Do Higher Plasma Omega-3 Levels Predispose to Prostate Cancer? Results from the Intermountain INSPIRE Biobank Registry
Jeffrey L Anderson1*, Viet T Le2, Mohit Jain3, Raymond O McCubrey1, Stacey Knight1, Daniel Bride1, Mahan Najhawan3, Khoi Dao3, Jeramie D Watrous3, Tami L Bair1, Benjamin D Horne1,4, Joseph B Muhlestein1, John F Carlquist1, Kirk U Knowlton1
1Intermountain Medical Center Heart Institute, Intermountain Healthcare, Salt Lake City, USA
2Intermountain Medical Center Heart Institute, Rocky Mountain University of Health Professionals, Utah, USA
3University of California San Diego, California, USA
4Stanford University, Stanford, California, USA
*Corresponding author: Jeffrey L Anderson, Intermountain Medical Center Heart Institute, Intermountain Healthcare, Salt Lake City, USA
Received: 09 June 2021; Accepted: 23 June 2021; Published: 25 April 2022
Article Information
Citation: Jeffrey L Anderson, Viet T Le, Mohit Jain, Raymond O McCubrey, Stacey Knight, Daniel Bride, Mahan Najhawan, Khoi Dao, Jeramie D Watrous, Tami L Bair, Benjamin D Horne, Joseph B Muhlestein, John F Carlquist, Kirk U Knowlton. Do Higher Plasma Omega-3 Levels Predispose to Prostate Cancer? Results from the Intermountain INSPIRE Biobank Registry. Cardiology and Cardiovascular Medicine 5 (2022): 206-216.
View / Download Pdf Share at FacebookAbstract
Background: Omega-3 fatty acid supplements are widely used for cardiovascular (CV) prevention. However, a prominent report proposed that total and high-grade prostate cancer (PrCA) risk increased with increasing levels of docosahexaenoic acid (DHA) and trended to with eicosapentaenoic acid (EPA).
Objective: Given public health implications, we prospectively tested this finding in the INSPIRE Registry.
Methods: Plasma samples from men enrolled in the INSPIRE Registry who developed incident PrCA during follow-up were selected and matched ~2:1 with men of similar age and entry date who were free of PrCA throughout follow-up. Plasma lipid fractions were analyzed by rapid throughput liquid chromatography-mass spectrometry. Omega-3 levels in the 2 groups were compared.
Results: We identified samples from 87 men (age: 67.3 +/- 8.6 y) who developed PrCA (at 4.7 +/- 3.7 y) and matched them to 149 samples from control men (age: 66.2 +/- 8.6 y); follow-up averaged 10.1 +/- 3.8 y and 13.5 +/- 3.7 y, respectively. Entry levels of both EPA (p=0.012) and DHA (p=0.047) were significantly lower in PrCA patients than in controls. Levels also distinguished high-grade but not fatal PrCA.
Conclusions: PrCA risk did not rise with increasing EPA and DHA levels; rather, findings suggest protective potential. These results are in keeping with the cancer safety of omega-3 supplements in recent randomized trials. However, our results go beyond supplementation to directly address the relationship of PrCA risk with circulating omega-3 levels, and they imply that supplementation may be based on potential CV benefits apart from concerns for PrCA risk.
Keywords
<p>Cardiovascular Prevention; DHA; EPA; Omega-3 Fatty Acid; Supplementation; Prostate Cancer</p>
Article Details
1. Introduction
The promotion of long-chain ω-3 polyunsaturated fatty acid (PUFA) (commonly, omega-3 fatty acid) intake for cardiovascular (CV) prevention [1, 2] has enjoyed widespread and long-held popularity and endorsement both in the form of dietary fish [3] and nutritional supplements. However, controversy remains as to the most effective formulations and doses and also as to the impact on cancer risk [4-7]. Prostate cancer (PrCA) is the most common cancer occurring in men, accounting for almost 1 in 5 new male cancer diagnoses in the United States, where an estimated 165,000 new cases and 29,000 deaths due to prostate cancer occur annually [8].
In contrast to the expectation of general health benefits from dietary and supplemental omega-3 fatty acids, a prominent analysis of the SELECT trial reported that plasma levels of long-chain fatty acids correlated positively with the development of prostate cancer [9, 10]. Countering the concerns of an association of omega-3 levels and prostate cancer risk is a notable body of evidence reporting increased fish and fish oil consumption to be protective against prostate cancer [4, 11-14]. Given these ongoing and unresolved public health questions and concerns about potential risks versus benefits of increasing consumption of omega-3 fatty acids, we undertook to prospectively test the association of plasma EPA and DHA concentrations with incident prostate cancer in men enrolled and sampled in the Intermountain Healthcare INSPIRE Registry.
2. Material and Methods
2.1 Study objectives, design and endpoints
The primary objective of this study was to compare plasma concentrations of EPA and DHA in men who subsequently developed prostate cancer to those in matched controls who didn’t throughout long-term follow-up. This case-control study prospectively tested whether men developing prostate cancer would have higher levels of EPA and DHA at baseline in samples previously collected for the INSPIRE Registry [10]. Our null hypothesis was that EPA and DHA levels are not related to increased prostate cancer risk. Secondary objectives were: 1) to compare docosapentaenoic acid (DPA) and total omega-3 fatty acids (DHA + DPA + EPA) by PrCA group, and 2) to compare plasma concentrations for PrCA that (a) were high grade and (b) that proved fatal within 5 years to that of their matched, non-PrCA controls. (All-cause death was used because we did not have access in all fatal cases to cause-specific death information, i.e., for fatal prostate cancer).
2.2 Study subjects
Subjects were selected from the Intermountain Heart Institute’s INSPIRE Registry (formerly, the Intermountain Heart Collaborative Study) [15, 16]. INSPIRE Registry subjects are adults who presented to the catheterization laboratories of LDS Hospital or Intermountain Medical Center for clinical determination of the presence and extent of coronary artery disease (CAD) by coronary angiography and who provided written informed consent for blood sampling and clinical follow-up for inclusion in cardiovascular research studies.
For this study, the INSPIRE Registry was searched for all patients who, during long-term follow-up, developed prostate cancer, as identified by ICD-9/10 diagnostic codes. These patients then were matched ~2:1 to a randomly selected cohort of men of similar age, entry date and as long or longer follow-up days, but who were free of prostate cancer during long-term follow-up. While we attempted to match each case to 2 controls, a few lacked a second control match, and for others plasma sample testing failed quality control, resulting in an average of 1.7 controls to each case. This INSPIRE Registry study complied with the Declaration of Helsinki and was approved by the Intermountain Healthcare Institutional Review Board with a waiver of consent for the present analysis.
2.3 Plasma fatty acid sampling, storage, and measurement
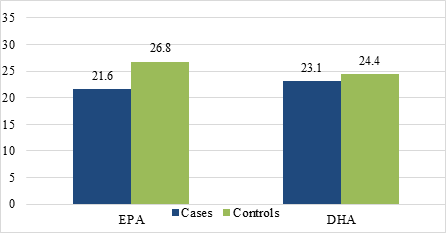
At the entry healthcare encounter, fasting blood was obtained, placed on ice, and transferred to the Intermountain Molecular Biology Laboratory, where plasma was separated and stored at -20 degrees C until analysis. Determinations of DHA, EPA, and DPA were performed at the University of San Diego’s metabolomics laboratory. Plasma lipid fractions were analyzed by rapid throughput liquid chromatography-mass spectrometry (LC-MS) and measured in arbitrary spectral units (ASU) as previously described [17]. In order to provide a reference to plasma concentrations for fatty acids, 100 samples, randomly selected from our plasma bank with ASU results, were analyzed for EPA and DHA by Boston Heart Diagnostics. Using these results, the following conversion equation was generated for EPA: EPA concentration (mcg/ml) = 1.642 + 0.8154 x ASU (x 10-7), yielding an average EPA concentration in our case and control cohorts of 21.52 and 27.07 mcg/ml, respectively. For DHA, the conversion equation was: -12.560 + 2.598 x ASU (x 10-7), which yielded average concentrations in cases and controls of 49.13 and 55.03 mcg/ml, respectively. All analyses were done using the ASU for comparisons both as continuous variables and categorized as quartiles.
2.4 Statistical considerations
Continuous variables are summarized as means ± standard deviations for normally distributed variables and as medians with interquartile ranges for non-normally distributed ones. Discrete variables are summarized as frequencies. Fatty acid levels in the 2 groups were compared by the Wilcoxon rank sum test. Other comparisons used t-tests and chi-square analysis as appropriate for continuous outcomes and logistic regression for cancer outcomes. Multivariable analyses were done adjusting for baseline differences. Analyses were performed in R (version 4.0.3). Two-tailed p-values are presented, with 2-sided p≤0.05 designated as significant for the primary comparisons.
3. Results
3.1 Characteristics of case and control subjects
Plasma samples from 87 men (age: 67.3 +/- 8.6 y) were identified in the INSPIRE Registry database who developed PrCA during long-term follow-up (at 4.6 +/- 3.7 y). These were matched to 149 samples from control men who did not. Characteristics of cases and controls are compared in Table 1 and were similar except for a history of hyperlipidemia. Race was predominantly European/Caucasian in both groups (>95%). Over one-fifth in both groups were smokers. Follow-up averaged 10.1 years in prostate cancer cases and 13.5 years in controls.
3.2 Plasma omega-3 concentrations in cases and controls
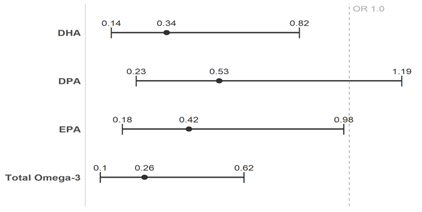
Entry plasma concentrations of DHA, DPA, and EPA for cases and control subjects are shown in Table 2 and Figure 1. Concentrations of both EPA (p=0.012) and DHA (p=0.047) were significantly lower in PrCA patients than in age- and entry-matched control men and trended lower with DPA (p=0.070). The odds ratios and 95% confidence intervals for developing prostate cancer, comparing highest versus lowest quartile of omega-3 concentrations, are shown in Table 3 and Figure 2 and demonstrated that for DHA (p = 0.016), EPA (p = 0.047), and total omega-3 concentrations (p = 0.002), those in the highest quartile had significantly lower risk of developing prostate cancer relative to those in the lowest quartile, and risk trended to be lower for DPA (p = 0.120). Full adjustment, including for hypertension, hyperlipidemia, diabetes, BMI, and obstructive coronary artery disease (present/absent) did not appreciably change the odds ratios for DHA, EPA, or DPA (Table 3, Figure 2).
3.3 Total omega-3 concentrations by prostate cancer status
Total omega-3 levels, defined as the sum of DHA, DPA, and EPA concentrations, are compared by incident prostate cancer status in Table 3 and Figure 2. Results showed that higher total omega-3 levels were significantly associated with reduced PrCA risk. The adjusted model found the odds of having PrCA to be 0.26 (95% CI: 0.10, 0.62) for those in the highest quartile of omega-3 compared to the lowest quartile.
3.4 Plasma omega-3 and prostate cancer grade and mortality
Table 4 presents results of omega-3 concentrations by PrCA grade (A) and fatality (5-year mortality) (B). Results showed that all 3 omega-3 analyte and total omega-3 concentrations were significantly lower in individuals with high grade cancer versus their matched controls. Omega-3 concentrations in those with fatality were not significantly different than their matched controls.
|
Characteristic |
Prostate Cancer Cases |
Controls |
P-value |
|
N |
87 |
149 |
|
|
Age, years (mean +/- SD) |
67.3 +/- 8.6 |
66.2 +/- 8.6 |
0.32 |
|
Race (N, % Caucasian) |
83 (95.4) |
143 (96.0) |
1.00 |
|
Current/former smokers (N, %) |
17 (19.5) |
35 (23.5) |
0.59 |
|
BMI (mean +/- SD) |
30.1 +/- 16.7 |
28.5 +/- 5.0 |
0.38 |
|
History of Hypertension (N, %) |
78 (89.7) |
122 (81.9) |
0.16 |
|
History of Hyperlipidemia (N, %) |
64 (73.6) |
68 (45.6) |
<0.001 |
|
History of Diabetes (N, %) |
25 (28.7) |
33 (22.1) |
0.33 |
|
CAD Status (N, %) |
49 (56.3) |
95 (63.8) |
0.32 |
|
Years of follow-up (mean +/- SD) |
10.1 +/- 3.8 |
13.5 +/- 3.7 |
<0.001 |
|
Years to PrCA diagnosis (mean +/- SD) |
4.7 +/- 3.7 |
-- |
-- |
Table 1: Characteristics of Prostate Cancer Incident Cases and Controls.
|
DHA:a |
||||||
|
Group |
Min. |
Q1 |
Median |
Mean |
Q3 |
Max. |
|
Cases |
13.19 |
19.29 |
23.09 |
23.74 |
26.54 |
50.28 |
|
Controls |
12.73 |
19.83 |
24.42 |
26.02 |
32.68 |
50.15 |
|
DPA: |
||||||
|
Group |
Min. |
Q1 |
Median |
Mean |
Q3 |
Max. |
|
Cases |
2.73 |
4.60 |
5.94 |
5.99 |
7.32 |
11.73 |
|
Controls |
2.10 |
4.86 |
6.24 |
6.66 |
7.97 |
13.99 |
|
EPA:b |
||||||
|
Group |
Min. |
Q1 |
Median |
Mean |
Q3 |
Max |
|
Cases |
5.45 |
15.93 |
21.61 |
24.38 |
31.57 |
77.81 |
|
Controls |
4.51 |
16.98 |
26.77 |
31.18 |
41.06 |
103.61 |
|
a, b Includes acid and acetate of DHA and EPA, respectively. Results in arbitrary spectral units x 10-7. |
||||||
Table 2: Baseline (entry) concentrations of DHA and EPA in PrCA cases and controls.
|
Omega-3 metabolite |
Unadjusted |
Adjusted |
||
|
Odds Ratio |
95% CI |
Odds Ratio |
95% CI |
|
|
DHA |
0.36 |
[0.15, 0.81] |
0.34 |
[0.14, 0.82] |
|
DPA |
0.54 |
[0.24, 1.17] |
0.53 |
[0.23, 1.19] |
|
EPA |
0.44 |
[0.19, 0.98] |
0.42 |
[0.18, 0.98] |
|
Total Omega-3 |
0.26 |
[0.11, 0.59] |
0.26 |
[0.10, 0.62] |
Table 3: Unadjusted and fully adjusted hazard ratios and 95% confidence intervals for developing prostate cancer, comparing highest versus lowest quartile of omega-3 concentrations.
*Age, entry date and follow-up time-matched case and control cohorts were further adjusted for smoking, race, hypertension, hyperlipidemia, diabetes, body mass index (BMI), coronary artery disease status.
|
Omega-3 |
High-grade PrCA |
Controls |
P-value |
|
N |
26 |
42 |
|
|
DHA |
23.63 +/- 7.29 |
27.81 +/- 9.25 |
0.046 |
|
DPA |
5.57 +/- 1.82 |
7.04 +/- 2.44 |
0.02 |
|
EPA |
22.09 +/- 10.60 |
33.67 +/- 18.50 |
0.005 |
|
Total |
51.29 +/- 18.96 |
68.52 +/- 28.14 |
0.006 |
|
Omega-3 |
Fatal Cases |
Controls |
P-value |
|
N |
17 |
28 |
|
|
DHA |
24.14 +/- 4.34 |
25.67 +/- 8.17 |
0.668 |
|
DPA |
5.49 +/- 1.76 |
6.26 +/- 2.51 |
0.39 |
|
EPA |
22.33 +/- 9.70 |
29.30 +/- 17.72 |
0.259 |
|
Total |
52.03 +/- 15.00 |
61.89 +/- 26.38 |
0.363 |
Table 4: Plasma omega-3 concentrations in high-grade PrCA and fatal* cases versus their controls.
4. Discussion
4.1 Summary of study findings
Use of high throughput liquid chromatography-mass spectroscopy is increasingly being used as a method to explore in a targeted or non-targeted fashion the relationship of a multitude of metabolites, including lipid metabolites, to physiologic and disease processes [18, 19]. Using this method, our prospective test of the reported positive association of EPA and DHA plasma levels with PrCA incidence failed to confirm an adverse association. Rather, our findings suggest a potential protective effect. Moreover, our results go beyond ω-3 PUFA supplementation to directly address the impact on PrCA risk of circulating omega-3 levels themselves, irrespective of how they were attained.
4.2 Literature comparisons and meta-analysis
Our findings are consistent with recent randomized trials of omega-3 supplementation (JELIS, VITAL, REDUCE-IT), which did not note increased cancer or non-CV death risks with supplementation [5, 6, 20] although prostate cancer was not specifically addressed. In contrast, an analysis of the SELECT trial reported that plasma levels of long-chain ω-3 PUFA correlated positively with the development of PrCA, i.e., both total and high-grade cancer risk increased with increasing levels of DHA and trended to increase with EPA [10]. The authors supported their findings in SELECT with a meta-analysis of earlier studies, consisting of their earlier trial [9] and 5 other smaller studies [10]. In contrast to concerns of an association of omega-3 levels and PrCA risk is a body of evidence supporting increased ω-3 PUFA in fish and fish oil consumption as protective against PrCA [4, 11-14]. Further complicating the association between omega-3 fatty acids and PrCA are the relationships among dietary sources (fish) versus oral supplements, oral intake versus plasma concentrations, plasma versus prostate tissue concentrations, in vivo metabolic interconversions [4], and questions of associations versus causation. Differing results of cross-sectional versus prospective trials and of associations with PrCA incidence versus stage versus mortality also add uncertainty to a net benefit/risk assessment [4]. Our study adds reassurance that EPA and DHA do not add substantial risk to prostate cancer and may be protective. Evidence for the impact of DPA is less conclusive; however, with emerging evidence favoring EPA over mixed EPA and DHA supplements for cardiovascular prevention, data for EPA become particularly relevant [6, 7, 20].
4.3 Mechanisms
A number of beneficial mechanisms of long-chain ω-3 PUFA on CV health have been proposed, including anti-inflammatory, anti-thrombotic, membrane-stabilizing, and anti-arrhythmic effects [2, 4]. Although the true CV benefit of standard omega-3 fatty acid supplements recently has been called into question [5, 7], it remains mechanistically unclear why long-chain ω-3 PUFA should increase the risk of prostate cancer. Nevertheless, the reported findings of the SELECT and earlier trials have invited mechanistic considerations. Why the adverse effect should be relatively specific to PrCA among other cancers also is unclear. However, the biology of nutritional supplements is complex, and clinical outcomes are unpredictable and require direct investigation. Recent trials [6, 7, 20] and basic research have suggested potential differential pathophysiological effects of EPA and DHA based on their contrasting plasma membrane effects [21]: EPA preserves membrane structure and cholesterol distribution and inhibits lipid oxidation and cholesterol crystal formation, impacting signal transduction for inflammation and vasodilation. In contrast, DHA, which is concentrated in brain and retina, increases membrane fluidity, disorders lipid structure, and reduces antioxidant effects. In this regard, the SELECT trial results did not find significant correlations between plasma EPA and either low-grade or high-grade PrCA, whereas DHA was linked to high-grade but not low-grade PrCA risk. However, our data do not support an adverse effect on PrCA incidence and grade of either EPA or DHA, with both showing preventive potential (Figure 2). Therefore, additional clinical trials and basic research will be needed to determine (patho)physiological mechanisms specifically related to ω-3 PUFA and prostate health and disease.
4.4 Implications
These results suggest that supplementation of long-chain ω-3 PUFA may be based on their potential CV benefits apart from concerns for PrCA risk. Given recent trial evidence arguing against an important CV protective effect of mixed omega-3 fatty acids, e.g., as fish oil supplements [5, 7], evidence for the safety and efficacy of EPA specifically becomes of particular importance [6, 20]. Our study argues against withholding EPA supplementation because of safety concerns for an increased risk of PrCA in men.
4.5 Limitations
Study limitations include those of observational studies, including the risk of uncontrolled confounding. This was addressed by using a matched case-control design and further adjusting for relevant baseline demographics. Another limitation is its moderate sample size, limiting the power for subgroup analyses. Also, findings are limited to subjects primarily of European (Caucasian) ancestry. We used ASU rather than plasma concentrations in our analyses and presentation of results to preserve internal consistency. We did not have serial samples to account for temporal changes in omega-3 fatty acids although these levels tend to be stable over time. Strengths include prespecified hypothesis testing applied to a prospectively enrolled cohort of CV registry subjects with long-term follow-up through comprehensive electronic medical records and entry plasma samples, which provide superior ability over supplement trials and dietary questionnaires to ascertain circulating omega-3 analyte levels.
5. Conclusion
In this moderate-sized case-control study with long-term follow-up, we found that EPA and DHA levels were not positively related to incident PrCA risk or severity; rather, findings suggested protective potential for EPA and DHA. These results are consistent with recent randomized trials of omega-3 supplementation, which did not note increased cancer or non-CV death risk. Our findings add to these by specifically addressing PrCA risk. Moreover, results go beyond supplementation by directly addressing the impact on PrCA risk of circulating omega-3 levels themselves, and they imply that supplementation can be based on potential CV benefits apart from concerns for PrCA risk.
Acknowledgments
Conflicts of interest: Authors affirm no financial conflicts of interest relevant to this manuscript.
Funding: This study was supported by internal departmental funds. It did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
References
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354 (1999): 447-455.
- Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58 (2011): 2047-2067.
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 74 (2019): e177-e232.
- McCarty MF, DiNicolantonio JJ, Lavie CJ, O'Keefe JH. Omega-3 and prostate cancer: examining the pertinent evidence. Mayo Clin Proc 89 (2014): 444-450.
- Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N Engl J Med 380 (2019): 23-32.
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 380 (2019): 11-22.
- Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH randomized clinical trial. JAMA 324 (2020): 2268-2280.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 68 (2018): 7-30.
- Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, et al. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol 173 (2011): 1429-1439.
- Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst 105 (2013): 1132-1141.
- Torfadottir JE, Valdimarsdottir UA, Mucci LA, Kasperzyk JL, Fall K, Tryggvadottir L, et al. Consumption of fish products across the lifespan and prostate cancer risk. PLoS One 8 (2013): e59799.
- Wynder EL, Fujita Y, Harris RE, Hirayama T, Hiyama T. Comparative epidemiology of cancer between the United States and Japan. A second look. Cancer 67 (1991): 746-763.
- Dewailly E, Mulvad G, Pedersen HS, Hansen JC, Behrendt N, Hansen JPH. Inuit are protected against prostate cancer. Cancer Epidemiol Biomarkers Prev 12 (2003): 926-927.
- Prener A, Storm HH, Nielsen NH. Cancer of the male genital tract in Circumpolar Inuit. Acta Oncol. 35 (1996): 589-593.
- Taylor GS, Muhlestein JB, Wagner GS, Bair TL, Li P, Anderson JL. Implementation of a computerized cardiovascular information system in a private hospital setting. Am Heart J 136 (1998): 792-803.
- Muhlestein JB, May HT, Bair TL, Prescott MF, Horne BD, White R, et al. Relation of elevated plasma renin activity at baseline to cardiac events in patients with angiographically proven coronary artery disease. Am J Cardiol 106 (2010): 764-769.
- Watrous JD, Niiranen TJ, Lagerborg KA, Henglin M, Xu YJ, Rong J, et al. Directed non-targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipids. Cell Chem Biol 26 (2019): 433-442.
- Cheng S, Shah SH, Corwin EJ, Fiehn O, Fitzgerald RL, Gerszten RE, et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ Cardiovasc Genet 10 (2017): e000032.
- Demler OV, Liu Y, Luttmann-Gibson H, Watrous JD, Lagerborg KA, Dashti H, et al. One-year effects of omega-3 treatment on fatty acids, oxylipins, and related bioactive lipids and their associations with clinical lipid and inflammatory biomarkers: Findings from a Substudy of the Vitamin D and Omega-3 Trial (VITAL). Metabolites 10 (2020): 431.
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369 (2007): 1090-1098.
- Mason RP, Libby P, Bhatt DL. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler Thromb Vasc Biol 40 (2020): 1135-1147.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 