CD4+CD25+Treg Cells Prolong the Survival Time of Heart Allograft Via Induction Lymphocyte Apoptosis and Modulation the Ratio of T Cell Subsets
Jinguo Zhu*, 1, Lihua Xiong2
1Department of cardiothoracic surgery, The second affiliated hospital of shantou university medical college, GuangDong, 515040, China.
2Department of cardiothoracic surgery, The second affiliated hospital of Sun Yat-Sen university, GuangDong, 510120, China.
*Corresponding author: Jinguo Zhu, Department of cardiothoracic surgery, The second affiliated hospital of shantou university medical college, GuangDong, 515040, China
Received: 10 February 2022; Accepted: 18 February 2022; Published: 04 March 2022
Article Information
Citation:
Jinguo Zhu, Lihua Xiong. CD4+CD25+Treg Cells Prolong the Survival Time of Heart Allograft Via Induction Lymphocyte Apoptosis and Modulation the Ratio of T Cell Subsets. Cardiology and Cardiovascular Medicine 6 (2022): 71-82.
View / Download Pdf Share at FacebookAbstract
Background: CD4+CD25+regulatory T cells (CD4+CD25+ Treg cells) play major roles in immune regulation. Previous studies showed CD4+CD25+ Treg cells can maintain peripheral immune tolerance and increase the survival time of transplanted organs. However, the biological characteristics and the functional roles of these CD4+CD25+ Treg cells in transplantation tolerance remain unknown. The current study was conducted to observe the effect of CD4+CD25+ Treg cells on heart allograft in rats and to investigate the underlying mechanism of the CD4+CD25+ Treg cells.
Methods: 5 x 107 spleen cells of SD rats were inoculated into the thymus gland of Wistar rats. The level of CD4+CD25+ Treg cells was examined by the flow cytometry method, and the biological activity of CD4+CD25+ Treg cells was detected by the 3H-TdR method. Hearts were transplanted from SD rats (donors) to Wistar rats (recipients) and the animals were assigned into four groups: HT, HT+Ii,HT+Treg, HT+Treg+Ii. At various time points after the transplantation, the transplanted hearts were collected and histologically examined. The rate of lymphocyte apoptosis and T cell subsets in the peripheral blood of Wistar rats were analyzed with flow cytometry.
Results: The CD4+CD25+ Treg cells in Wistar rats were sharply increased, and these CD4+CD25+ Treg cells significantly extended the survival time: The mean survival time of the transplanted hearts was 8.1 ± 1.2 days, 35.7 ± 4.7 days, 53.7 ± 6.2 days, 75.7 ± 11.3 days in the group of HT, HT+Ii, HT+Treg, or HT+Ii+Treg, respectively (n = 12-14/group). Among them, the survival time between HT and HT+Treg or betwee
Keywords
<p>Heart transplantation; CD4+CD25+Treg cells; Lymphocyte apoptosis; T cell subsets</p>
Article Details
1. Introduction
Organ transplantation brings hopes for patients with end-stage diseases that cannot be treated by routine methods. A variety of methods have been developed in clinical practice to prevent the occurrence of rejection reactions in organ transplantation. However, these methods can’t prevent the chronic dysfunction of transplanted organs, which ultimately leads to rejection. It is thus very important to find out new immuno-suppressors that can prevent transplantation rejections. It was reported that Donor-specific tolerance to a rat heterotopic cardiac allograft has been achieved by the pretransplantation intrathymic injection of donor splenocytes and a single intraperitoneal injection of antilymphocyte serum. Permanent tolerance is achieved without subsequent immunosuppression therapy [1]. Recently, researchers have focused their studies on dendritic cells and Th cells. CD4+CD25+ Treg cells are among the major players in immune regulation. Studies show they can maintain peripheral immune tolerance and prolong the survival time of transplanted organs [2-3]. Thymic-derived CD4+CD25+Treg cells can be expanded ex vivo, and it has been proven that they can suppress transplantation rejection and graft versus host disease in trials. These cells express receptors, which can be activated by interleukins such as IL-2 or IL-4. IL-2 and IL-4 can promote the induction of transplantation tolerance and the alloantigen-specific CD4+CD25+Treg cells. Through activation of the Th1 and Th2 pathways, CD4+CD25+ Treg cells produce more potent antigen-specific Treg cells that only suppress specific donor rejection. However, the biological characteristics and the immune regulatory function of these CD4+CD25+ Treg cells in transplantation tolerance are not known. This study was conducted to observe the effect of CD4+CD25+ Treg cells on heart allograft in rats and planned to investigate the underlying mechanism of CD4+CD25+Treg cells.
2. Methods
2.1 Isolation of spleen cells
The spleens of the Sprague-Dawley(SD) rats were rinsed in 10 ml PBS solution. The spleens were then put in 200 mesh, milled, and filtered twice. The cell suspension was then centrifuged at 1500 r/min at 4°C for 10 min. After resuspension, the concentration of the spleen cells was adjusted to 1.25×108/ml. Cell viability was examined by Trypan blue staining and visualized by microscope.
2.2 Preparation of antilymphocyte serum
White rabbits were purchased from the Medical Experimental Animal Center of Sun Yat-sen University and maintained in laminar-flow, specific pathogen-free animal facilities at the Sun Yat-sen University. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University. Spleen cells (2.5 x 107) of SD rats were subcutaneously injected into the neck and back area of white rabbits (n=5). After three weeks, 5 ml of blood was collected to detect the level of anti-spleen cell polyclonal antibody from these rabbits. When the level of anti-spleen cell polyclonal antibody was over 1:1000, the rabbits were euthanized (the rabbit was anesthetized by a single intraperitoneal injection of 2% Pentobarbital Sodium (40mg/kg) and the blood was collected from their hearts. Rabbit’s blood was stored under 4°C for 24 hours, and then centrifuged at 6000 r/min. The serum (supernatant) was saved under -20°C for further analysis.
2.3 Intrathymic inoculation and detection of the CD4+CD25+ Treg cells
fifty million spleen cells from SD rats were inoculated into the thymuses gland of each Wistar rat, and a total of 6 rats were inoculated. The amount of the CD4+CD25+ Treg cells in Wistar rats at different time points (0, 5, 10, 15, 20, and 25 days post-inoculation) was detected by previously published methods [4].
2.4 Detection of CD4+ CD25+ Treg cells activity by the 3H-TdR method
five mg/L anti-CD3 antibody and 1 x 105 SD spleen cells (treated by Mitomycin) were put into each well of a 96-well plate, a total of 30 wells. These wells were randomly assigned into 3 groups with 10 wells per group. The first group was added Wistar rats’CD4+ CD25- T cells at 2 x 104/well. The second group was added Wistar rats’ CD4+CD25+T cells at 2 x 104/well. The third group was added both the Wistar rats’ CD4+CD25+T cells and the Wistar rats’CD4+ CD25- T cells, each at 2 x 104/well. The spleen cells were used as stimulating cells, CD4+ CD25− T cells were used as reaction cells, and the CD4+ CD25+ T cells were used as immune modulation cells, and splenocytes of SD rat, CD4+CD25-T cell of Wistar rats, CD4+CD25+T cells of Wistar rats, CD4+CD25-T cells plus CD4+CD25+T cells of Wistar rats used as control groups. After the reaction system of the allogeneic mixed lymphocyte culture was established, cells were incubated for 3 days. MTT assay was performed after 3 days by adding 20μl MTT per well and incubating for 6 hours, to detect the inhibitory effect of the CD4+ CD25+ Treg cells.
2.5 The procedure of heart transplant
SD and Wistar rats (250 - 300 g, 8 weeks, male) were purchased from the Medical Experimental Animal Center of Sun Yat-sen University and maintained in laminar-flow, specific pathogen-free animal facilities at the Sun Yat-sen University. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University. SD and Wistar rats were used as donors and recipients, respectively. All Wistar rats were randomly assigned into four groups (n=15/group): heart transplantation group (HT group); heart transplantation and intrathymic inoculation group (HT+Ii group); heart transplantation and treatment with CD4+CD25+Treg cells group (HT+Treg group), and heart transplantation and treatment with CD4+CD25+Treg cells and intrathymic inoculation group (HT+Treg+Ii group). 5 x 107 allogeneic spleen cells of SD rats were inoculated into thymuses gland 21 day before operation and 1ml antilymphocyte serum intraperitoneal injected 1 day before operation in wistar rats of HT+Ii and HT+Treg+Ii group. 1×106 CD4+CD25+Treg cells injection from caudal vein 1 day before operation in wistar rats of HT+Treg and HT+Treg+Ii group. Animal surgery was performed by following the previously published method [3]. In brief, the SD rats (donors) were anesthetized by a single intraperitoneal injection of 2% Pentobarbital Sodium (40mg/kg). A midline incision was made on the thoracic wall. After the heart was exposed, the donor’s superior cava was divided to bleed. After clamping the aorta, cardioplegia was perfused. A profound local cardiac cooling was induced with ice water. The donor’s heart was then harvested. The same anesthetic method was applied onto the Wistar rats (recipients) and followed by a midline incision in the abdominal wall. After using standard vascular microsurgical techniques, the recipient’s abdominal aorta was anastomosed end-to-side to the donor’s abdominal aorta and the recipient’s inferior vena cava was anastomosed to the donor’s pulmonary artery. The viability of the graft was monitored by every day checking of palpable heartbeat. After reaching the endpoint of the experimental, the rats were anesthetized by a single intraperitoneal injection of 2% Pentobarbital Sodium (40mg/kg), the transplanted heart was harvested. Graft rejection was defined as the cessation of heartbeat.
2.6 Isolation of mononuclear cells
Peripheral blood of Wistar rats in the experimental groups was collected from the caudal vein (0.5 ml/rat) at day 0, 5, 10, 20, 40, 60, and 80 after transplantation surgery. The mononuclear cells peripheral blood was prepared by gradient density centrifugation as we described previously [4]. The cells were examined by an Accuri C6 flow cytometer and the data were analyzed by a C Flow Plus Analysis software (Ann Arbor, MI, USA).
2.7 Lymphocyte apoptosis assay
Lymphocyte apoptosis was detected by phosphatidylserine externalization in the cell membrane using the annexin V /propidium iodide (PI) assay on 0, 5, 10, 20, 40, 60, and 80 days after the transplantation surgery. The PBMCs were collected as previously published [4]. The concentration of the antibody used in the assay was based on the instruction of the assay kits. All samples were analyzed by Accuri C6 flow cytometry and a C Flow Plus Analysis software (Ann Arbor, MI, USA).
2.8 T-cell subsets assay
The T-cell subsets were identified by the double-positive staining of the membrane-specific markers CD3+, CD4+, and CD8+. The percentage of T-cell subsets in the peripheral blood from all experimental groups was analyzed on 0, 5th, 10th, 20th, 40th, 60th, and 80th day following the transplantation surgery. The PBMCs were collected as described above, re-suspended with PBS, and stained with fluoresceinisothiocyanate(fiTC)-conjugated antibodies against rat CD3, CD4, CD8, and the PE-conjugated anti-rat antibodies (Caltag, USA) for 30 min at 4°C in dark. Isotype matched IgG was used as negative controls. The concentration of antibodies was used by following the manufacture’s instructions. All samples were analyzed by using the Accuri C6 flow cytometry and the CFlow Plus Analysis software (Ann Arbor, MI, USA).
2.9 Histological examination of the heart
Hearts of the Wistar rats were served as the control group. Samples of transplanted hearts were from the four experimental groups mentioned above at the different time (endpoint is heartbeat cessation).The experiments were performed as previously described [4]. Briefly, all heart tissue samples were fixed in 4% buffered formalin solution overnight, embedded in paraffin, sec-tioned (5–6 μm thickness) under a microtome, and stained with hematoxylin and eosin (H&E) by using the standard method. All samples were analyzed under light microscopy in a blinded fashion. The criteria for evaluation of rejection was based on the guideline of the International Society for Heart and Lung Transplantation.
2.10 Statistical analysis
All results were presented here with mean ± SEM. A comparison between the two groups was evaluated by Student’s t-test. One-way ANOVA was used to evaluate the significance of the difference between multiple groups. Survival curves were generated by the Kaplan–Meier method and difference in survival between groups used the log-rank test. P values <0.05 were considered to be statistically significant.
3. Results
3.1 CD4+CD25+ Treg cells assay
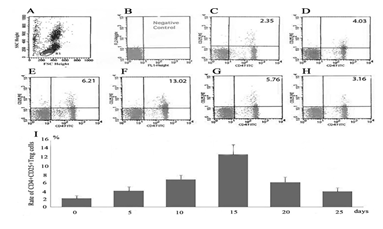
Treg cells in the peripheral blood from the intrathymically inoculated rats were analyzed at different time points (day0, 5, 10, 15, 20, and 25) (fig. 1). Mononuclear cells were detected by flow cytometry. The number of CD4+CD25+Treg cells were increased 5 and 10 days after intrathymic inoculation. On day 15, the number of CD4+CD25+Treg cells reached the peak value. After that, the level of the CD4+CD25+Treg cells in recipient rats gradually decreased on 20 and 25 days. CD4+CD25+Treg cells at Day 15 (12.32±2.63%) is significantly higher than day 5 (4.42±1.07%, n=6, p < 0.05), day 10 (6.32±1.12% n=6, p < 0.05), day 20(5.75±1.38% n=6, p < 0.05), and day 25 (3.26±0.92% n=6, p < 0.05) respectively.
figure 1: Qantitative analysis of CD4+CD25+Treg cells. (A) Flow cytometric plots and its gate(R1). (B)Flow cytometric plots negative control. (C) Representative flow cytometric plots on day 0. (D) Representative flow cytometric plots on day 5. (E) Representative flow cytometric plots on day 10. (F) Representative flow cytometric plots on day 15. (G) Representative flow cytometric plots on day 20. (H) Representative flow cytometric plots on day 25. (I) Qantitativelyanalysized of CD4+CD25+Treg cells and summarized. Data are mean±SEM (n = 4–6), P<0.05.
3.2 CD4+CD25+ Treg cells activity assay
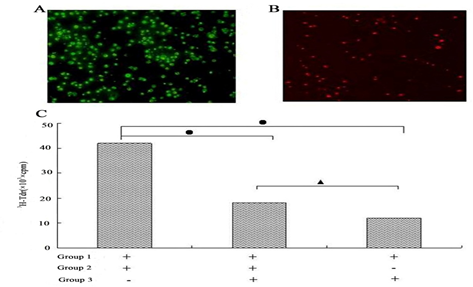
The purity of the isolated CD4+CD25+ and CD4+CD25- T cells was assessed by flow cytometry analysis. As shown in fig. 2A&2B, the purity of CD4+CD25-T cells was 99.5% and the purity of CD4+CD25+ T cells was 96.2%. Using 3H-TdR method to detect the activity of CD4+ CD25+ Treg cells, we found that the CD4+CD25+ T cells were able to significantly suppress the proliferation of CD4+CD25- Treg cells, as presented in fig. 2C, The number of CD4+CD25+ T cells in all control groups were not significantly changed.
figure 2: CD4+CD25+Treg cells Immunosuppressive function detection. (A)Fluorescence of CD4+CD25-T cells:The purity of CD4+CD25-T cells was 99.5%. (B)Fluorescence of CD4+CD25+T cells: The purity of CD4+CD25+ T cells was 96.2%. (C) 3H-TdR: [3H] thymidine; cpm: counts per minute: Group 1: SD rats’s pleen cells; Group 2: Wistarrats’CD4+ CD25− T cells; Group 3: Wistarrats’ CD4+ CD25+Treg cells. The data shows CD4+CD25+ T cells suppression CD4+CD25- T cellsproliferationeffect significantly, •P<0.001; ▲P<0.05.
3.3 Lymphocyte apoptotic assay
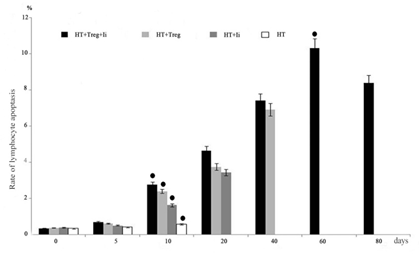
Lymphocyte apoptosis was examined by flow cytometry. The percentage of annexin-V+ PI+ cells was sharply increased in both the HT+Treg and HT+Treg+Ii groups. The rate of lymphocyte apoptosis was shown in fig. 3. The significant difference between HT+Treg+Ii and HT+Treg, HT+Treg+Ii and HT+ Ii, HT and HT+Treg+Ii, HT and HT+Treg, or HT and HT+Ii was observed (p>0.05) in each comparison.
3.4 T-cell subsets and CD3+CD4+/CD3+CD8+ analysis
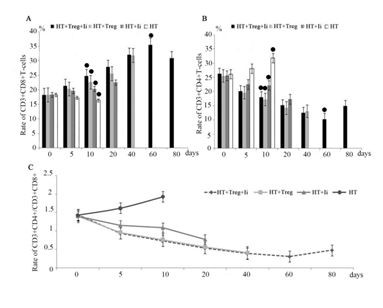
CD3+CD4+ T cells and CD3+CD8+ T-cell subsets in the peripheral blood from all of the experimental groups were analyzed at different time points (days 0, 5, 10, 20, 40, 60, and 80). The mononuclear cells in the peripheral blood were detected by the flow cytometry. The percentage of CD3+CD8+, CD3+CD4+T-cell subsets was changed as shown in fig. 4A and fig. 4B. The rate of CD3+CD4+/CD3+CD8+ was shown as fig. 4C.
figure 4: Detected the CD3+CD4+T cells, CD3+CD8+T cells by flow cytometry and analysis the rate of CD3+CD4+/CD3+CD8+. (A) Rate of CD3+CD8+T cells expressed at different time in four groups . (B) Rate of CD3+CD4+T cells expressed at different timein four groups. (C) showed the change of rate of CD3+CD4+/CD3+CD8+ at different time. All data were expressed as mean±SEM, •p>0.05.
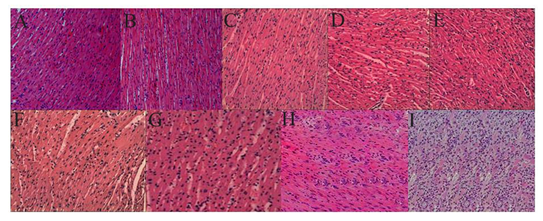
3.5 Pathological changes in transplanted hearts
The pathological changes of the transplanted hearts were shown in fig. 5, and the representative results in each group were displayed in fig. 5A-Q. The CD4+CD25+ Treg cells were able to improve the pathological properties in the transplanted hearts by showing better protection in the HT+Treg+Ii group than in the HT+Treg group or HT+Ii group.
figure 5: Histological assay of transplanted hearts. (A) The histology of the transplanted hearts from HT group at day 5. (B) The histology of the transplanted hearts from HT group at day 10. (C) The histology of the transplanted hearts from HT+Ii group at day 20. (D) The histology of the transplanted hearts from HT+Ii group at day 30. (E) The histology of the transplanted hearts from HT+Ii group at day 40. (F) The histology of the transplanted hearts from HT+Treg group at day 50. (G) The histology of the transplanted hearts from HT+Treg group at day 60. (H) The histology of the transplanted hearts from HT+Ii+Treg group at day 70. (I) The histology of the transplanted hearts from HT+Ii+Treg group at day 80. Hearts were evaluated for rejection according to the International Society for Heartand Lung Transplantation standard. 0: normal; IA:focal perivascular; IB: interstitial infiltrate without myocyte damage; II: multifocalinfiltrate with myocyte damage; IIIA: diffuse polymorphousinfiltrate with extensive myocyte damage; IIIB: diffuse polymorphousinfiltrate with extensive myocyte damageand/oredema; IV: diffuse polymorphousinfiltrate with extensive myocyte damage and/oredemaand/or hemorrhage and/or vasculitis. Each experimental group comprises of 12-14 samples. All samples were analyzed under light microscopy in a blinded fashion.
3.6 Survival time of heart allografts
The survival time of the transplanted hearts was 8.1 ± 1.2 days in the HT group (n = 15), 35.7 ± 4.7 days in the HT+Ii group (n = 15), 53.7 ± 6.2 days in the HT+Treg group (n = 15), and 75.7 ± 11.3 days in the HT+Ii+Treg group (n = 15), The survival curve of Kaplan–Meier was shown in fig. 6. This data showed that the survival time of the transplanted hearts in the HT+Ii+Treg group, HT+Treg group, and HT+Ii group were significantly longer (P < 0.001) than the HT group. The survival time of transplanted hearts in the HT+Ii+Treg group is longer than the HT+Ii group (P < 0.05), and the HT+Ii+Treg group is longer than the HT+Treg group (P < 0.05).
figure 6 The Kaplan–Meier curve shows the survival time of transplanted hearts. The survival time of the transplanted hearts in the HT+Ii+Treg group (n=15), HT+Treg group (n=15), and HT+Ii group (n=15) were significantly longer (P < 0.001) than HT group (n=15). The survival time of transplanted hearts in HT+Ii+Treg group is longer than the HT+Ii group (P < 0.05), and the HT+Ii+Treg group is longer than the HT+Treg group (P < 0.05).
4. Discussion
CD4+CD25+ Treg cells play an important role in the induction and maintenance of immune tolerance in experimental transplant models. Numerous studies showed that CD4+CD25+ Treg cells are essential for the induction and maintenance of immunologic self-tolerance and transplant tolerance [5-6]. In this report, we focused on the suppressive properties of CD4+CD25+ Treg cells in immune regulation and explored their therapeutic potential in clinical transplantation. Several studies[7-8] have shown that CD4+CD25+ Treg cells can effectively prevent transplantation rejection and actively induce transplantation tolerance. However, the underlying mechanisms of the protective effect of CD4+CD25+ Treg cells in transplantation are not fully understood. In this report we found that CD4+CD25+ Treg cells can delay the occurrence of the pathological changes and prolong the survival time of cardiac allografts. This was due to that CD4+CD25+ Treg cells can downregulate immune function of the receiver and improve immune tolerance of the transplanted heart. The CD4+CD25+Treg cells are recognized as a major subset of the immune cells possessing potent suppressive properties [9]. Our data showed that CD4+CD25+ Treg cells can significantly suppress the proliferation of CD4+CD25- T cells shown in fig. 2C. Several groups demonstrated that apoptosis of immune cells induced by CD4+CD25+ Treg cells is one of the mechanisms of immune suppression [10-11]. Lymphocyte apoptosis is another important immunoregulatory mechanism of maintaining homeostasis in the immune system in vivo [12-14], which could be an effective strategy to reduce transplantation rejection and induce transplantation tolerance. It was reported that tolerant rats had higher numbers of lymphocytes apoptosis in the transplantation models[15-17]. The purpose of this study was to set up a model to investigate the effect of CD4+CD25+ Treg cells on the recipient immunoregulation in cardiac allotransplantation from SD rats to Wistar rats. We observed that the percentage of lymphocyte apoptosis was significantly increased in the HT+Treg+Ii and HT+Treg groups.
CD3+CD4+T cells and CD3+CD8+T cells are the major T-cells subsets in peripheral blood. They are essential parts of the immune system[18-19]. It has been reported that graft rejection mediated by alloreactive CD4+ T cells is a major obstacle to transplantation tolerance. CD8+ T cells can induce graft tolerance by restraining the function of activated CD4+ T cells [20-21]. Our results showed that CD4+CD25+ Treg cells suppressed the proliferation of CD4+CD25- T cells (fig. 2C) and altered the percentage of CD3+CD8+, CD3+CD4+T-cell subsets (fig.4A and fig.4B). These results indicate CD4+CD25+ Treg cells can directly regulate CD3+CD4+T cells and CD3+CD8+T cells. The interaction between CD3+CD4+T cells and CD3+CD8+T cells changed the ratio of CD3+CD4+ to CD3+CD8+. The ratio of CD3+CD4+ to CD3+CD8+ is independently associated with T-cell activation [22-23]. The CD3+CD4+/CD3+CD8+ ratio (< 1) has been accepted as a marker of immune system suppression, and is associated with diseases such as autoimmune disease, tumors, HIV, etc. However, the relationship between the ratio of CD3+CD4+/CD3+CD8+ and transplantation reje-ction has not yet been identified.
In our report, we found that the ratio of CD3+CD4+/CD3+CD8+ was between 1.5 and 2.0 in the HT group, between 1.0 and 1.5 in the HT+Ii group, between 0.5 and 1.0 in both HT+Treg and HT+Treg+Ii groups. This data indicates that the immune response was sharply suppressed in HT+Treg and HT+Treg+Ii groups. In contrast, the immune response was only mildly suppressed in the HT+Ii group and was normal in the HT group.
5. Conclusion
In summary, CD4+CD25+ Treg cells can induce lymphocyte apoptosis and regulate the ratio of T-cell subsets, leading to the suppression of the immune system and delayed immune response. These changes prolong the mean survival time of the heart allograft. Therefore, it is promising that CD4+CD25+ Treg cells can induce heart transplantation tolerance. The underlying mechanisms include the induction lymphocyte apoptosis and the modulation the ratio of T cell subsets by CD4+CD25+ Treg cells.
Abbreviations
HT heart transplantation
Ii intrathymic inoculation
PI propidium iodide
3H-TdR [3H] thymidine
Cpm counts per minute
SD Sprague–Dawley
Acknowledgments
The manuscript was edited by Dr. Huibin Tang, Stanford University School of Medicine.
Ethical approval
Ethical approval for this study was obtained from the Institutional Ethical Committee (approval #16, 26/10/2013).
Animal welfare
The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Competing interests
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
Funding
This work was not sported by a funding agency
References
- Z Shen,M Mohiuddin,V J DiSesa. Suppressor Cells and Intrathymic Inoculation of Donor Alloantigens in Cardiac Transplantation. Ann Thorac Surg.1995; 60 (6):1683-1685.
- Oluwole SF, Chowdhury NC, Fawwaz RA. Induction of donor-specific unresp-onsiveness to rat cardiac allografts by intrathymic injection of UV-B-irradiated donor spleen cells. Transplantation 55 (1993): 1389-95.
- Zhu J, Chen S, Wang J, et al. Splenectomy increases the survival time of heart allograft via developing immune tolerance.J Cardiothorac Surg 8 (2013): 129.
- Zhu J, Gao B. Simvastatin combined with aspirin increases the survival time of heart allograft by activating CD4(+)CD25(+) Treg cells and enhancing vascular endothelial cell protection. Cardiovasc Pathol 24 (2015): 173-178.
- Dutta P, Dart M, Roenneburg DA, et al. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant 11 (2011): 1296-301.
- Kim JI, O'connor MR, Duff PE, et al. Generation of adaptive regulatory T cells by alloantigen is required for some but not all transplant tolerance protocols.Transplantation 91 (2011): 707-713.
- Kim HR, Oh SK, Lim W, et al. Immune enhancing effects of Echinacea purpurea root extract by reducing regulatory T cell number and function. Nat Prod Commun 9 (2014): 511-514.
- Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin-2 in Foxp3-expressing regulatory T cells. Nat Immunol 6 (2005): 1142-1151.
- Zheng S G,Wang J H, Gray D, et al. Natural and induced CD4+CD25+ educate CD4+CD25- cells to develop supp ressive activity:The role of IL-2, TGF-β, and IL-10. J Immunol 172 (2004): 5213-5221.
- Gertel-Lapter S, Mizrachi K, Berrih-Aknin S, et al. Impairment of regulatory T cells in myasthenia gravis: studies in an experimental model. Autoimmun Rev 12 (2013): 894-903
- Shu CC, Wang JY, Wu MF et al. Attenuation of lymphocyte immune respo-nses during Mycobacterium avium complex-induced lung disease due to increasing expr-ession of programmed death-1 on lymp-hocytes. Sci Rep 7 (2017): 42004.
- Gonzalez N, Bensinger SJ, Hong C, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31 (2009): 245–258.
- 13 Radu CG, Shu CJ, Nair-Gill E, et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat Med 14 (2008): 783–788.
- Rothlin CV, Ghosh S, Zuniga EI, et al. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131 (2007): 1124–1136.
- Horst AK, Neumann K, Diehl L , et al. Modulation of liver tolerance by conven-tional and nonconventional antigen-prese-nting cells and regulatory immune cells. Cell Mol Immunol 13 (2016): 277-92.
- McGaha TL, Karlsson MC. Apoptotic cell responses in the splenic marginal zone: a paradigm for immunologic reactions to apo-ptotic antigens with implications for auto-immunity. Immunol Rev 263 (2016): 26-43.
- Zhou F, Zhang GX, Rostami A. Apoptotic cell-treated dendritic cells induce immune tolerance by specifically inhibiting development of CD4? effector memory T cells. Immunol Res 64 (2016): 73-81.
- Jurcevic S, Juif PE, Hamid C, et al. Effects of multiple-dose ponesimod, a selective S1P1 receptor modulator, on lymphocyte subsets in healthy humans.Drug Des Devel Ther 28 (2016): 123-131.
- Monticelli S, Äijö T, Trifari S. Approaches to Detect microRNA Expression in T Cell Subsets and T Cell Differentiation. Methods Mol Biol 1514 (2017): 153-172.
- Arroyo Hornero R, Betts GJ, Sawitzki B, et al. CD45RA Distinguishes CD4+CD25+CD127-/low TSDR Deme-thylated Regulatory T Cell Sub-populations With Differential Stability and Susceptibility to Tacrolimus-Mediated Inh-ibition of Sup-pression. Transplantation 101 (2017): 302-309.
- Yu F, Hao Y, Zhao H, et al. Distinct Mitochondrial Disturbance in CD4+T and CD8+T Cells From HIV-Infected Patients. J Acquir Immune Defic Syndr 74 (2017): 206-212.
- Chan ES, Landay AL, Brown TT, et al. Differential CD4+ cell count increase and CD4+: CD8+ ratio normalization with maraviroc compared with tenofovir. AIDS 30 (2016): 2091-7.
- Bellissimo F, Pinzone MR, Celesia BM, et al. Baseline CD4/CD8 T-Cell Ratio Predicts Prompt Immune Restoration Upon cART Initiation. Curr HIV Res 14 (2016): 491-496.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 