given that VEGF is important for normal endothelial cell function, its blockade was expected to induce endothelial cell dysfunction, stimulate apoptosis-resistant endothelial cell proliferation and consequently cause PH [93]. Indeed, the SU5416/chronic hypoxia model is the most used multiple stimuli model to study both PH pathogenesis and treatment. Interestingly, this model demonstrated resistance to some drugs commonly used in PAH patients, being thus refractory to treatment as it is verified in most PAH patients [8, 93, 95]. However, a limitation of SU5416/chronic hypoxia model is the absence of perivascular inflammation, a key feature of human PAH [10, 11]. Another relevant multiple model consists in MCT administration coupled with pneumonectomy, which add to the MCT model vascular characteristics the presence of neointimal lesions [73]. Nevertheless, it requires technical skills associated with the experimental procedure.
Conclusion and Future Perspectives
The complexity of the molecular mechanisms underlying PH pathogenesis and the diverse etiology that characterizes this disease makes the implementation of animal models a truly demanding task. Although a “gold” animal model in PH research does not exist, there are several animal models available nowadays, each one presenting specific features of the disease, allowing the investigation of key aspects of the disease. These models are important not only for the discovery and exploration of the molecular pathways underlying disease pathogenesis but also for the assessment of therapeutic suitability to treat PH patients.
However, caution should be taken when translating data from animal models to the human context considering the different aspects of the disease in animal models. So, efforts should continue to be done in the development of animal models that more exactly mimic the features of each group of human PH. The current trend is the use of two animal models, such as MCT and chronic hypoxia, to demonstrate that data obtained are model independent and to facilitate data translation to the human clinical set. The simultaneous use of distinct PH animal models to test an experimental therapy is expected to continue and even increase once raises the hypothesis of its suitability for PH patients.
Acknowledgements
This work was supported by Fundação para a Ciência e a Tecnologia (FCT, Portugal), European Union, QREN, FEDER and COMPETE for funding the Organic Chemistry Research Unit (QOPNA) (UID/QUI/00062/2013), the Cardiovascular R&D Unit (UID/IC/00051/2013) and the post-graduation student (grant number SFRH/BD/91067/2012).
Conflicts of Interest
The authors report no conflicts of interest.
References
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 6 (2013): 34-41.
- McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol 65 (2015): 1976-1997.
- Wilkins MR. Pulmonary hypertension: the science behind the disease spectrum. Eur Respir Rev 21 (2012): 19-26.
- West J, Hemnes A. Experimental and transgenic models of pulmonary hypertension. Compr Physiol 1 (2011): 769-782.
- Zanjani KS. Platelets in pulmonary hypertension: a causative role or a simple association? Iran J Pediatr 22 (2012): 145-157.
- Colvin KL and Yeager ME. Animal models of pulmonary hypertension: matching disease mechanisms to etiology of the human disease. J Pulm Respir Med 4 (2014): 198.
- Ryan JK, Bloch K and Archer SL. Rodent models of pulmonary hypertension: harmonisation with the world health organisation's categorisation of human PH. Int J Clin Pract Suppl 172 (2011): 15-34.
- Maarman GS, Lecour G, Butrous F, Thienemann and Sliwa K. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet? Pulm Circ. 3 (2013): 739-56.
- Das M, Fessel J, Tang H, West J. A process-based review of mouse models of pulmonary hypertension. Pulm Circ 2 (2012): 415-433.
- Zhao L. Chronic hypoxia-induced pulmonary hypertension in rat: the best animal model for studying pulmonary vasoconstriction and vascular medial hypertrophy. Drug Discov Today Dis Models 7 (2010): 83-88.
- Ryan JJ, Marsboom G, Archer SL. Rodent models of group 1 pulmonary hypertension. Handb Exp Pharmacol 218 (2013): 105-149.
- Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest 106 (2000): 733-738.
- Barman SA, Zhu S, White RE. RhoA/Rho-kinase signaling: a therapeutic target in pulmonary hypertension. Vasc Health Risk Manag 5 (2009): 663-671.
- Shelub I, van Grondelle A, McCullough R, Hofmeister S, Reeves JT. A model of embolic chronic pulmonary hypertension in the dog. J Appl Physiol Respir Environ Exerc Physiol 56 (1984): 810-815.
- Vonk Noordegraaf A, Galiè N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev 20 (2011): 243-253.
- Van Albada ME, Schoemaker RG, Kemna MS, Cromme-Dijkhuis AH, R. Van Veghel, et al. The role of increased pulmonary blood flow in pulmonary arterial hypertension. Eur Respir J 26 (2005): 487-93.
- Hirata M, Ousaka D, Arai S, Okuyama M. Tarui S, et al. Novel model of pulmonary artery banding leading to right heart failure in rats. Biomed Res Int 2015 (2015): 1-10.
- Dias-Neto M, Luísa-Neves A, Pinho S, Gonçalves N, Mendes M, et al. Pathophysiology of infantile pulmonary arterial hypertension induced by monocrotaline. Pediatr Cardiol 2015. 36 (2015): 1000-1013.
- Umar S, Steendijk P, Ypey DL, Atsma DE, van der Wall EE, et al. Novel approaches to treat experimental pulmonary arterial hypertension: a review. J Biomed Biotechnol 2010 (2010): 702836.
- Nogueira-Ferreira RR, Vitorino R. Ferreira and T. Henriques-Coelho. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm Pharmacol Ther 35 (2015): 8-16.
- Schoental R, Head MA. Pathological changes in rats as a result of treatment with monocrotaline. Br J Cancer 9 (1955): 229-237.
- Dumitrascu R, Koebrich S, Dony E, Weissmann N, Savai R, et al. Characterization of a murine model of monocrotaline pyrrole-induced acute lung injury. BMC Pulm Med 8 (2008): 25.
- Okada M, Yamashita C and Okada K. Establishment of canine pulmonary hypertension with dehydromonocrotaline. Importance of larger animal model for lung transplantation. Transplantation 60 (1995): 9-13.
- Zeng GQ, Liu R, Liao HX, Zhang XF, Qian YX, et al. Single intraperitoneal injection of monocrotaline as a novel large animal model of chronic pulmonary hypertension in Tibet minipigs. PLoS One 8(2013): e78965.
- Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, et al. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 302 (2012): L363-L369.
- Naeije R, Dewachter L. [Animal models of pulmonary arterial hypertension]. Rev Mal Respir 24 (2007): 481-496.
- Meyrick B and Reid L. Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol 100 (1980): 151-178.
- Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, et al. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101 (1998): 2468-2477.
- Janssens SP, Thompson BT, Spence CR, Hales CA. Polycythemia and vascular remodeling in chronic hypoxic pulmonary hypertension in guinea pigs. J Appl Physiol 71 (1991): 2218-2223.
- Marsboom GR and Janssens SP. Models for pulmonary hypertension. Drug Discov Today Dis Models 1 (2004): 289-296.
- Weimann J, Zink W, Gebhard MM, Gries A, Martin E, et al. Effects of oxygen and nitric oxide inhalation in a porcine model of recurrent microembolism. Acta Anaesthesiol Scand 44(2000): 1109-1115.
- Zagorski J, Debelak J, Gellar M, Watts JA and Kline JA. Chemokines accumulate in the lungs of rats with severe pulmonary embolism induced by polystyrene microspheres. J Immunol 171 (2003): 5529-5536.
- Jones AE, Watts JA, Debelak JP, Thornton LR, Younger JG, et al. Inhibition of prostaglandin synthesis during polystyrene microsphere-induced pulmonary embolism in the rat. Am J Physiol Lung Cell Mol Physiol. 284 (2003): L1072-L1081.
- Weimann J, Zink W, Schnabel PA, Jakob H, Gebhard MM, et al. Selective vasodilation by nitric oxide inhalation during sustained pulmonary hypertension following recurrent microembolism in pigs. J Crit Care 14 (1999): 133-140.
- Rondelet B, Kerbaul F, Motte S, Van Beneden R, Remmelink M, et al. Bosentan for the prevention of overcirculation-induced experimental pulmonary arterial hypertension. Circulation 107 (2003): 1329-1335.
- Corno AF, Tozzi P, Genton CY and Von Segesser LK. Surgically induced unilateral pulmonary hypertension: time-related analysis of a new experimental model. Eur J Cardiothorac Surg 23(2003): 513-517.
- Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, et al. In utero placement of aortopulmonary shunts: a model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation 92 (1995): 606-613.
- Dias CA, Assad RS, Caneo LF, Abduch MCD, Aiello VD, et al. Reversible pulmonary trunk banding. II. An experimental model for rapid pulmonary ventricular hypertrophy. J Thorac Cardiovasc Surg 124 (2002): 999-1006.
- Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, et al. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med 183 (2011): 1402-1410.
- Hilliker KS, Bell TG, Roth RA. Pneumotoxicity and thrombocytopenia after single injection of monocrotaline. Am J Physiol 242 (1982): H573-H579.
- Sugita T, Hyers TM, Dauber IM, Wagner WW, Mcmurtry IF, et al. Reeves Lung vessel leak precedes right ventricular hypertrophy in monocrotaline-treated rats. J Appl Physiol Respir Environ Exerc Physiol 54 (1983): 371-374.
- Rosenberg HC and Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol 255(1988): H1484-H1491.
- Ito KM, Sato M, Ushijima K, Nakai M and Ito K. Alterations of endothelium and smooth muscle function in monocrotaline-induced pulmonary hypertensive arteries. Am J Physiol Heart Circ Physiol 279 (2000): H1786-H1795.
- Azoulay E, Eddahibi S, Marcos E, Levame M, Harf A, et al. Granulocyte colony-stimulating factor enhances alpha-naphthylthiourea-induced pulmonary hypertension. J Appl Physiol 94 (1985): 2027-2033.
- Hill NS, O'brien RF and Rounds S. Repeated lung injury due to alpha-naphthylthiourea causes right ventricular hypertrophy in rats. J Appl Physiol Respir Environ Exerc Physiol 56 (1984): 388-396.
- Ortiz LA, Champion HC, Lasky JA, Gambelli F, Gozal E, et al. Enalapril protects mice from pulmonary hypertension by inhibiting TNF-mediated activation of NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol 282 (2002): L1209-L1221.
- Bowden DH. Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest 50 (1984): 487-488.
- Harrison JH Jr, Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther 243 (1987): 1185-1194.
- Lynch DA, Hirose N, Cherniack RM and Doherty DE. Bleomycin-induced lung disease in an animal model: correlation between computed tomography-determined abnormalities and lung function. Acad Radiol 4 (1997): 102-107.
- Curtis J, Kim G, Wehr NB, Levine RL. Group B streptococcal phospholipid causes pulmonary hypertension. Proc Natl Acad Sci U S A 100 (2003): 5087-5090.
- Navarrete CT, Devia C, Lessa AC, Hehre D, Young K, et al. The role of endothelin converting enzyme inhibition during group B streptococcus-induced pulmonary hypertension in newborn piglets. Pediatr Res 54 (2003): 387-392.
- Carpenter DT, Larkin HR, Chang AS, Morris E, O'neill JT, et al. Superoxide dismutase and catalase do not affect the pulmonary hypertensive response to group B Streptococcus in the lamb. Pediatr Res 49 (2001): 181-188.
- Chu D, Sullivan CC, Du L, Cho AJ, Kido M, et al. A new animal model for pulmonary hypertension based on the overexpression of a single gene angiopoietin-1. Ann Thorac Surg 77 (2004): 449-456.
- Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104 (2009): 236-244.
- Greenway S, Van Suylen RJ, Du Marchie Sarvaas G, Kwan E, Ambartsumian N, et al. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol 164 (2004): 253-262.
- Maclean MR, Deuchar GA, Hicks MN, Morecroft I, Shen S, et al. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation 109 (2004): 2150-2155.
- Le Cras TD, Hardie WD, Fagan K, Whitsett JA, Korfhagen TR. Disrupted pulmonary vascular development and pulmonary hypertension in transgenic mice overexpressing transforming growth factor-alpha. Am J Physiol Lung Cell Mol Physiol 285 (2003): L1046-L1054.
- Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, et al. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 280 (2001): L39-L49.
- Hansmann G, Wagner RA, Schellong S, De Jesus Perez VA, Urashima T, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator–activated receptor-? activation. Circulation 115 (2007): 1275-1284.
- West J, Fagan K, Steudel W, Fouty B, Lane K, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94 (2004): 1109-1114.
- Dempsey EC, Wick MJ, Karoor V, Barr EJ, Tallman DW, et al. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am J Pathol 174 (2009): 782-796.
- Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, et al. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 115 (2007): 1260-1268.
- Le Cras TD, Kim DH, Gebb S, Markham NE, Shannon JM, et al. Abnormal lung growth and the development of pulmonary hypertension in the Fawn-Hooded rat. Am J Physiol 277 (1999): L709-L718.
- Martinez-Lemus LA, Hester RK, Becker EJ, Jeffrey JS, and Odom TW. Pulmonary artery endothelium-dependent vasodilation is impaired in a chicken model of pulmonary hypertension. Am J Physiol 277 (1999): R190-R197.
- Xiang RP, Sun WD, Zhang KC, Li JC, Wang JY, et al. Sodium chloride-induced acute and chronic pulmonary hypertension syndrome in broiler chickens. Poult Sci 83 (2004): 732-736.
- Cawthon D, Iqbal M, Brand J, Mcnew R and Bottje WG. Investigation of proton conductance in liver mitochondria of broilers with pulmonary hypertension syndrome. Poult Sci 83 (2004): 259-265.
- Iqbal M, Cawthon D, Wideman Jr RF, and Bottje WG. Lung mitochondrial dysfunction in pulmonary hypertension syndrome. I. Site-specific defects in the electron transport chain. Poult Sci 80 (2001): 485-495.
- Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121 (2010): 2747-2754.
- Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. Faseb J 15 (2001): 427-438.
- Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, et al. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 184 (2011): 1171-82.
- Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 175 (2007): 1280-1289.
- Miyata M, Sakuma F, Ito M, Ohira H, Sato Y, et al. Athymic nude rats develop severe pulmonary hypertension following monocrotaline administration. Int Arch Allergy Immunol 121 (2000): 246-52.
- Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, et al. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 151 (1997): 1019-1025.
- White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, et al. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293 (2007): L583-L590.
- Ivy DD, Mcmurtry IF, Colvin K, Imamura M, Oka M, et al. Development of occlusive neointimal lesions in distal pulmonary arteries of endothelin B receptor-deficient rats: a new model of severe pulmonary arterial hypertension. Circulation 111 (2005): 2988-2996.
- Galié N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 61 (2004): 227-237.
- Izikki M, Hanoun N, Marcos E, Savale L, Barlier-Mur AM, et al. Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: effects on hypoxic pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 293 (2007): L1045-L1052.
- Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest 105 (2000): 1555-1562.
- Keegan A, Morecroft I, Smillie D, Hicks MN, Maclean MR. Contribution of the 5-HT(1B) receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT(1B)-receptor knockout mice and the 5-HT(1B/1D)-receptor antagonist GR127935. Circ Res 89 (2001): 1231-1239.
- Mathew R. Inflammation and pulmonary hypertension. Cardiol Rev 18 (2010): 67-72.
- Henriques-Coelho T, Oliveira SM, Moura RS, Roncon-Albuquerque Jr R, Neves AL, Thymulin inhibits monocrotaline-induced pulmonary hypertension modulating interleukin-6 expression and suppressing p38 pathway. Endocrinology 149 (2008): 4367-4373.
- Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, et al. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 34 (2009): 662-668.
- Heresi GA, Aytekin M, Hammel JP, Wang S, Chatterjee S, et al. Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J 43 (2014): 912-914.
- Savale L, Tu L, Rideau D, Izziki M, Maitre B, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 10 (2009): 6.
- Hagen M, Fagan K, Steudel W, Carr M, Lane K, et al. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 292 (2007): L1473-L1479.
- Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II a receptor member of the TGF-beta family. J Med Genet 37 (2000): 741-745.
- Yasuda T, Tada Y, Tanabe N, Tatsumi K, West J. Rho-kinase inhibition alleviates pulmonary hypertension in transgenic mice expressing a dominant-negative type II bone morphogenetic protein receptor gene. Am J Physiol Lung Cell Mol Physiol 301 (2011): L667-L674.
- Anderson L, Lowery JW, Frank DB, Novitskaya T, Jones M, et al. Bmp2 and Bmp4 exert opposing effects in hypoxic pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol 298 (2000): R833-R842.
- Ambartsumian N, Grigorian M, Lukanidin E. Genetically modified mouse models to study the role of metastasis-promoting S100A4(mts1) protein in metastatic mammary cancer. J Dairy Res 72 (2005): 27-33.
- Dempsie Y, MacLean MR. The influence of gender on the development of pulmonary arterial hypertension. Exp Physiol 98 (2013): 1257-1261.
- Leong XF, Ng CY, Jaarin K. Animal Models in Cardiovascular Research: Hypertension and Atherosclerosis. Biomed Res Int 2015 (2015): 528757.
- Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297 (2009): L1013-L1032.
- Nicolls MR, Mizuno S, Taraseviciene-Stewart L, Farkas L, Drake JI, et al. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm Circ 2 (2012): 434-442.
- Firth AL, Mandel J, Yuan JX. Idiopathic pulmonary arterial hypertension. Dis Model Mech 3 (2010): 268-273.
- Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 291 (2006): L668-L676.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 