Oncostatin M, Serpins, and Oxidative Stress in Extracellular Matrix Remodeling and Arteriovenous Fistula Maturation
Nathaniel DeMarco1, Vikrant Rai1, Daniel R Wilson1, Devendra K Agrawal1*
1Department of Translational Research, College of Osteopathic Medicine of the Pacific Western University of Health Sciences, Pomona, CA 91766
*Corresponding author: Devendra K Agrawal, Professor and Director, Department of Translational Research, Western University of Health Sciences, 309 E. Second Street, Pomona, California 91766-1854, USA.
Received: 03 April 2023; Accepted: 10 April 2023; Published: 20 April 2023
Article Information
Citation:
Nathaniel DeMarco, Vikrant Rai, Daniel R Wilson, Devendra K Agrawal. Oncostatin M, Serpins, and Oxidative Stress in Extracellular Matrix Remodeling and Arteriovenous Fistula Maturation. Cardiology and Cardiovascular Medicine. 7 (2023): 129-140.
View / Download Pdf Share at FacebookAbstract
End-stage renal disease is a crippling diagnosis that generally requires dialysis to prolong life. To facilitate filtration of patient’s blood in dialysis, surgical formation of an arteriovenous fistula (AVF) is commonly performed. Maturation of the AVF is required to allow for successful dialysis. However, AVFs commonly fail to mature, leading to the fistula closure, the necessity for another fistula site, and markedly increased morbidity and mortality. The current literature concerning molecular mechanisms associated with AVF maturation failure supports the role of inflammatory mediators involving immune cells and inflammatory cytokines. However, the role of oncostatin M (OSM), an inflammatory cytokine, and its downstream targets are not well investigated. Through inflammation, oxidative stress, and hypoxic conditions, the vascular tissue surrounding the AVF undergoes fibrosis, stenosis, and wall thickening, leading to complete occlusion and nonfunctional. In this report, first we critically review the existing literature on the role of OSM in the most common causes of early AVF failure - vascular inflammation, thrombosis, and stenosis. We next consider the potential of using OSM as a therapeutic target, and finally discuss therapeutic agents targeting inflammatory mediators involved in OSM signaling to potentiate successful maturation of the AVF.
Keywords
<p>Arterio-Venous Fistula; Hypoxia; Inflammation; Oncostatin M; Oxidative Stress; Stenosis; Thrombosis</p>
Article Details
1. Introduction
Chronic kidney disease (CKD) is highly prevalent disease within the US, affecting 1 in 7 adults [1]. In a recent study from 2019, the global estimated prevalence of CKD is 13.4%, and the combined effect of cardiovascular risk shows a direct effect on the global burden of morbidity and mortality worldwide [2]. As of 31 December 2018, approximately 0.7 million patients (785,883) were under treatment for end-stage renal disease (ESRD – GFR < 15mL/min) in the United States. This prevalence has more than tripled between 1990 and 2018 [1]. The causes of CKD are variable; however, diabetes mellitus and hypertension are among the critical factors leading to general hyperfiltration of the nephrons and their eventual destruction, culminating in kidney failure. As of now, there are two standard forms of treatment for ESRD: kidney transplant and dialysis. For the later, there are additional subcategories, peritoneal dialysis, and hemodialysis. With respect to hemodialysis, one procedure involves creation of an arteriovenous fistula (AVF), usually on the patient’s forearm by which to connect an external filter. This allows for direct connection of the arterial and venous systems, a high to low pressure system. Maturation of the AVF is defined as the complete opening of fistula. However, Maturation of the AVF is the complete opening of the fistula, as is essential to allow for the proper flow of filtered blood back into the body. This process takes ~4-6 months following the surgical procedure. However, complications often arise in the form of non-maturation of the AVF and resultant fistula closure [3]. Such failure occurs in approximately 28-53% of patients [4]. With such failure, further procedures must be done to open more sites for potential dialysis, which entail increased patient morbidity and mortality. Of note, when investigating the causes of AVF maturation failure, the crosstalk between the chronic inflammatory processes plays a critical role in the early AVF closure. The inflammatory response to tissue injury is characterized by the induction of cytokines and chemokines that serve different functions at the site of injury. The pro-inflammatory cytokines involved in this process include tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 [5, 6]. The innate and adaptive immune response and perivascular inflammation play critical roles in the pathogenesis of AVF maturation or failure [7-11]. However, the role of oncostatin M (OSM) has not been discussed in the literature, even though it is an IL-6-like cytokine that is involved in the pathogenesis of a variety of inflammatory diseases [12] including thrombosis and atherosclerosis, and is also a marker for coronary artery disease [13, 14]. Since chronic inflammation plays a critical role in AVF maturation failure, OSM involved in thrombosis and atherosclerosis may play a crucial role in this process. This review comprehensively discusses the role of OSM in vascular disease and proposes that its potential role in AVF maturation failure may be a target for future therapies that promote AVF maturation by modulating OSM and its downstream signaling processes.
2. AVF Maturation and Failure
Of studies involving arteriovenous fistulas, most concentrate on the clinical classification of AVF maturation. The clinical definition of successful AVF maturation in humans is when the fistula maintains a flow of 600 mL/min post 6 weeks of surgery, is located a maximum of 6mm from the skin, and maintains a diameter of >6 mm [15], whereas there is no clinical definition of failure. However, there are conserved mechanisms through which AVF maturation failure may occur. For example, failed AVF has been shown to exhibit early thrombosis to which ESRD patients are already subject to an increased risk due to metabolic abnormalities [16]. Additionally, this hypercoagulable state might be contributed to further through the actions of downstream pro-inflammatory mediators, such as plasminogen activator inhibitor-1 (PAI-1) [17]. Hypercoagulable states that cause early vessel thrombosis due to inflow problems (juxta-anastomosis stenosis or accessory vein) are the most common cause of AVF maturation failure [18]. In addition to early thrombosis, vessel wall thickening due to neointimal hyperplasia can directly contribute to the narrowing of both the fistula and the outflow vein. In response to injury by way of surgical creation of the AVF, with a well-regulated inflammatory response can occur. With this, the adventitial myofibroblast undergoes signaling to form collagen, matrix metalloproteinases (MMPs), and collagen to strengthen the fistula and outflow vessel wall [19]. But dysfunction of inflammatory responses and/or prolonged inflammation results in excessive neointimal hyperplasia (NIH) and eventual fistula stenosis or closure. In conjunction with this finding, the deposition of collagen can be seen with initial fibrotic changes in the vessel wall that result in the stiffening of the vasculature. Martinez et al. [20] established that an increase in medial fibrosis through excessive fibrotic remodeling was a key risk factor in non-maturation of the AVF due to a decrease in vascular elasticity and distensibility that compromises high blood flow. Since inflammation underlies the pathogenesis of thrombosis, NIH, and stenosis, OSM being a pro-inflammatory cytokine may be involved in these pathologies.
3. Oncostatin M (OSM)
Oncostatin M (OSM) is a member of the IL-6 family of cytokines and is primarily known for its effects on cell growth [21]. While cell growth is one of its more characteristic effects, OSM has been shown to also influence various functions and pathological processes in multiple body systems [22]. These effects are transduced from signaling via receptor complexes composed of gp130 and a ligand-specific receptor subunit [23, 24]. The binding of gp130 may occur weakly; however, it is the LIFR and OSM receptor binding that maintains a high binding affinity [25-28]. Since gp130 is ubiquitously expressed in all tissues, LIFR and OSMR expression allows for effective, cell-specific effects of OSM [22]. For example, OSMR is highly expressed in fibroblasts, endothelial cells, smooth muscle cells, osteoblasts, adipocytes, hepatocytes, mesothelial cells, glial cells, and epithelial cells, which allow the functional diversity that OSM has been shown to have [23, 24]. The signal transduction pathway of OSMR is initiated by Janus kinases (JAKs), which transphosphorylate each other at conserved tyrosine residues. This phosphorylation forms a docking site for signal transducers and activator of transcription (STATs). With this, JAKs phosphorylate STATs to form hetero- or homodimers where they may translocate to the nucleus to act as transcription factors [29]. OSM, produced by T cells and macrophages, plays a key role in regulation of inflammation in the event of tissue damage [23, 30]. When inflammation becomes chronic, pathological conditions often arise in the form of tuberculosis, ARDS, autoimmune diseases, inflammatory bowel disease, and atherosclerosis [13, 14, 31]. With our interest on vasculature tissue injury, a brief review of the progression of atherosclerosis and its close association with OSM is pertinent.
3.1 OSM and Inflammation
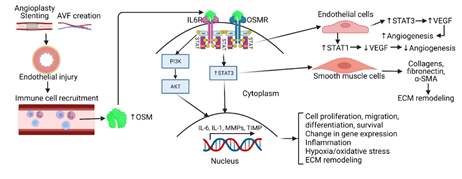
Tissue injury and subsequent repair is a dynamic process that involves inflammation and eventual regeneration. However, if this response becomes chronic and dysregulated it may lead to fibrosis, as well as other organ dysfunctions [32]. In the inflammatory response, various chemokines and cytokines are secreted by recruited immune cells to modulate the repair response. Among these molecules is oncostatin-M, an IL-6-like cytokine, involved in growth regulation, differentiation, gene expression, and cell survival, as well as tissue remodeling [12]. Under inflammatory conditions (Figure 1), OSM is secreted by monocytes and polymorphonuclear cells (PMNs) [33, 34]. Once expressed, OSM functions are mediated by interactions with the OSM receptor (OSMR), with signals transduced by JAK/STAT3 pathways. Additionally, through signaling cascades involving this JAK/STAT motif, OSM through OSMR strongly induces STAT3 activation and increases cardiovascular angiogenesis through actions of vascular endothelial growth factor (VEGF) promoting an increase in blood flow [28] (Figure 1). Later studies indicate a modulatory relationship between OSM and VEGF, in which STAT3 increases VEGF expression, but STAT1 inhibits VEGF expression [35]. Additionally, OSM has been shown to contribute to cardiovascular extracellular matrix (ECM) deposition through the up- regulation of tissue inhibitors of matrix metalloproteinases (TIMPs) [36] (Figure 1). These studies have shown that smooth muscle cells (SMCs) deficient in OSMR-b have decreased sizes of necrotic areas in response to tissue injury. This suggests that OSM is implicated in the proliferation and migration of SMCs during injury [37]. Although OSM has been shown to increase angiogenesis, the necrotic areas that arise during injury show decreased vasculature which induce a subsequent increase in oxidative stress.
Figure 1: Oncostatin-M (OSM) secretion, downstream signaling, and regulation of molecular functions. Vascular tissue manipulation through stenting or arteriovenous fistula (AVF) creation leads to endothelial injury and subsequent immune cell recruitment including monocytes, macrophages, and neutrophils. The recruited immune cells secrete OSM which on binding with OSM-receptor (OSMR) leads to activation of downstream signaling that involve signal transducers and activator of transcription (STAT)3, phosphatidylinositol 3-kinases (PI3K), and protein kinase B (PKB/Akt). This regulates various molecular and cellular events including angiogenesis, extracellular matrix (ECM) remodeling, cell proliferation, migration, change in gene expression, and inflammation. Interleukin 6 receptor (IL6R), vascular endothelial growth factor (VEGF), and alpha-smooth muscle actin (α-SMA).
3.2 OSM and Oxidative Stress
With increased oxidative stress, there is potential for the surrounding ECM to undergo remodeling. This process is normal within the context of embryonic development, with importance in cell growth, differentiation, survival, and morphogenesis [38]. However, with improper regulation, pathological conditions may arise. One such condition is known as fibrosis, involving excessive ECM deposition, scar formation, and eventual impairment of organ function [35, 36]. Of course, fibrosis is a condition marked by high levels of oxidative stress, seen in both cardiac and pulmonary models [39, 40]. Past studies have shown that deposition of collagen to the ECM depends on the severity of oxidative stress present, with higher oxidative stress levels favoring collagen accumulation [41-43]. Additionally, the stability of key ECM components, such as fibronectin, has been implicated in the production of reactive oxygen species (ROS) [41, 44, 45]. The modifications made through oxidative stress reduced cell adhesion and increased the proliferation of smooth muscle cells in vitro, suggesting oxidative stress plays a key role in ECM function, organization, and stability [45]. Correlation of ROS and ECM remodeling is significant to discuss and investigate in the context of OSM and AVF maturation because of the strong association of fibrosis with AVF maturation failure [20, 46] and OSM with ECM remodeling in various diseases, such as scleroderma, idiopathic pulmonary fibrosis, and, despite a paucity of literature, likely in correlation with AVF as well [47, 48]. Looking at inflammation in atherosclerotic lesions, the major sources of oxidative stress are mitochondria and non-phagocytic NADPH oxidase [49, 50]. The reactive oxygen species (ROS) that arise are associated with many diseases, including many cardiovascular diseases. Within mitochondria, ROS are produced via the reduction of oxygen to the molecule O2•- [51]. Past studies have shown that the major producers of O2•- are complex I and complex III of the electron transport chain [52-54]. Through this increase in mitochondrial ROS, greater VSMC proliferation was seen, leading to the formation of plaques [55]. However, it has also been documented that mitochondrial dysfunction is implicated in ischemic injury via plaque rupture through mechanisms involving the opening of the permeability transition pore (PTP) [56-58]. When the PTP opens for a prolonged period, mitochondrial matrix swelling occurs with an eventual rupture of the mitochondrial membrane [51]. Rupture of the membrane causes a release of proapoptotic molecules in the intermembrane space, leading to a caspase-dependent and caspase-independent cell death with eventual atherosclerotic plaque rupture [51, 59]. In plaques susceptible to rupture, there is typically a necrotic core with a thin, fibrous cap containing macrophages and T-lymphocytes, which correlates with the inflammatory basis of atherosclerosis [60]. Additionally, the plaques have arterial walls that thicken beyond the diffusion limit for oxygen, leading to hypoxic regions [61]. These hypoxic regions lead to increased expression of the transcription factor, hypoxia-inducible factor-1 (HIF-1), which plays a key role in adaptation to hypoxic conditions [62]. In hypoxic conditions, HIF-1α, one of the two heterodimer subunits of HIF-1, stabilizes and translocates to the nucleus to heterodimerize with HIF-1β. With normal adaptation, HIF-1 regulates multiple genes involved in neovascularization, cell proliferation, survival, and cell recruitment [63-65]. In human atherosclerotic plaques, HIF-1α is closely associated with macrophages and associated with an inflammatory plaque phenotype [61, 66]. This suggests that HIF-1α, along with increased ROS, leads to plaque instability. However, the specific molecular mechanism for this phenomenon is not well-studied. Immune cell infiltration, vascular SMCs, and endothelial cells play an important role in AVF vessel wall remodeling and maturation and hypoxia is a common deterrent. Additionally, the effects of OSM are context dependent. For example, past studies have shown the pro-inflammatory effects of OSM within the CNS, as well as its neuroprotective effects in mitochondrial dysfunction. Additionally, OSM has been shown to induce a hypoxic state in hepatic cells, producing a chronic inflammatory phenotype [67-69]. Thus, the effect of the OSM-hypoxia axis mediated by mitochondrial dysfunction on AVF maturation should be investigated further to uncover the specific mechanisms within the context of AVFs.
4. OSM Signaling, Serpins, and AVF Maturation
4.1 Serpins and Vascular Disease
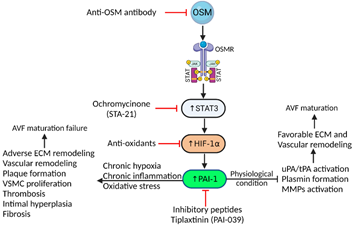
From the onset of tissue injury to the subsequent increase in oxidative stress effects of OSM have been shown to go a step further to induce plasminogen activator inhibitor-1 (PAI-1), a regulator of fibrinolysis and proteolysis [70, 71]. PAI-1, when present at elevated levels within fibrotic tissues, prevents proteolytic activities and contributes to decreased collagen degradation and tissue fibrogenesis [72]. The cross-play between elevated ROS and fibrogenesis in different organs is due to activation of TGF-β, a collagen stimulator, as well as PAI-1 expression [73-75]. These processes can be seen within the context of skin and lung fibrosis. Additionally, hypoxia plays a significant role in fibrogenesis [76]. Previous studies have shown that hypoxia and oxidative stress go hand in hand, as hypoxia causes mitochondrial dysfunction which leads to increased production of ROS. Additionally, hypoxia-inducible factor-1 alpha (HIF-1α) protein production is increased [77]. This increase in HIF-1α leads to an increase in PAI-1 [78, 79] (Figure 2). All these processes ultimately contribute to fibrosis, ECM remodeling, and subsequent instability and dysfunction of intimal tissue and eventual formation of atherosclerotic plaques. While the fibrotic effects of PAI-1 have been well characterized in a variety of tissues, little is known about its effects on ECM remodeling and fibrosis in vascular tissue. When looking specifically at AVF maturation failure, past studies have shown increased expression of HIF-1α, VEGF, MMP-2, as well as TIMP-1 along with the stenosed AVF outflow veins in rats showcasing a typical phenotype for AVF maturation failure [80]. The role of PAI-1 in vascular remodeling, thrombosis, and cardiovascular disease is summarized below in Table 1.
|
Reference |
Aim of the study |
Experimental strategy |
Outcome |
|
[81] |
To investigate the effects of recombinant PAI-1 on intimal hyperplasia after intimal injury |
Rats with carotid artery injury were injected mutant form of PAI-1 intraperitoneally and intimal hyperplasia was examined |
Recombinant PAI-1 inhibits proteases and binding of VSMCs with vitronectin, thereby decreasing VSMCs migration. Recombinant PAI-1 attenuates intimal hyperplasia after intimal injury |
|
[82] |
To investigate the effects of PAI-1 on the mural, adaptive response to hypertension |
Patients with essential hypertension and gender-matched normotensive patients had brachial intima-media thickness (IMT), flow-mediated dilation (FMD), and PAI-1 antigen in blood measured. |
IMT and FMD correlated positively in hypertensive patients, FMD correlated inversely with wall stress, and IMT correlated inversely with PAI-1. This supports the hypothesis that PAI-1 attenuates increases in neointimal vascular smooth muscle cellularity |
|
[83] |
To investigate the hypothesis that PAI-1 inhibition prevents neointimal hyperplasia after arterial injury |
A wire-injury model was made in WT and PAI-1 (-/-) mice. The mice were injected intraperitoneally with IMD-0354, an IKK inhibitor, and intimal hyperplasia was examined |
Thickened intima was observed in WT arteries, while thickening was suppressed in PAI-1 (-/-) arteries. PAI-1 is an essential factor in the progression of vascular remodeling and its inhibition may prevent restenosis after arterial injury |
|
[84] |
To determine the vein wall response when exposed to increased and decreased plasmin activity |
Stasis thrombi were created in a mouse IVC ligation model in uPA (-/-) and PAI-1 (-/-) |
Thrombi were larger in uPA (-/-) mice and smaller in PAI-1 (-/-) mice with 8-day plasmin levels increased three-fold compared with WT. Therefore, plasmin activity is critical for thrombus resolution and PAI-1 plays a role in the prolongation of thrombi. |
|
[85] |
To investigate the role of the plasminogen activator system in coronary vascular remodeling during long-term nitric oxide synthase inhibition |
WT, PAI-1 (-/-), and t-PA (-/-) mice were treated with N(omega)-nitro-L-arginine methyl ester (L-NAME) |
PAI-1 deficiency protects against L-NAME-induced hypertension and perivascular fibrosis |
|
[86] |
To investigate the regulation of vein-graft (VG) thrombin activity by PAI-1 |
VGs from WT, PAI-1 (-/-), and PAI-1 transgenic mice were implanted into WT, PAI-1 (-/-), and PAI-1 transgenic arteries. VG remodeling was then assessed 4 weeks after. |
Thrombin activity and thrombin-induced proliferation of PAI-1 deficient venous smooth muscle cells (SMCs) were significantly greater than that of WT SMCs. Thus challenging the hypothesis that PAI-1 drives non-thrombotic obstructive diseases. |
|
[87] |
To investigate the role of PAI-1 in the progression of venous thromboembolism (VTE) in pancreatic cancer patients |
Plasma levels of PAI-1 were measured via ELISA in pancreatic cancer patients and mice bearing human tumors. The resolution of an acquired VTE was measured in the mice. |
Mice bearing PANC-1 tumors had the highest levels of PAI-1 and exhibited impaired venous thrombus resolution 8 days after IVC stasis when compared with nontumor controls. |
|
[88] |
To investigate the specific determinants of clot lysis time (CLT) |
Plasma levels of PAI-1, plasminogen, thrombin-activatable fibrinolysis inhibitor (TAFI), prothrombin, and a2-antiplasmin were measured in thrombosis patients and healthy control subjects. |
After adjusting for acute-phase proteins, TAFI and PAI-1 remained associated with thrombosis. |
Table 1: Key findings of PAI-1 in vascular remodeling, thrombosis, and cardiovascular diseases.
Of note, the findings from the studies reported in Table 1 also suggest the paradoxical dual role of PAI-1, in both inhibiting NIH and promoting maladaptive remodeling [89]. Thus, it is imperative to discuss the relationship between OSM and PAI-1 in the context of vascular disease and AVF maturation. Investigating the role of PAI-1 in AVF maturation is also important as early thrombosis in AVF is due to a hypercoagulable state with increased PAI-1 expression reported near the thrombus. Additionally, current studies have shown links between PAI-1 and age-related diseases such as inflammation, atherosclerosis, obesity, and Werner syndrome [90]. While these links have yet to be confirmed with proper studies, the association of PAI-1 with aging provides another reason to fully investigate the implications of PAI-1 in AVF maturation.
4.2 OSM and PAI-1
As stated previously, PAI-1 is a key downstream player in the OSM-OSMR pathway for cellular effects, mainly, as a regulator of fibrinolysis and proteolysis [71]. OSM increases PAI-1 protein significantly in both human coronary artery and aortic smooth muscle cells [70]. However, the mechanistic aspects of how OSM binding to its receptor induce PAI-1 were not identified. In a separate study looking at human trophoblast cell lines, it was found that HIF-1α induced a robust expression of PAI-1 during hypoxic conditions [91]. This further highlights the relationship between OSM and PAI-1 amid conditions conducive to oxidative stress and hypoxia within the vasculature. The previously cited study of outflow veins within rat AVF junctions [80], the presence of HIF-1α in increased levels further strengthens the association between OSM and production of hypoxic conditions, as well as robust association with PAI-1 and AVF junction failure. Plasminogen activator inhibitor (PAI) is a member of the serine protease inhibitor (serpin) family and is an inhibitor of serine proteases, urokinase-type plasminogen activator, and tissue-type plasminogen activator [72]. PAI-1 is synthesized in various cells, including vascular endothelial cells, macrophages, cardiomyocytes, and fibroblasts, with an activated and latent half-life of 32 and 7 minutes, respectively [92]. Of note in the structure of PAI-1 is a functional domain coded by exon 8, called the reactive center loop (RCL), which is required for inhibition of tPA/uPA activity [93]. In normal conditions, PAI-1 blocks uPA/tPA activation, plasmin formation, and plasmin-dependent MMP activation, thus protecting ECM proteins from proteolytic degradation [94, 95]. With dysfunction, this protective action may become deleterious if unregulated. As stated previously, ESRD patients exhibit hypercoagulability through native states of metabolic dysfunction. Such hypercoagulable state - in conjunction with PAI-1 effects on inhibition of key activators of clot breakdown, tPA/uPA, and early thrombosis - can be seen as a major risk factor for the failure of AVF maturation (Figure 2). These findings are important in the context of AVF maturation failure because hypercoagulability induces early thrombosis and maturation failure due to decreased blood flow through the inflow artery [8-10].
Figure 2: Schematics of possible targets in OSM-HIF1α-PAI-1 axis to enhance vascular remodeling and AVF maturation. Targeting circulating OSM in chronic inflammation may attenuate downstream signaling cascade and may enhance favorable vascular remodeling. Among downstream signals, targeting STAT3, oxidative stress, and PAI-1 may modulate cascade toward favorable ECM and vascular remodeling via inhibition of plasmin formation and overexpression of MMPs. Oncostatin-M (OSM), Oncostatin M receptor (OSMR), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), hypoxia-inducible factor-1-alpha (HIF-1α), extra cellular matrix (ECM), vascular smooth muscle cells (VSMCs), matrix metalloproteinases (MMPs).
Past studies have shown that activation of PAI-1 modulates ROS-induced fibrogenesis in various cell types [96, 97]. Looking at specific fibrotic conditions, PAI-1 has been studied within the context of the growth of keloid scars. Experimental studies showed fibroblasts derived from keloids had synthesized elevated levels of collagen and PAI-1 [98]. The ratio between PAI-1 and uPA was pathologically high which caused decreased degradation of fibrin and other ECM proteins to ultimately contribute to dermal fibrogenesis [99]. Additionally, response to injury in keratinocytes in the epidermis has been shown to be significantly lower when PAI-1 was knocked out in mice [100]. It has been suggested that this is possibly due to PAI-1 inhibiting cell migration through interactions with vitronectin, a key player in the regulation of proteolytic ECM degradation [101]. PAI-1 has also been studied within the context of lung fibrosis. Numerous studies have shown that PAI-1 deficiency leads to a protective effect in the lungs, preventing fibrotic tissue from forming from excess fibrin, and induction by bleomycin [102-105]. However, it should be noted that these studies also found that this is only due to increased fibrinolytic activity, not altered cell migration due to comparable amounts of leukocytes present in both bleomycin-treated wildtype and PAI-1 deficient lungs [104]. Additionally, Wilderberding et al. [106] found that levels of bleomycin-induced collagen accumulation were comparable between fibrinogen-deficient and heterozygous control mice. All of this suggests that PAI-1 plays a key role in lung fibrogenesis via the active suppression of proteolytic functions of uPA/tPA/plasmin [103, 104, 107, 108] (Figure 2). These studies involving PAI-1 in connection with fibrogenesis of keloid scars, as well as lung fibrosis, showcase the versatility of PAI-1 in producing a fibrosis phenotype through different tissue types. As this review serves to elucidate the molecular mechanisms governing AVF maturation failure, it is important to note past studies that have shown fibrosis as a key factor in preventing full maturation of the AVF junction. Simone et al. [109] have shown that AVF failure has direct associations with adventitial fibrosis as seen in their analysis of adventitial extracellular matrix deposition. Additionally, Martinez et al. [20] have demonstrated that pre-existing levels of medial fibrosis in native veins were independent of the post-operative medial fibrosis in the failed AVF. This further supports the idea that fibrotic remodeling of the AVF wall is a key determinant of maturation failure.
5. Translational Significance and Future Perspective
AVF maturation failure is subject to various factors that seem to be amplified by an ESRD patient’s dysfunctional metabolic state. Within the context of the factors discussed in this review, targeting OSM-mediated inflammation and hypoxic stress may be of particular therapeutic significance to promote successful AVF maturation. Further, an increase in oxidative stress through OSM-OSMR interactions leads to an increase in ECM and collagen deposition and an eventual thickening and stiffening of the vasculature [34, 110]. Additionally, a study involving an anti-OSM antibody was used to inhibit the overexpression of OSMR within squamous cell carcinoma tissue [111]. While this is a different tissue of interest, it is noteworthy that this technique may potentially be used to attenuate the inflammatory response of OSM in vascular tissue. Indeed, we propose that OSM and OSMR are potential therapeutic targets, through inhibition, to attenuate effects of ECM remodeling during the AVF maturation process. PAI-1 also has pro-coagulation and pro-fibrotic effects [20, 96, 97]. While the fibrotic effects of PAI-1 were studied in tissues other than vascular tissue, it is important to note that high levels of PAI-1 have been found in AVF tissues, thus highlighting PAI-1 as another potential therapeutic target for the attenuation of AVF maturation failure. Among the already known PAI-1 inhibitors, PAI-039 (Tiplaxtinin) is the most well-studied and effective inhibitor of PAI-1 but has side effects of provoking bleeding disorders during administration [112, 113] (Figure 2). Given these side effects, it would be desirable to develop therapeutic agents with the same mechanism as PAI-039 yet without adverse properties. Moreover, further studies must be done to assess how often a given treatment may be administered to maintain a patent AVF graft. The association of PAI-1 with overall cardiovascular disease underscores the importance of identifying other potential inhibitors that may attenuate processes that contribute to AVF maturation failure. Several studies have addressed the targeting of PAI-1 to control cardiovascular disease processes such as hypertension or pathological clotting [114, 115]. Additionally, previous studies have explored other inhibitors, such as RCL-mimicking peptides that would reduce PAI-1's ability to trap the target proteinase (Figure 2). However, these peptides, as well as a variety of other low molecular weight inhibitors, had decreased efficacy in the presence of vitronectin [114]. These results highlight the structural plasticity of PAI-1 and the difficulty in attempts to identify an efficient inhibitor. Kairuz et al. demonstrated in vivo reduction of PAI-1, as well as inflammatory processes such as macrophage accumulation and intimal thickening via treatment with an intra-luminal C-type natriuretic peptide, a peptide known for its antiproliferative effects [116]. Furthermore, Tiplaxtinin (PAI-039) has been shown to antagonize the anti-fibrinolytic activity of PAI-1 [109]. Simone et al. [109] went further to assess the mechanism by which Tiplaxtinin operates. They found that elastase-cleaved PAI-1, a peptide structurally similar to Tiplaxtinin, promotes VSMC apoptosis in vitro and reduced neointimal formation in vivo. That a cleaved PAI-1 has effects like an already established molecule such as Tiplaxtinin suggests potential therapies that promote cleavage of PAI-1 and thereby induce anti-stenotic processes may be viable. Another potential target may be to look through a broader lens at the JAK/STAT3 motif that is integral to OSM cell signaling. Of particular interest may be modulators of STAT3 specific inhibitors such as Ochromycinone (STA-21) (Figure 2). STA-21 historically has been used in the treatment of idiopathic pulmonary fibrosis, with proven decreases in STAT3 activity, as well as IL-6 attenuation after treatment [117]. While this therapeutic target may have other effects due to the widespread localization of STAT3 tyrosine kinase proteins, it is worth investigating the anti-inflammatory effects of Ochromycinone in relation to patency maintenance of arteriovenous fistulas.
6. Conclusion
Successful AVF maturation is a seemingly coordinated process that involves a balance of many different molecular mechanisms. Through tissue injury, inflammation is to be expected response. However, when this inflammatory response becomes dysregulated, proinflammatory mediators such as OSM potentiate failure of AVF maturation through a variety of mechanisms that are currently not well studied. With respect to cardiovascular disease, PAI-1 is a key mediator of pathogenic processes such as atherosclerosis and hypertension, with high expression being noted in atherosclerotic lesions and vasculature endothelium [17]. Additional systematic reviews studying PAI-1 as a marker for major adverse cardiovascular events (MACE) showed elevated plasma PAI-1 antigen levels in association with MACE [118]. Furthermore, Morrow et al. [119] have shown a clear association between thrombotic diseases, inflammation, and a dramatic elevation of PAI-1. These associations have clear implications for our better understanding of the development of disease processes directly linked to AVF maturation failure. This further highlights the importance of investigating the OSM-PAI-1 pathway. The studies included in this review depict the potential mechanisms for AVF maturation failure within the milieu of inflammation, oxidative stress, and fibrotic changes through vascular smooth muscle cell remodeling. While there are very few studies connecting these concepts concerning OSM and AVF maturation failure, the existing literature allows for inferences that there are potential therapeutic targets involving the OSM-PAI-1HIF-1α axis and various mediators in-between that may attenuate mechanisms involved in AVF maturation failure or potentiate success of AVF maturation.
Concept and Design
ND, VR, DKA; Literature Search: ND and VR; Critical review and interpretation of the findings: ND, VR; Drafting the article: ND, VR, DKA; Revising and editing the manuscript: VR, DRW, DKA; Final approval of the article: ND, VR, DRW, DKA.
Funding
This work was supported by the research grants R01HL144125 and R01HL147662 to DKA from the National Heart, Lung, and Blood Institute, National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable since the information is gathered from published articles.
Acknowledgments
None.
References
- Control for Disease Control and Prevention. Chronic kidney disease in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention 3 (2019).
- Lv JC, Zhang LX. Prevalence and Disease Burden of Chronic Kidney Disease. Adv Exp Med Biol 1165 (2019): 3-15.
- Asensio JA, Dabestani PJ, Miljkovic SS, et al. Traumatic penetrating arteriovenous fistulas: a collective review. Eur J Trauma Emerg Surg 48 (2022): 775-789.
- Asif A, Roy-Chaudhury P, Beathard GA. Early arteriovenous fistula failure: a logical proposal for when and how to intervene. Clin J Am Soc Nephrol 1 (2006): 332-339.
- Satish M, Kumar G, Agrawal DK. Pro-inflammatory and pro-resolving mechanisms in the immunopathology of arteriovenous fistula maturation. Expert Rev Cardiovasc Therapy 17 (2019): 369-376.
- Nguyen M, Thankam FG, Agrawal DK. Sterile inflammation in the pathogenesis of maturation failure of arteriovenous fistula. J Mol Med (Berl) 99 (2021): 729-741.
- Rai V, Singh H, Agrawal DK. Targeting the Crosstalk of Immune Response and Vascular Smooth Muscle Cells Phenotype Switch for Arteriovenous Fistula Maturation. Int J Mol Sci. 2022;23 (2022): 12012.
- Samra G, Rai V, Agrawal DK. Heterogeneous Population of Immune cells Associated with Early Thrombosis in Arteriovenous Fistula. J Surg Res (Houst) 5 (2022): 423-434.
- Samra G, Rai V, Agrawal DK. Innate and adaptive immune cells associate with arteriovenous fistula maturation and failure. Can J Physiol Pharmacol 100 (2022): 716-727.
- Rai V, Agrawal DK. Transcriptomic Analysis Identifies Differentially Expressed Genes Associated with Vascular Cuffing and Chronic Inflammation Mediating Early Thrombosis in Arteriovenous Fistula. Biomedicines 10 (2022).
- Rai V, Agrawal DK. Transcriptional and epigenetic factors associated with early thrombosis of femoral artery involved in arteriovenous fistula. Proteomes 10 (2022): 14.
- Tanaka M, Miyajima A. Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol 149 (2003): 39-52.
- Ikeda S, Sato K, Takeda M, et al. Oncostatin M is a novel biomarker for coronary artery disease - A possibility as a screening tool of silent myocardial ischemia for diabetes mellitus. Int J Cardiol Heart Vasc 35 (2021): 100829.
- Kastl SP, Speidl WS, Katsaros KM, et al. Thrombin induces the expression of oncostatin M via AP-1 activation in human macrophages: a link between coagulation and inflammation. Blood 114 (2009): 2812-2818.
- Vascular Access Work G. Clinical practice guidelines for vascular access. Am J Kidney Dis 48 (2006): S176-247.
- Hu H, Patel S, Hanisch JJ, et al. Future research directions to improve fistula maturation and reduce access failure. Semin Vasc Surg 29 (2016): 153-171.
- Tjarnlund-Wolf A, Brogren H, Lo EH, et al. Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke 43 (2012): 2833-2839.
- MacRae JM, Dipchand C, Oliver M, et al. Arteriovenous Access Failure, Stenosis, and Thrombosis. Can J Kidney Health Dis 3 (2016): 2054358116669126.
- Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol 31 (2011): 2391-2396.
- Martinez L, Duque JC, Tabbara M, et al. Fibrotic Venous Remodeling and Nonmaturation of Arteriovenous Fistulas. J Am Soc Nephrol 29 (2018): 1030-1040.
- Rose TM, Bruce AG. Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci U S A 88 (1991): 8641-8645.
- West NR, Owens BMJ, Hegazy AN. The oncostatin M-stromal cell axis in health and disease. Scand J Immunol 88 (2018): e12694.
- Richards CD. The enigmatic cytokine oncostatin M and roles in disease. ISRN Inflamm 2013 (2013): 512103.
- Hermanns HM. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev 26 (2015): 545-558.
- Liu J, Modrell B, Aruffo A, et al. Interactions between oncostatin M and the IL-6 signal transducer, gp130. Cytokine 6 (1994): 272-278.
- Gearing DP, Bruce AG. Oncostatin M binds the high-affinity leukemia inhibitory factor receptor. New Biol 4 (1992): 61-65.
- Mosley B, De Imus C, Friend D, et al. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J Biol Chem 271 (1996): 32635-32643.
- Fossey SL, Bear MD, Kisseberth WC, et al. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer 11 (2011): 125.
- Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci 117 (2004): 1281-1283.
- Zarling JM, Shoyab M, Marquardt H, et al. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A 83 (1986): 9739-9743.
- Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20 (2014): 1126-1167.
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6 (2014): 265sr6.
- Grenier A, Dehoux M, Boutten A, et al. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 93 (1999): 1413-1421.
- Hurst SM, McLoughlin RM, Monslow J, et al. Secretion of oncostatin M by infiltrating neutrophils: regulation of IL-6 and chemokine expression in human mesothelial cells. J Immunol 169 (2002): 5244-5251.
- Albasanz-Puig A, Murray J, Namekata M, et al. Opposing roles of STAT-1 and STAT-3 in regulating vascular endothelial growth factor expression in vascular smooth muscle cells. Biochem Biophys Res Commun 428 (2012): 179-184.
- Weiss TW, Kvakan H, Kaun C, et al. The gp130 ligand oncostatin M regulates tissue inhibitor of metalloproteinases-1 through ERK1/2 and p38 in human adult cardiac myocytes and in human adult cardiac fibroblasts: a possible role for the gp130/gp130 ligand system in the modulation of extracellular matrix degradation in the human heart. J Mol Cell Cardiol 39 (2005): 545-551.
- Zhang X, Li J, Qin JJ, et al. Oncostatin M receptor beta deficiency attenuates atherogenesis by inhibiting JAK2/STAT3 signaling in macrophages. J Lipid Res 58 (2017): 895-906.
- Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 341 (2010): 126-140.
- Li L, Zhao Q, Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol 68-69 (2018): 490-506.
- Cheresh P, Kim SJ, Tulasiram S, et al. Oxidative stress and pulmonary fibrosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1832 (2013): 1028-1040.
- Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280 (2001): C53-60.
- Zhao W, Zhao T, Chen Y, et al. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem 317 (2008): 43-50.
- Liu C, Yang Q, Fang G, et al. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse. Mol Med Rep 13 (2016): 2999-3008.
- Lee HB, Yu MR, Song JS, et al. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int 65 (2004): 1170-1179.
- Nybo T, Cai H, Chuang CY, et al. Chlorination and oxidation of human plasma fibronectin by myeloperoxidase-derived oxidants, and its consequences for smooth muscle cell function. Redox Biol 19 (2018): 388-400.
- Allon M, Litovsky S, Young CJ, et al. Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58 (2011): 437-443.
- Marden G, Wan Q, Wilks J, et al. The role of the oncostatin M/OSM receptor beta axis in activating dermal microvascular endothelial cells in systemic sclerosis. Arthritis Res Ther 22 (2020): 179.
- Richards CD, Botelho F. Oncostatin M in the Regulation of Connective Tissue Cells and Macrophages in Pulmonary Disease. Biomedicines 7 (2019).
- Luft R, Landau B. Mitochondrial medicine. Journal of internal medicine 238 (1995): 405-421.
- Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 8 (2002): 132-140.
- Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100 (2007): 460-473.
- Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of physiology 552 (2003): 335-344.
- Chen Q, Vazquez EJ, Moghaddas S, et al. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278 (2003): 36027-36031.
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiological reviews 80 (2000): 315-360.
- Madamanchi NR, Moon SK, Hakim ZS, et al. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol 25 (2005): 950-956.
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341 (1999): 233-249.
- Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res 61 (2004): 372-385.
- Di Lisa F, Canton M, Menabo R, et al. Mitochondria and reperfusion injury. The role of permeability transition. Basic Res Cardiol 98 (2003): 235-241.
- Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Annals of the New York Academy of Sciences 1047 (2005): 248-258.
- Sakakura K, Nakano M, Otsuka F, et al. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ 22 (2013): 399-411.
- Sluimer JC, Gasc JM, van Wanroij JL, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol 51 (2008): 1258-1265.
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med 7 (2001): 345-350.
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3 (2003): 721-732.
- Weidemann A, Johnson R. Biology of HIF-1α. Cell Death & Differentiation 15 (2008): 621-627.
- Gao L, Chen Q, Zhou X, et al. The role of hypoxia-inducible factor 1 in atherosclerosis. J Clin Pathol 65 (2012): 872-876.
- Vink A, Schoneveld AH, Lamers D, et al. HIF-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis 195 (2007): e69-75.
- Houben E, Hellings N, Broux B. Oncostatin M, an Underestimated Player in the Central Nervous System. Front Immunol 10 (2019): 1165.
- Battello N, Zimmer AD, Goebel C, et al. The role of HIF-1 in oncostatin M-dependent metabolic reprogramming of hepatic cells. Cancer Metab 4 (2016): 3.
- Chang SH, Hwang CS, Yin JH, et al. Oncostatin M-dependent Mcl-1 induction mediated by JAK1/2-STAT1/3 and CREB contributes to bioenergetic improvements and protective effects against mitochondrial dysfunction in cortical neurons. Biochim Biophys Acta 1853 (2015): 2306-2325.
- Demyanets S, Kaun C, Rychli K, et al. The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI 3-kinase and ERK1/2-dependent pathways. Am J Physiol Heart Circ Physiol 293 (2007): H1962-1968.
- Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost 3 (2005): 35-45.
- Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. Journal of cellular physiology 227 (2012): 493-507.
- Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal 10 (2008): 303-319.
- Liu RM, Choi J, Wu JH, et al. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. J Biol Chem 285 (2010): 16239-16247.
- Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med 48 (2010): 1-15.
- Ueno M, Maeno T, Nomura M, et al. Hypoxia-inducible factor-1alpha mediates TGF-beta-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 300 (2011): L740-752.
- Louis K, Hertig A. How tubular epithelial cells dictate the rate of renal fibrogenesis? World J Nephrol 4 (2015): 367-373.
- Zhang Q, Wu Y, Ann DK, et al. Mechanisms of hypoxic regulation of plasminogen activator inhibitor-1 gene expression in keloid fibroblasts. J Invest Dermatol 121 (2003): 1005-1012.
- Zhang Q, Wu Y, Chau CH, et al. Crosstalk of hypoxia-mediated signaling pathways in upregulating plasminogen activator inhibitor-1 expression in keloid fibroblasts. J Cell Physiol 199 (2004): 89-97.
- Misra S, Shergill U, Yang B, et al. Increased expression of HIF-1alpha, VEGF-A and its receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J Vasc Interv Radiol 21 (2010): 1255-1261.
- Wu J, Peng L, McMahon GA, et al. Recombinant plasminogen activator inhibitor-1 inhibits intimal hyperplasia. Arterioscler Thromb Vasc Biol 29 (2009): 1565-1570.
- Furumoto T, Fujii S, Nishihara K, et al. Maladaptive arterial remodeling with systemic hypertension associated with increased concentrations in blood of plasminogen activator inhibitor type-1 (PAI-1). Am J Cardiol 93 (2004): 997-1001.
- Tominaga Y, Miyagawa S, Kawamura T, et al. Mechanism of Cardiac Repair in Rat Myocardial Infarction Model Treated with Extracellular Vesicles from Differentiating Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circulation 144 (2021): A10495-A.
- Baldwin JF, Sood V, Elfline MA, et al. The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis. J Vasc Surg 56 (2012): 1089-1097.
- Kaikita K, Schoenhard JA, Painter CA, et al. Potential roles of plasminogen activator system in coronary vascular remodeling induced by long-term nitric oxide synthase inhibition. J Mol Cell Cardiol 34 (2002): 617-627.
- Ji Y, Strawn TL, Grunz EA, et al. Multifaceted role of plasminogen activator inhibitor-1 in regulating early remodeling of vein bypass grafts. Arterioscler Thromb Vasc Biol 31 (2011): 1781-1787.
- Hisada Y, Garratt KB, Maqsood A, et al. Plasminogen activator inhibitor 1 and venous thrombosis in pancreatic cancer. Blood Adv 5 (2021): 487-495.
- Meltzer ME, Lisman T, de Groot PG, et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 116 (2010): 113-121.
- Diebold I, Kraicun D, Bonello S, et al. The 'PAI-1 paradox' in vascular remodeling. Thromb Haemost 100 (2008): 984-991.
- Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther 28 (2010): e72-91.
- Meade ES, Ma YY, Guller S. Role of hypoxia-inducible transcription factors 1alpha and 2alpha in the regulation of plasminogen activator inhibitor-1 expression in a human trophoblast cell line. Placenta 28 (2007): 1012-1019.
- Reilly CF, Fujita T, Mayer EJ, et al. Both circulating and clot-bound plasminogen activator inhibitor-1 inhibit endogenous fibrinolysis in the rat. Arterioscler Thromb 11 (1991): 1276-1286.
- Gorlatova NV, Cale JM, Elokdah H, et al. Mechanism of inactivation of plasminogen activator inhibitor-1 by a small molecule inhibitor. J Biol Chem 282 (2007): 9288-9296.
- Hertig A, Berrou J, Allory Y, et al. Type 1 plasminogen activator inhibitor deficiency aggravates the course of experimental glomerulonephritis through overactivation of transforming growth factor beta. FASEB J 17 (2003): 1904-1906.
- Lackie PM. Molecular portfolios: cells interacting with matrix in repairing airway epithelium. Clin Exp Allergy 38 (2008): 1840-1843.
- Vayalil PK, Iles KE, Choi J, et al. Glutathione suppresses TGF-beta-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol 293 (2007): L1281-1292.
- Vayalil PK, Olman M, Murphy-Ullrich JE, et al. Glutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogen. Am J Physiol Lung Cell Mol Physiol 289 (2005): L937-945.
- Higgins PJ, Slack JK, Diegelmann RF, et al. Differential regulation of PAI-1 gene expression in human fibroblasts predisposed to a fibrotic phenotype. Exp Cell Res 248 (1999): 634-642.
- Tuan TL, Zhu JY, Sun B, et al. Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol 106 (1996): 1007-1011.
- Chan JC, Duszczyszyn DA, Castellino FJ, et al. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol 159 (2001): 1681-1688.
- Schvartz I, Seger D, Shaltiel S. Vitronectin. The international journal of biochemistry & cell biology 31 (1999): 539-544.
- Bauman KA, Wettlaufer SH, Okunishi K, et al. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest 120 (2010): 1950-1960.
- Eitzman DT, McCoy RD, Zheng X, et al. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 97 (1996): 232-237.
- Hattori N, Degen JL, Sisson TH, et al. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 106 (2000): 1341-1350.
- Senoo T, Hattori N, Tanimoto T, et al. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax 65 (2010): 334-340.
- Wilberding JA, Ploplis VA, McLennan L, et al. Development of pulmonary fibrosis in fibrinogen-deficient mice. Ann N Y Acad Sci 936 (2001): 542-548.
- Sisson TH, Hattori N, Xu Y, et al. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum Gene Ther 10 (1999): 2315-2323.
- Swaisgood CM, French EL, Noga C, et al. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol 157 (2000): 177-187.
- Simone S, Loverre A, Cariello M, et al. Arteriovenous fistula stenosis in hemodialysis patients is characterized by an increased adventitial fibrosis. J Nephrol 27 (2014): 555-562.
- Iglesias-De La Cruz MC, Ruiz-Torres P, Alcami J, et al. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int 59 (2001): 87-95.
- Kucia-Tran JA, Tulkki V, Scarpini CG, et al. Anti-oncostatin M antibody inhibits the pro-malignant effects of oncostatin M receptor overexpression in squamous cell carcinoma. J Pathol 244 (2018): 283-295.
- Elokdah H, Abou-Gharbia M, Hennan JK, et al. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization. J Med Chem 47 (2004): 3491-3494.
- Hennan JK, Elokdah H, Leal M, et al. Evaluation of PAI-039 [1-benzyl-5-[4-(trifluoromethoxy)phenyl]-1H-indol-3-yl(oxo)acetic acid], a novel plasminogen activator inhibitor-1 inhibitor, in a canine model of coronary artery thrombosis. J Pharmacol Exp Ther 314 (2005): 710-716.
- Sillen M, Declerck PJ. Targeting PAI-1 in Cardiovascular Disease: Structural Insights Into PAI-1 Functionality and Inhibition. Front Cardiovasc Med 7 (2020): 622473.
- Simon DI, Simon NM. Plasminogen activator inhibitor-1: a novel therapeutic target for hypertension? Circulation 128 (2013): 2286-2288.
- Kairuz EM, Barber MN, Anderson CR, et al. C-type natriuretic peptide (CNP) suppresses plasminogen activator inhibitor-1 (PAI-1) in vivo. Cardiovasc Res 66 (2005): 574-582.
- Waters DW, Blokland KEC, Pathinayake PS, et al. STAT3 Regulates the Onset of Oxidant-induced Senescence in Lung Fibroblasts. Am J Respir Cell Mol Biol 61 (2019): 61-73.
- Jung RG, Motazedian P, Ramirez FD, et al. Association between plasminogen activator inhibitor-1 and cardiovascular events: a systematic review and meta-analysis. Thromb J 16 (2018): 12.
- Morrow GB, Whyte CS, Mutch NJ. A Serpin With a Finger in Many PAIs: PAI-1's Central Function in Thromboinflammation and Cardiovascular Disease. Front Cardiovasc Med 8 (2021): 653655.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 