In Vitro Proof of Concept Evaluation of a Gravity Powered Novel Filtration Device to Effectively Process a Blood Analogue/Particle Mixture to Rapidly Produce a Clear Filtrate
Michael Bruce Horowitz1*, Brandon Repko2, Hieu Le3, Benjamin Bobo3, Henre Pande4
1HCA Florida First Coast Neurosurgery, HCA Florida-Orange Park Hospital 1825, Kingsley Avenue, Suite 170, Orange Park, FL 32073, United States
2Department of Radiology, Butler Medical Center, 1 Hospital Way, Butler, PA 16001, United States
3Retriever Medical, 6016 Agatha Christie Ave, Las Vegas, NV 89131, United State
4Jacksonville, Florida, United States
*Corresponding author: : Michael Bruce Horowitz, MD. HCA Florida First Coast Neurosurgery HCA Florida-Orange Park Hospital 1825 Kingsley Avenue Suite 170, Orange Park, FL 32073, United States
Received: 18 January 2024; Accepted: 24 January 2024; Published: 31 January 2024
Article Information
Citation: Michael Bruce Horowitz, Brandon Repko, Hieu Le, Benjamin Bobo, Henre Pande. In Vitro Proof of Concept Evaluation of a Gravity Powered Novel Filtration Device to Effectively Process a Blood Analogue/Particle Mixture to Rapidly Produce a Clear Filtrate. Cardiology and Cardiovascular Medicine. 8 (2024): 48-51.
View / Download Pdf Share at FacebookAbstract
Background: This study provides an In Vitro proof of concept evaluation of a gravity powered novel blood filtration device to effectively process a high viscosity whole blood analogue/particle mixture to rapidly produce a clear filtrate.
Methods: A novel proprietary gravity fed blood filtration system was used to process a particulate filled water/glycerol high viscosity whole blood analogue to determine the device’s ability to rapidly exclude foreign body and to produce a cleared filtrate.
Results: This study demonstrated 100% filtration of 2.3 mm, 63-75 micron and 355-425 micron spheres from a high viscosity blood analogue/particle mixture using a gravity fed three filter system. Filtration rate using a clear solution that mimics high viscosity whole blood occurred at a rate of 100 cc/4-7 seconds (> 500 cc/minute).
Conclusion: A gravity driven three filter device can rapidly process a high viscosity whole blood equivalent fluid/particulate mixture. Results from this and prior In vitro studies indicate that such a device may prove useful for the reinfusion of autologous blood lost during invasive procedures.
Trial Registration: This article does not report the results of a health care intervention on human subjects. .
Keywords
<p>Filtration; Blood</p>
Article Details
Abbreviations:
Millimeters; mm: Cubic centimeter; cc:
1. Introduction
The ability to efficiently and quickly filter and reinfuse autologous whole blood that is lost during surgical and interventional procedures may provide the opportunity to transfuse patients with their own non-fractionated blood. The authors’ have previously published a proof-of-concept study showing no cellular injury or coagulation changes when porcine and human blood was filtered using their proprietary gravity fed filtration system [1]. The study discussed below evaluated the system’s filtration reliability as well as its filtration rate.
2. Methods
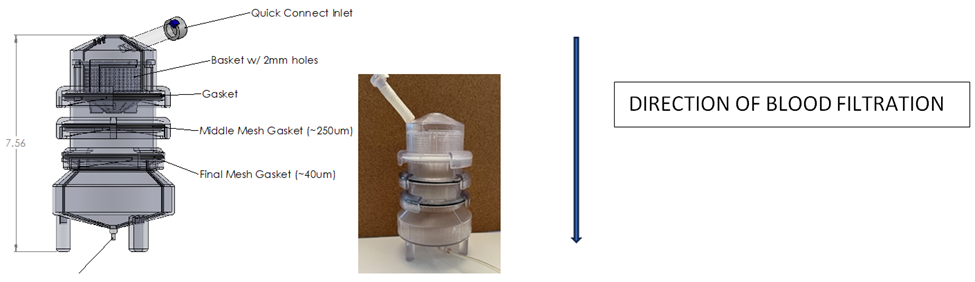
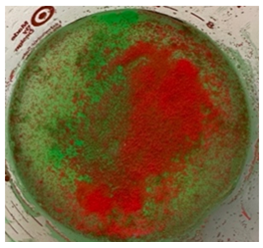
This study evaluated the ability for our three-filter system (Figure 1) to reliably exclude particles of various sizes from the final filtrate product that would be reinfused as an autologous whole blood transfusion. Because the sequentially placed filters measured 2mm, 250 micron and 40 micron in pore size, the filtered materials consisted of three different sized spherical particles each having a diameter of 2.3 mm, 63-75 micron and 355-425 micron. The 2.3 mm particles were composed of stainless steel while the 63-75 micron and 355-425 micron spheres were composed of green and red fluorescent microspheres, respectively (Cospheric, Somis, CA, USA). These round particles emit bright colors when illuminated by UV light.
In order to better identify the presence of stainless steel and fluorescent microspheres in the final filtrate, a transparent high viscosity blood analog was used as opposed to whole blood [2, 3]. This substitute was composed of 50 cc water mixed with 50 cc glycerol (Himedia Laboratories Pvt. Ltd, Nashik, India).
The stainless-steel spheres and fluorescent microspheres were added to the water/glycerol solution (Figure 2). This mixture was infused into the top section of the filtration device, allowed to process through the sequential filters, and enter the bottom collection chamber. Five separate 100 cc samples of the mixture were processed.
3. Results
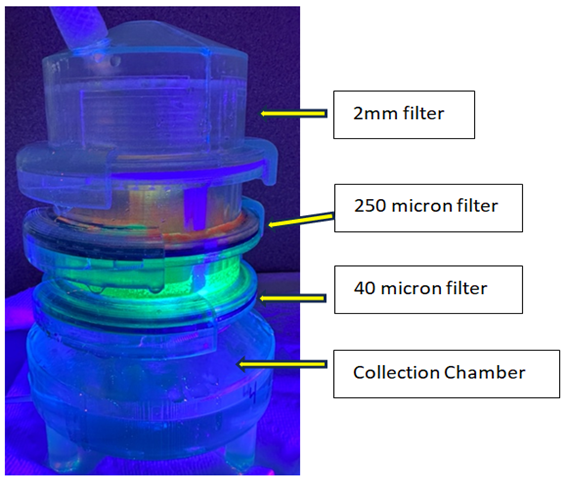
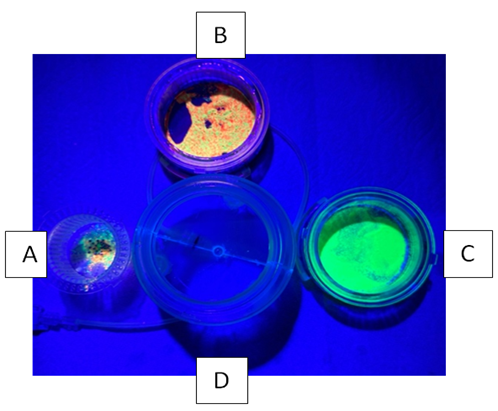
Five 100cc fluid/particle samples were tested. Filtration time to process each sample ranged between 4 -7 seconds. Following processing, the filter chambers and collection chamber were illuminated with UV light. The five individual filtration trials yielded identical results. All steel spheres remained proximal to the 2 mm filter as did a small volume of the smaller microspheres. Both fluorescing microspheres sizes collected proximal to the 250 micron filter and only the smallest microspheres collected proximal to the 40 micron filter. No particles entered the final filtrate in the collection chamber (Figures 3,4).
Figure 4: Disassembled filter device illuminated with UV light. Each chamber has captured the spheres while the bottom collection chamber contains fluid only. Chamber A holds the 2 mm filter and has captured a mixture of 2.3 mm, 63-75 micron and 355-425 micron spheres. Chamber B holds the 250 micron filter and has captured the 63-75 micron and 355-425 micron spheres. Chamber C holds the 40 micron filter and has captured the 63-75 micron spheres. Chamber D holds the final filtrate which is free of particulates.
4. Discussion
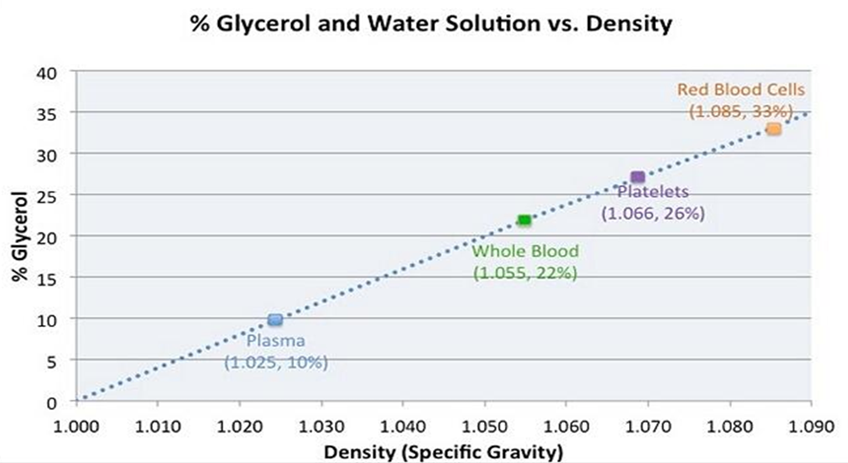
Evaluation of a novel medical device requires methodical documentation of safety and efficacy. Design is aided by proof-of-concept studies that guide further development and modification. Proof-of-concept studies also provide developers with an opportunity to determine what parameters will require final verification in larger GLP (Good Laboratory Practice) studies. Ideally, testing will not only answer critical questions regarding device efficacy and safety but will also provide information that clinicians need to determine if a device can meet their needs. Having published a prior study that indicated that the gravity fed three filtration system excluded formed thrombus from the final filtrate, did not damage porcine and human red blood cells and platelets, and did not disturb coagulation as measured by PT, PTT and INR we next chose to more specifically evaluate the filtration system’s ability to exclude manufactured metallic and fluorescent microspheres from the final filtrate. To aid with visualization, a transparent solution consisting of glycerol and water was utilized as a blood substitute. Based on Figure 5, some will argue that our choice of a 50/50 water/glycerol mixture exceeded the actual viscosity of whole blood [4]. This may well be true. We intentionally chose to use a blood analogue that exceeded the viscosity of whole blood and of PRBC so as to evaluate our filtration device under more rigid and strenuous conditions. Despite using a higher viscosity model, time to completely repeatedly filter 100 cc of the experimental mixture took 4-7 seconds (5 trials using a total of 500 cc). This corresponds to a filtration rate of >500 cc/minute.
This simple investigation has demonstrated, in a proof-of-concept model, that the proprietary blood filtration system seen in Figure 1 can remove particulate matter in a rapid fashion. Such information, combined with prior studies regarding the device’s atraumatic qualities helps the developer and end user better understand the system’s capability in enabling reinfusion of autologous whole blood in real life situations.
Conclusion:
A gravity driven three filter device can rapidly process a high viscosity whole blood equivalent fluid/particulate mixture. Results from this and prior in vitro studies indicate that such a device may prove useful for the reinfusion of autologous blood lost during invasive procedures.
DECLARATIONS
Consent:
This in vitro study did not require consent
No individual personal data was contained in this study.
Availability of Data and Materials:
The datasets used and or analyzed during the current study are available from the corresponding author on reasonable request. All data analyzed are included.
Competing Interests:
Michael Horowitz, MD (MH) is a device inventor, Co-Founder of Retriever Medical. He holds equity in Retriever Medical.
Brandon Repko, MD (BR) is a device inventor and Consultant for Retriever Medical. He holds equity in Retriever Medical.
Hieu Le (HL) is a device inventor and Vice President of Retriever Medical. He holds equitv in Retriever Medical.
Benjamin Bobo (BB) is a device inventor, Co-Founder of Retriever Medical, and CEO of Retriever Medical. He holds equity in Retriever Medical.
Henre Pande (HP) has no competing interests.
Funding:
This study was designed and funded by Retriever Medical, Inc. (Las Vegas, Nevada, USA)
Author Contributions:
Study design: MH, BR, HL and BB.
Study performance: MH, HP
Manuscript preparation: MH, BR, BB
References
- Horowitz M, Bobo B, Le H, Repko B. Extra-Corporeal Processing of Bovine, Porcine and Human Blood in Preparation for Autologous Re-Infusion Using a Disposable Filtration System: Initial Proof of Concept Study and Potential Applications for Human Autologous Blood Reinfusion in the Civilian and Combat Casualty Care Settings. Journal of Biology and Today's World 12 (2023): 001-006.
- Brookshiier KA, Tarbell JM. Evaluation of a transparent blood analog fluid: Aqueous Xanthan Gum/Glycerin. Biorheology 30 (1993): 107-116.
- Yousif MY, Holdsworth DW, Poepping TL. Deriving a blood-mimicking fluid for particle image velocimetry in Sylgard-184 vascular models. Annu Int Conf IEEE Eng Med Biol Soc (2009):1412-1415.
- Environmental Sensing Insights. Zebra Technologies (2023).


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 