Exercise in Patients with Chronic Angina Pectoris: Friend or Foe?
Ralf Dechend1, Hans-Georg Predel2,*
1Experimental and Clinical Research Center (ECRC) Charitè Campus Buch and HELIOS Klinikum Berlin Buch, Dept. of Cardiology and Nephrology, Germany
2Institute of Cardiology and Sports Medicine, German Sport University Cologne, Cologne, Germany
*Corresponding author: Hans-Georg Predel. Institute of Cardiology and Sports Medicine, German Sports University Cologne, Cologne, Germany
Received: 11 March 2022; Accepted: 21 March 2022; Published: 07 July 2022
Article Information
Citation: Ralf Dechend, Hans-Georg Predel. Exercise in Patients with Chronic Angina Pectoris: Friend or Foe?. Cardiology and Cardiovascular Medicine 6 (2022): 364-373.
View / Download Pdf Share at FacebookAbstract
Angina pectoris, a constricting pain resulting from inadequate oxygen supply to the heart, typically develops as a consequence of physical exertion or emotional stress in the presence of coronary artery disease (CAD). However, there is also evidence that physical activity is effective in the prevention of CAD and may also prevent angina in the long term. Guideline-based strategies for chronic stable angina aim to reduce symptoms and improve patient prognosis through lifestyle changes and appropriate medications and interventions. Physicians may have uncertainty and concerns around the safety of exercise regimens in patients with chronic angina, a factor complicated by the paucity of data related to this clinical condition. This narrative review discusses the importance of regular physical activity as a key component in the management of patients with stable angina, demonstrating cardioprotective effects in patients with CAD, as well as improving prognosis and physical fitness while maintaining an appropriate risk/benefit ratio. Given these benefits, current guidelines recommend 30–60 minutes of moderate-intensity aerobic activity for at least 5 days/week in patients with chronic coronary syndromes, personalized based on the ‘Frequency, Intensity, Time and Type’ (FITT) principle to derive optimal efficacy with the lowest risk; addition of resistance training 2 days/week can provide further benefits. We also highlight the importance of complementing pharmacological options with regular physical exercise in patients with stable angina, and how webbased technologies can help to overcome some of the barriers to exercise training and challenges associated with implementing cardiac rehabilitation programmes during the COVID-19 pandemic.
Keywords
<p>Angina, stable; angina unstable; coronary artery disease; exercise therapy; life style; pharmacotherapy; physical fitness; risk assessment</p>
Article Details
1. Introduction
Chest pain caused by coronary artery disease (CAD) is called angina pectoris. It is brought on by physical exertion or emotional stress and may be aggravated by additional stressors, such as cold weather or the ingestion of a heavy meal; the pain is usually relieved by rest. Angina pectoris was first described by William Heberden in 1772 as a “painful and most disagreeable sensation in the breast, which seems as if it would extinguish life, if it were to increase or to continue; but the moment they stand still, all this uneasiness vanishes” [1]. Heberden also noted that a patient “who set himself a task of sawing wood for half an hour every day and was nearly cured”, indicating the prognostic and symptomatic effects of exercise in a patient with persisting angina [2]. The definition of angina has essentially remained the same over the years, and anginal pain is described as constricting discomfort that usually occurs in the front of the chest, but may radiate to the neck, shoulders, jaw or arms [3].
It is widely known that regular physical activity, including systematic exercise, is an important component that may prevent or reduce the development of CAD and reduce cardiovascular (CV) and all-cause mortality and morbidity. Furthermore, evidence also indicates that physical activity may prevent CAD. One early study noted that male executive-grade civil servants who engaged in vigorous physical activity for at least 2 hours per week during their leisure time had a one-third lower risk of developing CAD and a marked reduction in symptoms of CAD compared with their inactive peers (Table 1) [4]. In general, modern guideline-based therapeutic strategies for chronic stable angina aim to reduce symptoms and improve patient prognosis through lifestyle changes (e.g. smoking cessation, healthy diet, healthy body weight and physical activity combined with appropriate medications and interventions) [5].
Table 1: Effect of vigorous physical exercise on prognosis and symptoms of coronary heart (CHD) disease in male executive-grade civil servants aged 40–64 years (Used with permission of Elsevier from Morris JN, Chave SP, Adam C, et al. Vigorous exercise in leisure-time and the incidence of coronary heart-disease. Lancet 1(1973):333-9, permission conveyed through Copyright Clearance Center, Inc. [4])
|
n (%) |
Men with first attack of CHD |
Matched controls |
|
(N = 214) |
(N = 428) |
|
|
Men doing vigorous exercise |
23 (10.7) |
111 (25.9) |
|
Men not doing vigorous exercise |
191 (89.2) |
317 (74.1) |
|
Form of vigorous exercise |
||
|
Active recreation |
5 (2.3) |
15 (3.5) |
|
Keep fit |
3 (1.4) |
15 (3.5) |
|
Hard physical work |
17 (7.9) |
73 (17.1) |
|
Vigorous getting about |
1 (0.5) |
18 (4.2) |
|
Climbing up 500+ stairs |
0 |
8 (1.9) |
|
Chest pain in men doing vigorous exercise |
(n = 23) |
(n = 111) |
|
Angina pectoris |
2 (8.7) |
5 (4.5) |
|
Other pain and discomfort |
7 (30.4) |
20 (18.0) |
|
No pain/discomfort |
14 (60.9) |
86 (77.4) |
|
Chest pain in men not doing vigorous exercise |
(n = 191) |
(n = 317) |
|
Angina pectoris |
14 (7.3) |
14 (4.4) |
|
Other pain and discomfort |
51 (26.7) |
63 (19.9) |
|
No pain/discomfort |
126 (66.0) |
240 (75.7) |
On the other hand, evidence for the association between physical exertion and the onset of myocardial infarction (MI) was provided by a case-control study, which showed that patients with acute MI were twice as likely to have engaged in strenuous physical activity at the onset of infarction than matched controls (adjusted odds ratio [OR] 2.1; 95% confidence interval [CI] 1.1–3.6) [6]. Intense exercise may trigger life-threatening ventricular arrhythmias in the presence of underlying CV disease [7]. Major CV adverse events associated with exercise include sudden cardiac arrest and sudden cardiac death, acute coronary syndromes (e.g. myocardial ischemia and MI), transient ischemic attacks and cerebrovascular accidents, and supraventricular tachyarrhythmias [7].
The case-control study by Willich et al. also showed that patients who exercised infrequently were more likely to have onset of MI after strenuous physical activity. The OR of having engaged in physical activity in the hour prior to onset of MI was significantly higher in individuals who engaged in exertional physical activity <4 times a week than in those who reported a usual frequency of physical activity of ≥ 4 times a week (OR 6.9; 95% CI 4.1–2.2 vs OR 1.3; 95% CI 0.8–2.2) [6].
Against the background of the controversial and potentially conflicting data with respect to the association between physical activity and stable angina, this narrative review presents a summary of the currently available literature on this topic (a free-form search was performed using PubMed and SPOLIT [a German-language database of the Federal Institute of Sport Science in Cologne]). Furthermore, we discuss the importance of regular physical activity for patients with stable angina as a complementary treatment strategy and the current recommendations for exercise prescription in these patients.
2. Angina in coronary artery disease
Angina is frequently the initial clinical manifestation of CAD in approximately half of patients, and adversely affects quality of life (QoL) [8]. Of note, the 2019 European Society of Cardiology (ESC) Guidelines for the diagnosis and management of chronic coronary syndromes (CSS) now include dyspnoea as one of the primary symptoms, equivalent to angina, and incorporate dyspnoea in the determination of pretest probability [5]. In addition, the presence of angina increases the risk of major CV events by approximately?2-fold [8].
Although the exact aetiology of angina is not well defined, it is thought to result from a deficiency in myocardial oxygen supply, and is usually caused by atherosclerotic CAD, which restricts blood flow and, therefore, oxygen supply to the heart muscle [3, 9]. Patients with angina episodes that are stable over 3–6 months are considered to have stable angina [10], while angina at rest or on minimal exertion in the absence of acute cardiomyocyte injury/necrosis describes unstable angina [11].
Angina is often under-recognized by cardiologists and general practitioners (GPs). Beltrame et al. [12] showed a substantial discordance when comparing physicians’ and patients’ assessments of angina control. GPs considered patients' angina to be optimally controlled in 80% of cases, despite the high prevalence of frequent angina symptoms described by the patients. Among the patients with weekly and daily angina, GPs felt that 48% and 37%, respectively, were optimally controlled. This under-recognition may result in less aggressive treatment escalation and potentially poorer angina control [13].
In the APPEAR study in patients with CAD (n = 1257), angina was under-recognized in 43.3% of the 411 patients who reported angina in the previous month [13]. Patients with under-recognized angina were significantly less likely to undergo treatment escalation than those whose angina was appropriately recognized (8.4% vs 39.1%; p < 0.001) [13]. The likelihood of angina being under-recognized is higher if angina occurs less frequently (OR for monthly vs daily/weekly angina 1.69; 95% CI 1.12–2.56) [14].
Up to 80% of patients with CAD can develop warm-up angina, which is defined by reduced ischemia (ST-segment depression) or a raised ischemic threshold on second versus first exertion [15]. Studies have shown that it is safe for patients to exercise to the point of angina [15], and a warm-up period of 5–10 minutes may allow patients to safely engage in higher-intensity exercise training [16].
The exact mechanism of warm-up angina is unclear, but several mechanisms explaining the cardioprotective phenomenon have been proposed, including (1) improvement in myocardial perfusion; (2) increased myocardial resistance to ischemia, similar to ischemic preconditioning; (3) a reduction in myocardial work [15] and improvement in endothelial function resulting in increased coronary arterial reserve [17].
3. Role of exercise in the prevention and treatment of chronic coronary syndrome (CCS)
Exercise is defined as physical activity that is structured, repetitive and purposeful to improve or maintain physical fitness [7]. As exercise has numerous beneficial effects on the CV system and CV risk factors, it has also been referred to as a ‘polypill’ [5, 18-20]. Besides improving the prognosis of patients with CCS, the second principal aim of treatment is to improve QoL and to eliminate angina [13]. Regular physical activity and exercise training is protective in patients with CAD and improves prognosis and physical fitness [21, 22]. The STABILITY study showed that increased physical activity was associated with a lower mortality risk in patients with CAD (n = 15,486) [23]. Doubling of exercise volume lowered all-cause mortality (HR 0.90; 95% CI 0.87–0.93), CV mortality (HR 0.92; 95% CI 0.88–0.96) and non-CV mortality (HR 0.88; 95% CI 0.83–0.95) in these patients [23].
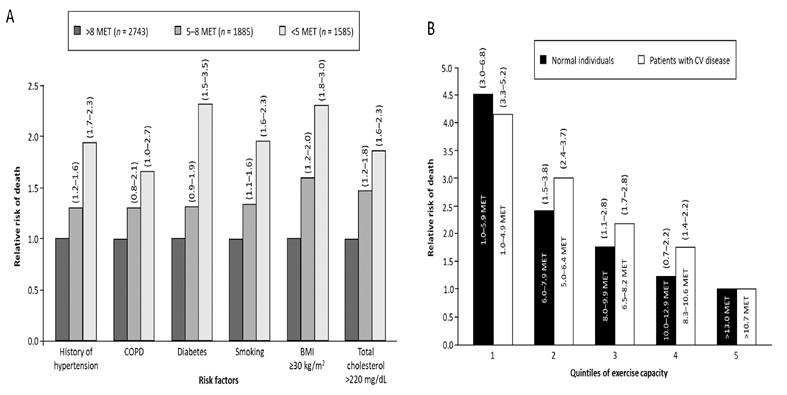
Exercise capacity is considered to be a strong prognostic factor in patients with and without CV disease [24]. Among 6,213 men referred for exercise testing for clinical reasons, 1,256 deaths were reported during a mean follow-up period of 6.2 years (average mortality rate 2.6%). After adjustment for age, peak exercise capacity was found to be the strongest predictor of a decreased risk of death in healthy individuals (n = 2534; HR 0.84; 95% CI 0.79–0.89; p < 0.001) and in patients with CV disease (n = 3679; HR 0.91; 95% CI 0.88–0.94; p < 0.001), with every one metabolic equivalents (MET) increase in exercise capacity conferring a 12% improvement in survival in the overall population.
The age-adjusted relative risk (RR) of any-cause mortality increased significantly as exercise capacity decreased, both in healthy individuals and patients with CV disease (Figure 1) [24]. A meta-analysis of 33 epidemiological studies showed that the risk of developing CAD was 14% lower (RR 0.86; 95% CI 0.77–0.96) in individuals who engaged in the equivalent of 150 minutes/week (550 kcal/week) of moderate-intensity leisure-time physical activity (recommended minimum in the US physical activity guidelines [25]) and 20% lower (RR 0.80; 95% CI 0.74–0.88) in those who engaged in the equivalent of 300 minutes/week (1000 kcal/week) compared with individuals who did not engage in leisure-time physical activity [26]. Interestingly, the association between leisure-time physical activity and the risk of CAD was stronger in women than in men (p = 0.03) [26].
Figure 1: Age-adjusted relative risk of death from any cause (A) among individuals with various risk factors in the overall population and (B) according to quintile of exercise capacity in normal individuals and patients with cardiovascular disease (from Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. New England Journal of Medicine 346 (2002) :793-801. Copyright © 2002 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society [24]). BMI, body mass index; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; MET, metabolic equivalents.
A recent, large (n = 130,843) prospective cohort study found that increasing recreational and non-recreational physical activity over a 6.9-year follow-up period lowered the risk of mortality and CV disease in low-, middle- and high-income countries [27]. Graded reductions in mortality were observed with low (<600 METs/week or <150 minutes/week of moderate intensity physical activity), moderate (600–3000 METs/week or 150–750 minutes/week) and high (>3000 METs/week or >750 minutes/week) physical activity levels. Both recreational and non-recreational activities were associated with CV benefits. A graded association was also seen between increasing physical fitness and lower CV risk [27]. These results were also confirmed by a multimodal exercise-based prevention program in CV-high risk patients demonstrating a significant reduction of coronary events [28].
In contrast, reduced physical activity has been shown to increase the risk of MI, ischemic heart disease and mortality. In the population-based Copenhagen city heart study, women who decreased leisure-time physical activity by at least one level had a higher risk of MI (hazard ratio [HR] 1.30; 95% CI 1.03–1.65), ischemic heart disease (HR 1.28; 95% CI 1.10–1.49) and all-cause mortality (HR 1.23; 95% CI 1.11–1.35) than women with an unchanged level of physical activity [29]. In this study, men who reduced physical activity by at least two levels had a higher risk of MI (HR 1.74; 95% CI 1.17–2.60), and those who reduced physical activity by at least one level had a higher risk of all-cause mortality (HR 1.15; 95% CI 1.04–1.28) [29].
Similarly, inactivity or sedentary behaviour has been associated with a higher risk of mortality in patients with CAD [30, 31]. Over 2 years’ follow-up in one study, patients with CAD (n = 1,746) who remained inactive or became inactive had a 4.9- and 2.4-fold higher risk of cardiac death, respectively, than patients who remained at least irregularly active (both p < 0.01) [30]. Over a 10-year follow-up period in a prospective cohort study in patients with CAD (n = 1038), the least active group of patients had an approximately?2-fold higher risk of major CV events and a 4-fold higher risk of both CV and all-cause mortality than the reference group of moderately frequent active patients [31]. However, a reverse J-shaped association was observed between physical activity levels and CV mortality, with the most frequently active patients who engaged in daily strenuous physical activity also having increased CV mortality [31].
Moreover, the Nord-Trøndelag Health Study (HUNT) found that regular and sustained physical activity is needed to derive the greatest benefit in terms of reductions in CV disease mortality [32]. The HUNT study assessed the association between physical activity, body mass index and all-cause and CV mortality in 3,307 patients with CAD over a 30-year follow-up period. The risk of CV mortality was significantly lower in patients who maintained a high level of physical activity (adjusted HR 0.62; 95% CI 0.43–0.89) or changed from inactive to high physical activity (adjusted HR 0.68; 95% CI 0.47–0.97) over the follow-up period [32].
Several underlying mechanisms for these favorable effects of regular moderate-to-vigorous physical activity are suggested. It has been demonstrated that regular physical activity reduces myocardial oxygen demand at rest and during any given submaximal workload. Among others, lowering of heart rate and systolic blood pressure and an increase of the period of diastole, during which coronary perfusion predominates, may be the underlying mechanisms [21]. Exercise also augments coronary blood flow by improving endothelial and coronary smooth muscle function, thereby enhancing coronary vasodilation [33]. Furthermore, exercise promotes coronary collateralization and attenuates the progression of coronary atherosclerosis [33, 34]. Exercise also improves the production of endothelial progenitor cells and increases nitric oxide delivery [21, 35]. Further benefits of regular exercise include improvements in atherosclerosis risk factors, endurance, muscle strength, mood and cognition, self-esteem and sleep quality [36].
Medically supervised, structured cardiac rehabilitation (CR) programs aim to stabilize, slow or even reverse the progression of CV disease, thereby reducing the risk of a future cardiac event [37]. Structured exercise and CR programmes have been shown to reduce total mortality by > 20%, CV mortality by > 26% and nonfatal MI by > 21% [21]. CR also reduces hospital admissions and improves psychological wellbeing, QoL and the CV risk profile [38]. Exercise-based CR may improve short-term exercise capacity in patients with stable angina [39]. A meta-analysis of seven studies (n = 581 patients) with a median follow-up of 12 months found evidence that exercise-based CR resulted in an improvement in exercise capacity (standard mean difference 0.45; 95% CI 0.20–0.70). A Cochrane analysis of randomized controlled trials, which exclusively examined patients with CCS and evaluated the effect of training-based CR over a period of up to 12 months, found evidence that such training resulted in a significant but moderate improvement in exercise capacity (standard mean difference 0.45; 95% CI 0.20–0.70) [39]. CR may also be particularly beneficial in frail patients [40]. Hence, older patients with CV disease should be assessed for frailty and encouraged to increase physical activity [40].
4. Exercise t’raining as adjunctive treatment for stable angina
There is a lack of information regarding the effects of exercise specifically in patients with symptomatic CAD, where angina is present and stable despite therapy. Several studies report that in spite of optimal medical and interventional therapy, around 50% of CAD patients remain symptomatic with angina periods. The angina frequency varies between monthly to weekly and up to daily [41]. The 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease do not make specific recommendations on patients with stable angina. Our scientific knowledge is largely derived from the subgroup of patients with persisting angina pectoris and only a few studies are available where specifically patients with angina were included; many of the available studies are 20 to 30 years old. Todd et al. investigated exercise training as an adjunct to pharmacological management of CAD with angina pectoris [42]. A 1-year high-intensity training programme in men with CCS improved exercise tolerance to a greater extent than atenolol, as indicated by an increase in treadmill time from 741 seconds at baseline to 1272 seconds with training versus 974 seconds with atenolol [42]. Thus, the anti-anginal effects of exercise training were as good as those achieved with β-blockers, indicating that exercise training may provide an alternative to pharmacological therapy [42]. Another study suggested that exercise training together with medical treatment could be an alternative to an interventional strategy in selected, motivated patients with CAD [43]. Among 101 patients randomized to 12 months of exercise training (20 minutes/day of bicycle ergometry) or standard percutaneous coronary intervention (PCI), exercise training was found to be associated with a higher rate of event-free survival (88% vs 70%; p = 0.023) and a greater increase in maximal oxygen uptake (p = 0.008) [43]. The costs associated with exercise training were also significantly lower than the cost per PCI (p < 0.001), with costs of $3,429 in the exercise training group and $6,956 in the PCI group in order to gain a clinical improvement of one Canadian Cardiovascular Society classification of angina class [43].
A study in 40 patients with PCI showed that regular high-intensity interval training (HIIT) significantly (p = 0.01) reduced restenosis (assessed as late luminal loss in the stented coronary segment), suggesting that medical therapy, mechanical revascularization and physical rehabilitation complement each other and should be a natural part of the treatment program for patients with CAD [44]. Exercise after PCI may improve left ventricular ejection fraction (LVEF) and reduce the risk of cardiac death, MI, coronary angioplasty, angina pectoris and restenosis [45]. Although, patients who undergo coronary stenting are routinely advised to resume exercise in a delayed and graduated fashion, a study in 21 patients with stable CAD showed that it was safe to perform maximal physical exercise immediately after PCI, as indicated by immediate normalization of objective physiological responses to exercise following PCI [46]. Thus, patients can start exercising soon after PCI, allowing them to derive the benefits associated with physical activity.
5. Safety aspects of exercise in patients with CCS
A strong endorsement for the safety of promoting exercise in patients with CAD and angina is that the 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease recommend “leisure-time exercise, below the angina and ischaemic thresholds, may be considered in individuals at high risk of exercise induced adverse events”. Also, these guidelines do not consider angina to be a high-risk feature in the context of exercise-induced adverse cardiac events in patients with atherosclerotic CCS [7].
A number of studies have examined the safety of exercise in patients with CCS/CV diseases and support the application of exercise as a diagnostic aid. In a safety study by Noel et al., vigorous endurance training was titrated so that at least 1 mm but no more than 3 mm of ST-segment depression was induced, in order to target and remain in the ischemic zone. These training sessions were well tolerated in patients with ischemic heart disease without apparent myocardial injury, significant arrhythmias or left ventricular dysfunction [16]. The risk of cardiac arrest during swimming was historically thought to account for a considerable number of deaths during exercise in patients with CAD. This was investigated in 25 patients with proven CAD and angina. After treadmill testing and Holter monitoring during swimming and jogging, the authors concluded that swimming bears a slight risk since one patient developed ischemia-induced ventricular tachycardia, requiring resuscitation during swimming. The authors still recommend swimming, but advise testing with treadmill and Holter [47].
6. Practical recommendations for exercise in stable angina
Given the benefits of physical activity/exercise, the ESC clinical guidelines recommend that patients with CCS should engage in 30–60 minutes of moderate-intensity aerobic activity for ≥5 days/week [5, 7]. Every 1 mL/kg/min increase in maximal oxygen consumption (VO2max) is associated with an approximately 15% reduction in the risk of death [48]. Similarly, the American Heart Association guidelines recommend 30–60 minutes of moderate-intensity physical activity (e.g. brisk walking) 7 days per week (minimum 5 days/week), supplemented by an increase in daily activities, such as walking breaks at work, gardening or household work [49]. Patients’ CV risk should be evaluated via assessment of their physical activity history and, where appropriate, an exercise test conducted to guide exercise prescription. For at-risk patients (i.e. those with recent acute coronary syndrome, revascularization or heart failure), medically supervised CR programmes are recommended. Also, the addition of resistance training 2 days/week may be reasonable for expanding physical activity in patients at moderate-to-high CV risk [49].
6.1 Exercise training in cardiac rehabilitation programs
Exercise-based CR is recommended by the ESC guidelines as an effective means for patients with CCS to achieve a healthy lifestyle and manage risk factors [5]. Exercise training is a cornerstone of CR, and aerobic interval training appears to be safe in patients with CAD and preserved and/or reduced LVEF [50, 51]. A meta-analysis of 15 studies found aerobic interval training to be superior to moderate continuous training for improving peak exercise capacity in these patients (1.60 mL/kg/min greater increase in exercise capacity; p = 0.03) [50]. Safe and progressive exercise programs are essential for improving functional capacity, which is a key prognostic indicator for patients with CV disease. Functional capacity, among other factors, measures the heart’s ability to deliver oxygen to tissues and the proficiency of tissues to utilize oxygen [52].
According to the 2020 ESC Guidelines on Sports Cardiology “individuals with risk factors should be restricted from competitive sport only when there is substantial risk of an adverse event, as indicated by functional tests, or when there is evidence of disease progression during serial evaluations” [7]. Furthermore, “exercise recommendations should be individually tailored based on the intensity of the exercise and the sporting discipline. Participation in competitive endurance, power, and mixed disciplines generally requires vigorous effort and is more likely to induce myocardial ischaemia, whereas leisure sports or intentional recreational exercise allows for greater control of physical effort. Individuals with a high risk of atherosclerotic CAD and asymptomatic individuals in whom CAD is detected at screening who participate in intensive exercise should be assessed with a maximal exercise test or functional imaging test on an annual basis” [7].
Due to heterogeneity within the population, exercise training should be personalized based on the ‘Frequency, Intensity, Time and Type’ (FITT) principle to derive the optimal effectiveness with the lowest associated risk [51]. The preferred type of exercise should be a global endurance training involving a large number of muscle groups (e.g. exercises on an ergometric bicycle or treadmill Nordic walking) combined with resistance training of specific muscle groups. The recommended frequency of exercise varies from three to five sessions/week and the recommended duration ranging from 30 minutes to 2 hours per session, with the length of programs fluctuating between 2 and 3 months. Of the four FITT factors, exercise intensity is the least standardized and may vary from exercises aimed at improving aerobic capacity (based on moderate-intensity training to facilitate activities of daily living) or improving performance with high-intensity exercises. The proposed intensity of exercise can range from 40% to 80%, or even up to 95%, of maximal capacity [51]. In patients with CAD, a graded exercise test is important for determining the exercise intensity level at which symptoms occur (reported as the ischemic or anginal threshold) [52]. Exercise intensity at each CR session should remain below the ischemic threshold (i.e. the exercise heart rate should be 10 bpm lower than the heart rate at which angina symptoms occur) [52].
Regarding heart rate analysis, peak heart rate and VO2 values from a maximal-effort graded exercise test are used to prescribe aerobic exercise intensity [51]. Commonly used indicators of exercise intensity are percentages of heart rate reserve (%HRR), VO2 reserve (%VO2R) and peak aerobic power (%VO2max) [51, 52]. Usually, the goal is to titrate exercise intensity to achieve 40–80% HRR, VO2max or VO2R [52]. Incremental cardiopulmonary exercise testing is the best tool for assessing exercise capacity, CV risk and functional capacity [51]. It is most useful for developing an individualized exercise prescription and for assessing the effectiveness of training during the program and after its completion [53]. Without a graded exercise test, the intensity goal for the first CR exercise session is a heart rate 20–30 bpm above resting, and a rate of perceived exertion monitored of within the range of 11 to 14 on the Borg scale [52]. The Borg scale is a subjective 6- to 20-point scale assessing the rate of perceived exertion, with a target of 12–16 (moderate to hard) [51, 52].
In patients with stable CCS, it is obligatory to individually tailor the training [54]. With respect to additive resistance training [51], it is recommended that resistance training is initiated at 30–50% of maximum voluntary contraction in order to avoid cardiac events and musculoskeletal complications, with an increase to 50–80% of the maximum muscle strength as CR progresses. Periodic evaluation permits accurate adjustment of the training program, while avoiding significant muscle injury or soreness [51]. Walking speed can also be used to personalize training especially in patients with reduced exercise capacity [51]. Self-selected walking velocity, representing a comfortable walking speed, ensures that walking is well tolerated and practiced with pleasure, thus encouraging compliance with lifestyle changes in patients with CAD. Walking tests, e.g. the 6-minute walking test or the shuttle-walking test, are well tolerated and can be used to assess the effects of exercise training in the context of a CR programme [51].
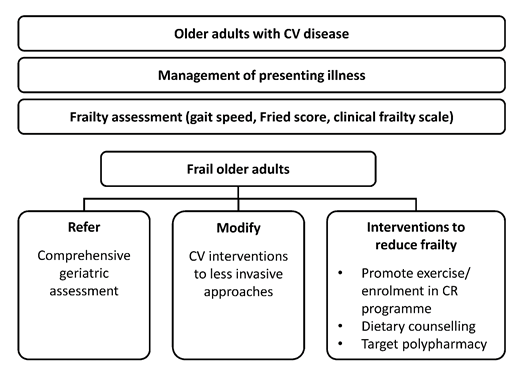
Patients with CV disease have been shown to achieve larger improvements in functional capacity with high-intensity exercises throughout CR [52]. One study found that among patients with stable CAD who signed up for CR (n = 39), VO2max after CR improved to a significantly greater extent with HIIT than with moderate-intensity continuous training (3.6 ± 3.1 vs 1.7 ± 1.7 mL/kg/min; p < 0.05) [55]. Thus, it is critically important that patients with CV disease progress towards achieving higher exercise loads throughout their CR program [52]. In older patients, specific deficits in physical activity can be identified and exercise-based CR may also aim to prevent and treat frailty (Figure 2) [40]. In addition, medical supervision and electrocardiographic (ECG) and blood pressure monitoring during the initial prescription sessions are beneficial [56].
Figure 2: Treatment algorithm for older adults with cardiovascular disease (from Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. European Heart Journal 35 (2014): 1726-31, by permission of the European Society of Cardiology [40]). CR, cardiac rehabilitation; CV, cardiovascular.
6.2 Barriers to exercise training
Despite the benefits associated with exercise training, referral and enrolment into CR program are limited [21]. Patient factors shown to negatively impact participation in CR programs include female sex, older age, lack of or limited health insurance, poor self-management, decreased health literacy, inadequate understanding of the disease and its treatment and a lack of perceived need. Failure of patient referral is the most significant factor for the poor use of CR programs. In order to improve CR uptake, it is recommended that hospital discharge plans for patients with myocardial ischemia should include referral to an outpatient CR program and close attention be given to follow-up appointments, while non-hospitalized patients should be referred by their physician. Details regarding CR programs should be made available in multiple languages, and home-based programs should be made available for low-risk patients with myocardial ischemia. The addition of home-based CR increases exercise frequency and may also help transition patients from center-based programs to self-care programs [21].
Another barrier to performing regular exercise is the patient’s avoidance of physical stress and activity for fear of precipitating an angina attack [57]. Medications that improve exercise tolerance or reduce the incidence of angina episodes can improve the uptake of exercise interventions. Prophylactic use of rapidly absorbed sublingual nitroglycerine tablets and oral nitroglycerine spray can improve exercise tolerance and reduce the incidence of MI [3, 5, 10, 57]. Also, calcium channel blockers have been shown to reduce the incidence of angina episodes, increase exercise duration and decrease nitroglycerine use [10]. β-Blockers inhibit sympathetic stimulation of the heart, reducing heart rate and contractility and resulting in decreased myocardial demand [42]. The effect of β-blockers on heart rate is minimal at rest, but the effect is more apparent during physical or emotional stress [9, 42]. In patients with chronic angina, extended-release ranolazine increased total exercise time up to 46 seconds after 1 week of monotherapy compared to placebo [58], and demonstrated a 23.7-second improvement in exercise duration when administered in combination with other antianginals compared with standard antianginal therapy alone [59]. CR programs could also be greatly improved by digital health tools [60]. Web-based technologies, such as video conferencing, and devices, including mobile phones and blood pressure, glucose and heart rate monitors, can be used to mitigate at least two barriers to participation in CR programs, namely cost and accessibility [21, 37]. The increased availability of smartphones and high-speed internet provides an opportunity to promote a major shift in CR programs, thereby improving the health of a larger number of individuals with cardiac disease [61]. Web-based technologies have the potential to favorably deliver all core components of CV risk reduction interventions. They can be used to enhance program compliance, provide ongoing feedback to patients and practitioners, and document program outcomes [21, 37]. An ideal digital program is one that can be delivered fully to patients’ homes, either as an add-on to, or replacement for, traditional center-based CR [60]. Home-based CR programs include the same core components as center-based ones, but the interventions are delivered mostly or entirely in the patient’s home [62]. Use of telemedicine tools (e.g. video-conferencing) in home-based programs could also overcome the disadvantages of a lack of direct supervision and socialization [62]. Invasive and non-invasive sensors can monitor various physiological parameters and thus improve the training in patients with cardiovascular disease. In the future, medication and physical activity prescription could be driven by artificial-intelligence algorithms. Furthermore, delivery of this highly personalized medicine represents a low burden on the healthcare system. These digital tools should be integrated into a system that is supported by a multidisciplinary team of CR providers. If required, video conferencing can enable swift contact between patients and healthcare facilities [60]. Privacy and data protection are of key importance in the context of digital technology [60].
7. The three cornerstones of the treatment of angina
The effective management of angina should comprise three key elements: 1. PCI (stent implant or balloon angioplasty) or coronary artery bypass graft surgery, 2. symptomatic pharmacological therapy, to reduce angina (such as a β-blocker, calcium channel blocker, or ranolazine, ivabradine, or long-acting nitrates); and 3. physical activity [63]. Cook and colleagues compared pre- and post-PCI exercise performance (exercising until rate-limiting angina or exhaustion) in 21 patients with stable CAD [46]. In addition to significantly improving many coronary and CV parameters (flow velocity, perfusion pressure, systolic blood pressure and myocardial workload) after PCI, exercise time significantly improved and rate-limiting angina symptoms significantly reduced, thereby rapidly normalizing the objective physiological response to exercise [46]. Few studies have examined the effect of combining the key elements of the management of angina, e.g. the combination of pharmacological treatment and exercise training. One such study investigated whether the addition of ranolazine to an exercise program could provide greater benefits than exercise alone in patients with CAD and stable, frequent angina [64]; it demonstrated that ranolazine would allow patients to exercise at greater intensities during training and derive greater clinical benefit.In patients receiving ranolazine, the improvement in aerobic capacity, physical activity and health-related QoL was significantly greater than that achieved with exercise alone. While this study involved a small number of patients (n = 37 randomized to treatment), it is unique in that it examined the benefit of combining two management approaches: medical therapy and exercise [64].
8. Angina and COVID-19
Post-acute coronavirus disease 2019 (COVID-19), also referred to as long-COVID, appears to be a multisystem disease that can occur after a relatively mild acute COVID infection [65]. It is characterized by persisting symptoms 12 weeks after an acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In addition to fatigue, chest pain is one of the most common complaints in these patients and it is critical to separate musculoskeletal and other non-specific chest pain from serious CV conditions [65].
Chest pain in patients with post-acute COVID-19 should be assessed in the same manner as any chest pain (i.e. by taking into account past medical history and risk factors, a physical examination and further investigations as necessary) [65, 66]. If the patient is acutely unwell or if the diagnosis is unclear, hospital referral may be required for further assessments and investigations [65]. The COVID-19 pandemic has resulted in the suspension of many CR services and programs. Given the health benefits of CR, cessation of these programs can result in dramatically adverse consequences in terms of public health [61]. Thus, there is an urgent need to develop remote home-based CR programs, both in clinical research and routine clinical care, as discussed in the section above [61]. Before resuming CR, cardiac assessment is recommended [65]. Upon recovery from mild COVID-19 illness, one week of low-level physical activity such as stretching and strengthening is suggested before commencing targeted CV sessions; patients with mild symptoms should limit their activity to slow walking, avoiding high-intensity training, while those with persistent symptoms (e.g. fatigue, cough, breathlessness and fever) should limit activity to 60% maximum heart rate until 2–3 weeks after their symptoms resolve [65]. The German guidelines for the management of patients with post-acute COVID suggest performing a 12-channel ECG and determination of blood count, and C-reactive protein, troponin and N-terminal pro B-type natriuretic peptide levels [67]. If these parameters are within the normal range, the risk of a cardiovascular event in the future is very low and physical exercise at moderate intensities can be resumed.
9. Conclusion
Regular physical activity is a key component in the management of CCS, including stable angina. Regular physical activity and exercise training provides cardioprotective benefits for patients with CAD and improves their physical fitness and QoL. Even irregular leisure-time physical activity reduces the risk of mortality in previously sedentary patients. Given the benefits of exercise on cardiac health in patients with CCS, current guidelines recommend 30–60 minutes of moderate-intensity aerobic activity combined with low-to-moderate intensity resistance training for at least 5 days/week. It should be noted that there are no specific recommendations for symptomatic patients with angina but only for CAD patients in general. Symptoms of angina induced by physical exercise should be targeted by concomitant pharmacological treatment. The use of digital technology will help to individually tailor the exercise therapy.
References
- De La Chapelle CE. The recognition of angina pectoris. Circulation 21 (1960): 1061-1064.
- MacAlpin RN and Kattus AA. Adaptation to exercise in angina pectoris. The electrocardiogram during treadmill walking and coronary angiographic findings. Circulation 33 (1966): 183-201.
- National Institute for Health and Care Excellence (2011). "Stable angina: management." 2021.
- Morris JN, Chave SP, Adam C, et al. Vigorous exercise in leisure-time and the incidence of coronary heart-disease. Lancet 1 (1973): 333-339.
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal 41 (2020): 407-477.
- Willich SN, Lewis M, Lowel H, et al. Physical exertion as a trigger of acute myocardial infarction. Triggers and Mechanisms of Myocardial Infarction Study Group. New England Journal of Medicine. 329 (1993): 1684-1690.
- Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. European Heart Journal 42 (2021): 17-96.
- Ohman EM. Clinical practice. Chronic stable angina. New England Journal of Medicine 374 (2016): 1167-1176.
- Winchester DE and Pepine CJ. Angina treatments and prevention of cardiac events: an appraisal of the evidence. European Heart Journal Supplements 17 (2015): G10-G18.
- Rousan TA and Thadani U. Stable angina medical therapy management guidelines: a critical review of guidelines from the European Society of Cardiology and National Institute for Health and Care Excellence. European Cardiology 14 (2019): 18-22.
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal (2020): 9.
- Beltrame JF, Weekes AJ, Morgan C, Tavella R and Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The Coronary Artery Disease in General Practice (CADENCE) Study. Archives of Internal Medicine 169 (2009): 1491-1499.
- Qintar M, Spertus JA, Gosch KL, et al. Effect of angina under-recognition on treatment in outpatients with stable ischaemic heart disease. European Heart Journal: Quality of Care and Clinical Outcomes 2 (2016): 208-214.
- Arnold SV, Grodzinsky A, Gosch KL, et al. Predictors of physician under-recognition of angina in outpatients with stable coronary artery disease. Circulation: Cardiovascular Quality and Outcomes 9 (2016): 554-559.
- Williams RP, Manou-Stathopoulou V, Redwood SR and Marber MS. 'Warm-up angina': harnessing the benefits of exercise and myocardial ischaemia. Heart 100 (2014): 106-114.
- Noel M, Jobin J, Marcoux A, et al. Can prolonged exercise-induced myocardial ischaemia be innocuous? European Heart Journal 28 (2007): 1559-1565.
- Bogaty P, Poirier P, Boyer L, Jobin J and Dagenais GR. What induces the warm-up ischemia/angina phenomenon: exercise or myocardial ischemia? Circulation 107 (2003): 1858-1863.
- Pinckard K, Baskin KK and Stanford KI. Effects of exercise to improve cardiovascular health. Frontiers in Cardiovascular Medicine 6 (2019): 69.
- Winzer EB, Woitek F and Linke A. Physical activity in the prevention and treatment of coronary artery disease. Journal of the American Heart Association 7 (2018): 1-12.
- Erbs S, Linke A and Hambrecht R. Effects of exercise training on mortality in patients with coronary heart disease. Coronary Artery Disease 17 (2006): 219-225.
- Boden WE, Franklin B, Berra K, et al. Exercise as a therapeutic intervention in patients with stable ischemic heart disease: an underfilled prescription. American Journal of Medicine 127 (2014): 905-911.
- Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 111 (2005): 369-376.
- Stewart RAH, Held C, Hadziosmanovic N, et al. Physical activity and mortality in patients with stable coronary heart disease. Journal of the American College of Cardiology 70 (2017): 1689-1700.
- Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. New England Journal of Medicine 346 (2002): 793-801.
- US Department of Health and Human Services. "Physical activity guidelines for Americans, 2nd edition” (2018).
- Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 124 (2011): 789-795.
- Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet 390 (2017): 2643-2654.
- Gysan DB, Millentrup S, Albus C, et al. Substantial improvement of primary cardiovascular prevention by a systematic score-based multimodal approach: A randomized trial: The PreFord-Study. Eur J Prev Cardiol 24 (2017): 1544-1554.
- Petersen CB, Gronbaek M, Helge JW, et al. Changes in physical activity in leisure time and the risk of myocardial infarction, ischemic heart disease, and all-cause mortality. European Journal of Epidemiology 27 (2012): 91-99.
- Lahtinen M, Toukola T, Junttila MJ, et al. Effect of changes in physical activity on risk for cardiac death in patients with coronary artery disease. American Journal of Cardiology 121 (2018): 143-148.
- Mons U, Hahmann H and Brenner H. A reverse J-shaped association of leisure time physical activity with prognosis in patients with stable coronary heart disease: evidence from a large cohort with repeated measurements. Heart 100 (2014): 1043-1049.
- Moholdt T, Lavie CJ and Nauman J. Sustained physical activity, not weight loss, associated with improved survival in coronary heart disease. Journal of the American College of Cardiology 71 (2018): 1094-1101.
- Bruning RS and Sturek M. Benefits of exercise training on coronary blood flow in coronary artery disease patients. Progress in Cardiovascular Diseases 57 (2015): 443-453.
- Zbinden R, Zbinden S, Meier P, et al. Coronary collateral flow in response to endurance exercise training. European Association for Cardiovascular Prevention and Rehabilitation 14 (2007): 250-257.
- Adams V, Lenk K, Linke A, et al. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arteriosclerosis, Thrombosis, and Vascular Biology 24 (2004): 684-690.
- Alpert JS. Exercise is just as important as your medication. American Journal of Medicine 127 (2014): 897-898.
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 124 (2011): 2951-2960.
- Dalal HM, Doherty P and Taylor RS. Cardiac rehabilitation. British Medical Journal 351 (2015): h5000.
- Long L, Anderson L, He J, et al. Exercise-based cardiac rehabilitation for stable angina: systematic review and meta-analysis. Open Heart 6 (2019): e000989.
- Singh M, Stewart R and White H. Importance of frailty in patients with cardiovascular disease. European Heart Journal 35 (2014): 1726-1731.
- Arnold SV, Morrow DA, Lei Y, et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circulation: Cardiovascular Quality and Outcomes 2 (2009): 344-353.
- Todd IC and Ballantyne D. Antianginal efficacy of exercise training: a comparison with beta blockade. British Heart Journal 64 (1990): 14-19.
- Hambrecht R, Walther C, Mobius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 109 (2004): 1371-1378.
- Munk PS, Staal EM, Butt N, Isaksen K and Larsen AI. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation A randomized controlled trial evaluating the relationship to endothelial function and inflammation. American Heart Journal 158 (2009): 734-741.
- Zhang H and Chang R. Effects of exercise after percutaneous coronary intervention on cardiac function and cardiovascular adverse events in patients with coronary heart disease: systematic review and meta-analysis. Journal of Sports Science and Medicine 18 (2019): 213-222.
- Cook CM, Ahmad Y, Howard JP, et al. Impact of percutaneous revascularization on exercise hemodynamics in patients with stable coronary disease. Journal of the American College of Cardiology 72 (2018): 970-983.
- Niebauer J, Hambrecht R, Hauer K, et al. Identification of patients at risk during swimming by Holter monitoring. American Journal of Cardiology 74 (1994): 651-656.
- Keteyian SJ, Brawner CA, Savage PD, et al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. American Heart Journal 156 (2008): 292-300.
- Fraker TD, Jr., Fihn SD, Gibbons RJ, et al. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation 116 (2007): 2762-2772.
- Pattyn N, Coeckelberghs E, Buys R, Cornelissen VA and Vanhees L. Aerobic interval training vs. moderate continuous training in coronary artery disease patients: a systematic review and meta-analysis. Sports Medicine 44 (2014): 687-700.
- Casillas JM, Gudjoncik A, Gremeaux V, et al. Assessment tools for personalizing training intensity during cardiac rehabilitation: literature review and practical proposals. Annals of Physical and Rehabilitation Medicine 60 (2017): 43-49.
- Mytinger M, Nelson RK and Zuhl M. Exercise prescription guidelines for cardiovascular disease patients in the absence of a baseline stress test. Journal of Cardiovascular Development and Disease 7 (2020): 1-3.
- Palermo P and Corra U. Exercise prescriptions for training and rehabilitation in patients with heart and lung disease. Annals of the American Thoracic Society 14 (2017): S59-S66.
- Schwaab B, Bjarnason-Wehrens B, Meng K, et al. Cardiac Rehabilitation in German Speaking Countries of Europe-Evidence-Based Guidelines from Germany, Austria and Switzerland LLKardReha-DACH-Part 2. Journal of Clinical Medicine 10 (2021): 4-7.
- Keteyian SJ, Hibner BA, Bronsteen K, et al. Greater improvement in cardiorespiratory fitness using higher-intensity interval training in the standard cardiac rehabilitation setting. Journal of Cardiopulmonary Rehabilitation and Prevention 34 (2014): 98-105.
- Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 128 (2013): 873-934.
- Hambrecht R, Berra K and Calfas KJ. Cardiology patient page. Managing your angina symptoms with nitroglycerin: what about exercise? Circulation 127 (2013): e642-645.
- Chaitman BR, Skettino SL, Parker JO, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. Journal of the American College of Cardiology 43 (2004): 1375-1382.
- Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. Journal of the American Medical Association 291 (2004): 309-316.
- Falter M, Scherrenberg M and Dendale P. Digital health in cardiac rehabilitation and secondary prevention: a search for the ideal tool. Sensors 21 (2020).
- Besnier F, Gayda M, Nigam A, Juneau M and Bherer L. Cardiac rehabilitation during quarantine in COVID-19 pandemic: challenges for center-based programs. Archives of Physical Medicine and Rehabilitation 101 (2020): 1835-1838.
- Ribeiro F and Santos M. Exercise-based cardiac rehabilitation in COVID-19 times: one small step for health care systems, one giant leap for patients. Revista Española de Cardiología (English Edition) 73 (2020): 969-970.
- Jain A, Wadehra V and Timmis AD. Management of stable angina. Postgraduate Medical Journal 79 (2003): 332-336.
- Willis LH, Slentz CA, Johnson JL, et al. Effects of Exercise Training With and Without Ranolazine on Peak Oxygen Consumption, Daily Physical Activity, and Quality of Life in Patients With Chronic Stable Angina Pectoris. American Journal of Cardiology 124 (2019): 655-660.
- Greenhalgh T, Knight M, A'Court C, Buxton M and Husain L. Management of post-acute covid-19 in primary care. British Medical Journal 370 (2020): m3026.
- Barker-Davies RM, O'Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. British Journal of Sports Medicine 54 (2020): 949-959.
- Koczulla AR, Ankermann T, Behrends U, et al. S1- Leitlinie Post-COVID/Long-COVID. The Association of the Scientific Medical Societies in Germany (2021): 1-76.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 