AQP-9, KCNJ11 and ABCC8 Gene Variants in Open Angle Glaucoma: A Hypothesis
Thumann G, M.D.1,2*, Sorgente N, Ph.D.3
1Experimental Ophthalmology, University of Geneva, 1205 Geneva, Geneva, Switzerland.
2Department of Ophthalmology, University Hospitals of Geneva, 1205 Geneva, Geneva, Switzerland.
2Elements Pharmaceuticals, Inc., Washington DC, USA.
*Corresponding Author: Gabriele Thumann, University of Geneva & University Hospitals of Geneva, Experimental Ophthalmology and Dept. of Ophthalmology, Rue Alcide-Jentzer 22, CH-1211 Geneva 14, Switzerland
Received: 05 May 2021; Accepted: 13 May 2021; Published: 18 May 2021
Article Information
Citation: Gabriele Thumann, Nino Sorgente. AQP-9, KCNJ11 and ABCC8 Gene Variants in Open Angle Glaucoma: A Hypothesis. Journal of Ophthalmology and Research 4 (2021): 161-173.
DOI: 10.26502/fjor.2644-00240034
View / Download Pdf Share at FacebookAbstract
Importance: Primary open angle glaucoma, an age-related, retinal neurodegenerative disease of unknown etiology, is treated by lowering intraocular pressure, even though elevated intraocular pressure is present in only about 60% of patients. Since we found that tolbutamide, which inhibits the opening of ATP-sensitive potassium channels, modulates aqueous dynamics with a significant increase in outflow, and since aquaporin-9 is essential for retinal ganglion cells survival, gene variants coding for the ATP-sensitive potassium channels and aquaporin-9 may participate in the development and progression of glaucoma.
Objective: To identify gene variants involved in ion and water transport in the trabecular meshwork of glaucoma donors.
Design: The study is a gene association study; since gene variants can be somatic or germline, the following genes KCNJ8, KCNJ11, ABCC8, and ABCC9, QP1, AQP4, AQP9, ATP1A1, KCNMA11, and CLCN3 associated to ATP-sensitive potassium channels and water transport in the trabecular meshwork were sequenced from DNA isolated from trabecular meshwork of glaucoma donors.
Setting: The study is a gene association study carried out with samples obtained through the Cooperative Human Tissue Network of which the DNA was isolated by the technical personnel at the Lions Vision Gift eye bank and analyzed by Admera Health LLC.
Participants: The only criteria for the ten donors (5 male, 5 females; 70-91 years of age) in the study was a diagnosis of primary open angle glaucoma and a consent form signed at their lifetime or by responsible relatives.
Main Outcomes and Measures: Of several missense variants, one was found in all 10 and three were found in nine trabecular meshwork samples. All variants, whether synonymous or missense, were germline.
Results: The AQP9 mi
Keywords
<p>Glaucoma; open-angle glaucoma; tolbutamide; ATP-sensitive potassium channels; ATP-sensitive potassium channel opener; ATP-sensitive potassium channel blocker; intraocular pressure; aqueous humor outflow.</p>
Article Details
1. Introduction
Glaucoma refers to a group of neurodegenerative ocular diseases that share a pathology characterized by retinal ganglion cell (RGC) degeneration [1], and progressive optic nerve atrophy that gradually lead to visual field loss and blindness. It is estimated that globally 65.5 million people are affected by glaucoma with about 5 million bilaterally blind [2]. Primary open angle glaucoma (POAG), the most common form of glaucoma, is associated with the progressive loss of RGC axons, along with supporting glia and vasculature, resulting in degeneration of the optic nerve head and loss of peripheral vision [3]. Advanced age and elevated intraocular pressure (IOP) are the main risk factors for the onset and progression of glaucoma even though lowering IOP in ocular hypertensive patients with no evidence of glaucoma reduces the development of glaucoma from 9.5% to 4.4% [4]. Nevertheless, 30-40% of patients with POAG present IOP values within the normal range [5, 6], indicating that increased IOP is not essential for neuronal degeneration. Since elevated IOP is the only modifiable risk factor, the aim of current therapeutic strategies is to lower IOP and include pharmacological treatments, surgical procedures, and laser treatment. However, in many patients RGCs’ degeneration continues in spite of treatments to lower IOP [7]. In fact, the risk of unilateral blindness in patients with POAG treated to lower IOP is estimated to be around 27% during a 20-year follow-up [8].
Although research is considerable, the pathological mechanisms involved in the onset and development of glaucoma are not understood. Recent research points to structural, metabolic, and functional glaucoma-driven changes in both the eye and the brain [9] and glaucoma deterioration may be already present in the eye and brain before substantial vision loss is detected clinically [10,11]. Some of the metabolic changes that are thought to underlie glaucoma pathology include calcium dysregulation [12], and alterations in glutamate and glutamine metabolism [13].
In previous work [14] we found that blockers of ATP-sensitive potassium (KATP) channels, e.g., sulfonylureas, lower IOP whereas drugs that activate KATP channels elevate IOP in rabbits. Using tolbutamide as a model sulfonylurea drug, we established that 0.5% tolbutamide applied topically to the eye lowered IOP and increased aqueous outflow. The finding that sulfonylurea drugs decrease IOP and modulate aqueous dynamics suggest that KATP channels in the eye may be mutated in POAG patients.
KATP channels are hetero-octamers consisting of four inwardly rectifying K+ channel subunits, Kir6.1 or Kir6.2, and four sulfonylurea receptors subunits, SUR1 or SUR2 or SUR2A, which belong to the family of ATP-binding cassette (ABC) transporters [15, 16]. KATP channels, which are inhibited by intracellular ATP and activated by ADP, oscillate between open and closed states that are determined by the ratio of ATP to ADP. While open-closed state of the KATP channels is primarily regulated by the level of ATP and ADP, several other factors appear to have modulatory effects [17,18].
A report that lactate is significantly elevated in the aqueous of glaucoma patients [19] indicates that in the eye energetics may be altered in glaucoma, especially considering that lactate is the preferred energy source for neurons [20-23]. The increased lactate in the aqueous suggests that the astrocyte-neuron lactate transfer shuttle [24, 25] is not operating effectively in the retina of glaucoma patients. In the brain and retina lactate is shuttled from astrocytes to neurons by a family of lactate/pyruvate monocarboxylate transporters (MCTs) [26, 27] with the cooperation of aquaporin-9 [27]. Even though the specific role of aquaporin-9 in the retina is not known, it is clear that aquaporin-9 is essential for the survival and function of RGCs [28-30].
Since POAG is a familial disease, genome-wide association studies have identified numerous gene variants associated with POAG, e.g., variants in the MYOC [31], the CDKN2B [32], the OPTN [33], the TBK1 [34], the ATXN233 genes, etc. However, there is no unifying hypothesis on how these gene variants may affect the metabolic neuronal ecosystem leading to neurodegeneration.
Since sulfonylureas, which are blockers of KATP channels, lower IOP by modifying aqueous dynamics and since aquaporin-9 is essential for deliver lactate to RGCs, we investigated whether variants of the KATP channels and aquaporin-9 genes may contribute to metabolic alterations that in glaucoma may be responsible for the neurodegeneration of RGCs. Specifically, we sequenced the genes that code for the KATP channels, i.e., the KCNJ 9, KCNJ 11, ABCC8 and ABCC9, and AQP9 gene that codes for aquaporin-9. Since age is a main risk factor for POAG, potential mutations could be somatic arising with age; therefore, we sequenced the exons of the KATP channel genes, aquaporin-9 and several other genes from DNA isolated from the trabecular meshwork tissue (TM) obtained from donors diagnosed with POAG.
2. Material and Methods
2.1 Trabecular meshwork tissue
TM tissue was obtained through the Cooperative Human Tissue Network (CHTN) and isolated by the technical personnel at the Lions Vision Gift (Portland, OR, USA) eye bank from 10 donors (5 males, 5 females; 70-91 years of age) that had been diagnosed with POAG. The isolated TM tissue was stored for subsequent sequencing in DNA/RNA Shield buffer (Zymo Research, Irvine, CA, USA).
2.2 Sequencing
TM tissue in DNA/RNA Shield buffer was shipped to Admera Health LLC (South Plainfield, NJ, USA). DNA from TM tissue was isolated using the QIAamp DNA mini kit per the manufacturer protocol (Qiagen, Germantown, USA). Custom probes were synthesized by Integrated DNA Technologies (Skokie, IL, USA) to target the exons of the following genes: KCNJ8 (codes for the Kir6.1 subunit of KATP channels), KCNJ11 (codes for the Kir6.2 subunit of KATP channels), ABCC8 (codes for the SUR1 subunit of KATP channels), ABCC9 (codes for the SUR2 subunit of KATP channels) , AQP1, AQP4, AQP9, ATP1A1 (codes for the alpha-1 subunit of Na+ /K+ ATPase), KCNMA1 (codes for the Potassium Calcium-Activated Channel Subfamily M Alpha 1), and CLCN3 (codes for Chloride Voltage-Gated Channel 3). Libraries were prepared with starting input of 250 ng of genomic DNA sheared using the Covaris S220 system (Covaris, Woburn, MA, USA). Libraries were combined into separate pools and targets were captured by hybridization using the Integrated DNA Technologies (Skokie, IL, USA) capture method. Quality and quantity checks were done on the Tape Station D1000 High Sensitivity and by the Qubit 2.0 dsDNA HS assay (Life Technologies, Grand Island, NY, USA). The average library size was approximately 400 bp. Sequencing was completed on the Illumina Miseq 300 Cycles to target 500x mean coverage. Data analysis, including genome alignment, was performed using the BWA software; variant discovery for the target regions was performed with the Genomic Analysis Toolkit (GATK, Broad Institute, Cambridge, MA, USA).
3. Results
In addition to several intronic and synonymous exonic variants of the gene sequenced, several germline missense variants were present (Table 1).
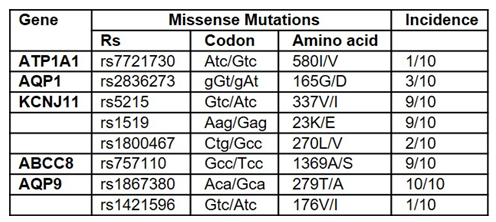
Table 1: Missense Variants in Trabecular Meshwork Tissue from Primary Open Angle Glaucoma Donors.
Two missense variants, rs5215 and rs5219, of the KCNJ11 gene, which codes for the Kir6.2 subunit of the KATP channels, and the missense variant rs757110 of the ABCC8 gene, which codes for the SUR1 subunit of the KATP channels, were found in 9 of 10 TM samples, and the AQP9 missense variant rs1867380 was found in all 10 TM samples. No missense variants were found in ABCC9, KCNJ8, AQP4, ATP1A1, and CLCN3.
4.Discussion
In the United States with 3 million diagnosed glaucoma patients, the average cost of treating glaucoma with pharmaceuticals in 2005 was $623 per patient per year for those with suspected or early-stage glaucoma and $2511 per patient per year for patients with end-stage disease [35]. Despite these numbers, the pathological mechanism is still not understood; thus, limiting treatment options to decreasing IOP pharmacologically or surgically.
Current drugs lower IOP but visual field loss continues [36], albeit at a lower rate, and many drugs are associated with significant side effects [37]. Since POAG often is not recognized by the patient until there is significant vision loss, treatment cannot be implemented too early to prevent RGC degeneration. Being able to identify patients before any RGC degeneration has occurred would allow treatment to begin before vision loss and pave the way for novel treatments.
The variants rs5215, rs5219 and rs757110 have been shown to be a risk for Type 2 diabetes (T2D) and heart disease [38-41] by increasing the hydrolysis of MgATP to MgADP [42, 43], which allows the KATP channel to remain open.
Since the rs5215 and rs5219 KCNJ11 variants are mild gain-of-function mutations, the cellular effects have been difficult to define; for the rs5215 variant (23K/E), studies have shown a significant reduction of sensitivity to ATP (wild-type KATP half-maximal inhibition = 71.0 ± 4.5 µmol/L ATP; 23K/E KATP half maximal inhibition = µmol/L 120.0 ± 5.2 µmol/L ATP) as well as an increase in open probability and reduced sulfonylurea sensitivity [44, 45]. The increase in the open probability of the channel increases the efflux of K+ into the extracellular space.
In considering the effect of the increased requirement of ATP to maintain the variant KATP channels in the normal open-closed oscillatory state, it is essential to consider the bioenergetics of the cell, the spatial distribution of mitochondria [46], the subcellular compartmentation of glycolytic and ATP-producing enzymes [47, 48], and the diffusion of ATP to cell-membrane compartments [49] limited by distance and impediment by the cellular cytoskeleton [45, 49]. The normal function of KATP channels is dependent on submembrane generated and not cellular bulk ATP as well as local factors such as availability of phosphocreatine [50-52]. The critical role of phosphocreatine for local modulation of KATP channels has been documented by Abrahams et al. [53] who showed that deletion of cytosolic creatine kinase, which transfers a phosphate to ADP to generate ATP, triggers channel opening in the presence of bulk ATP in cardiomyocytes.
4.1 Hypothesis. 23K/E and/or 337V/I KATP and/or 1369 A/S SUR variants; aquaporin-9 normal
In the aged TM the increased levels of ATP required to maintain the variant channel closed are not available resulting in increased efflux of K+, which leads to redistribution of ions, i.e. increased Na+, Cl-, and water influx into the cell and cellular swelling; the increased extracellular K+ is equilibrated by a decrease in water outflow to maintain extracellular osmolarity, which increases IOP without any significant harmful effects since the continuous inflow of fluid from the ciliary body and fluid outflow, even though restricted, prevents the development of a toxic milieu. In the retina, a tissue of high energy requirements, the high levels of lactate shuttled from glial cells to RGCs will generate enough ATP to normalize the open-closed state of the KATP variants while excess extracellular K+ is redistributed by spatial buffering [54, 55].
4.2 Kir6.2 23K/E and/or 337V/I and/or 1369 A/S SUR variants; aquaporin-9 279T/A variant Assumption
The aquaporin variant rs1867380 does not transport lactate as efficiently as normal aquaporin-9. The lack of sufficient ATP in the aged TM results in elevated IOP and cell swelling. However, for the neural retina, which depends on lactate for its energy needs [9,21,22], there are grave consequences since aquaporin-9 is essential to transport lactate from astrocytes to neurons [27-29] in concert with monocarboxylate transporters [27], which is converted to pyruvate to produce ATP [24].
The reduced lactate in RGCs results in reduced ATP, open KATP channels, and increased efflux of K+. Under normal physiological conditions glutamate, which is the predominant neurotransmitter in the mammalian central nervous system, is released in the synaptic cleft and transported into astrocytes [56] where it is converted to glutamine. The transport of one glutamate is accompanied by the uptake of three Na+ and one H+ and by the release in the extracellular space of one K+ molecule, which is then redistributed by astrocytes [55] since sustained exposure to elevated extracellular K+ causes hyperexcitability and significant neuronal death [57]. In fact, even a minor increase of extracellular potassium of 3 mEq/L decreases glutamate uptake by 40% [58].
The lack of lactate transport by aquaporin-9 limits the energy supply to RGCs, elevates extracellular K+, lactate and glutamate [59] creating a neurotoxic environment of elevated K+, glutamate [59-62], activation of the NLRP3 inflammasome cascade by the elevated K+ [63, 64], and edema [65]. In young individuals carrying the aquaporin variant 279T/A, glucose oxidation provides sufficient energy (ATP) for RGC function; however, with age the ability of neurons to utilize glucose as the energy source is lost, as it has been shown in brain [66], requiring the transport of lactose from astrocytes to meet the energetic needs of retinal neurons. The outcome of the KCNJ11, ABCC8 and AQP9 variants would be glaucoma and elevated IOP.
4.3 Normal KATP; aquaporin-9 279T/A variant
With normal KATP channels, the TM is not affected and the IOP will be in the normal range. However, the aquaporin-9 variant would have its effect on the retina. The decreased levels of ATP in neurons would result in open KATP channels, K+ and glutamate accumulation in the extracellular milieu as detailed above. The outcome would be normal IOP with RGC neurodegeneration, i.e., normal tension glaucoma.
4.4 Why Sulfonylureas treatment for Glaucoma
We have shown that tolbutamide, a first-generation sulfonylurea, lowers IOP in human glaucoma subjects, and increases aqueous formation and outflow via the trabecular meshwork-Schlemm’s canal with an approximately 200% higher outflow than formation [14]. Assuming that the hypothesis that high IOP and neurodegeneration result from KCNJ11, ABCC8 and AQP9 variants, sulfonylureas would block KATP channels, prevent K+ efflux, increase glutamate uptake, mitigate neurotoxicity [67, 68], reduce hydroxyl formation [69, 70] inhibit activation of the NLRP3 inflammasome [70], and reduce edema [71, 72].
4.5 Limitations
The authors are cognizant of the limitations of the study. The data on gene variants was obtained from TM tissue from glaucoma donors; however, we have IOP data from only one donor and we do not have a complete medical history for all donors. We have based our hypothesis on the results of the exonic sequence of several genes from 10 donors and even though 2 variants we found in 9/10 donors and one in 10/10 donors, it is possible that sequence analysis of a large population may show different results. It is also important to note that aquaporin-9 is not well studied; the AQP9 rs1867380 variant has been reported only in two publications that implicate it in the level of fetal hemoglobin in sickle cell disease [73,74]. For the hypothesis presented here, we have assumed that the AQP9 variant is not as effective as shuttling lactose to RGCs, which remains to be proven.
5. Conclusions
We have presented a hypothesis for the development of glaucoma based on the effect of sulfonylureas on the IOP of rabbits, on the effect of tolbutamide on human glaucoma patients [14], and on the presence of KCNJ11, ABCC8 and AQP9 gene variants in the TM of donors diagnosed with glaucoma. Limitations aside, the hypothesis can be platform for studies to prove, disprove or modify the hypothesis. If the AQP9 variant is responsible for the development of glaucoma, it can be used to design a genetic test to identify patient at-risk of glaucoma, institute treatment early while forgoing treatment of ocular hypertensive patients that do not have the AQP9 variant, and design gene therapeutic strategies.
Author Contributions
Both authors contributed equally to the study reported here.
Funding
Supported by personal funds.
Conflicts of interest
The authors are co-inventors of patent No: 10,780,068 “Methods and compositions for improving eye health”, and co-inventors of a Continuation-in-part application by the same title, application No.:16/938,628.
Nino Sorgente is the co-inventor on Patent No: 5,965,620 and 5,629,345 “Methods and compositions for ATP-sensitive K+ channel inhibition for lowering intraocular pressure”.
Nino Sorgente is President of the newly incorporated Elements Pharmaceuticals.
References:
- Vohra R, Tsai JC, Kolko M. The role of inflammation in the pathogenesis of glaucoma; Surv Ophthalmol 58 (2013): 311-320.
- Kapetanakis VV, Chan MP, Foster PJ, et al. Global variations, and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol 100 (2016): 86-93.
- Kwon YH, Fingertip JH, Kuehn MH, et al. Primary open-angle glaucoma. N Engl J Med 360 (2009): 1113–1124.
- Kass AM,Heuer KD, Higginbotham JE, et al. for the Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120 (2002): 701-713.
- Sommer A, Tielsch JM, Katz J, et al. Relationship Between Intraocular Pressure and Primary Open Angle Glaucoma Among White and Black Americans: The Baltimore Eye Survey. Arch Ophthalmol. 109 (1991): 1090-1095.
- Fan N, Wang P, Tang L, et al. Ocular blood flow and normal tension glaucoma. Biomed Res Int (2015): 308505.
- Brubaker RF. Delayed functional loss in glaucoma. LII Edward Jackson Memorial Lecture. Am J Ophthalmol. 121 (1996): 473-483
- Hattenhauer MG, Johnson DH, Ing HH et al. The probability of blindness from open-angle glaucoma. Ophthalmology 105 (1998): 2099-2104
- Murphy MC, Conner PI, Teng CY, et al. Retinal Structures and Visual Cortex Activity are Impaired Prior to Clinical Vision Loss in Glaucoma. Sci Rep.6 (2016): 31464.
- Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol 96 (2012): 47–52.
- Alasil T, Wang K, Yu F, et al. Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am J Ophthalmol 157 (2014): 953-959.
- He Y, Ge J, Tombran-Tink J. Mitochondrial Defects and Dysfunction in Calcium Regulation in Glaucomatous Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci 49 (2008): 4912-4922.
- Hu RG, Zhu Y, Donaldson P, et al. Alterations of glutamate, glutamine, and related amino acids in the anterior eye secondary to ischemia and reperfusion. Curr Eye Res 37 (2012): 633-643.
- Thumann G, Sorgente N, Kropp M, et al. Tolbutamide Eye Drops Increase Aqueous Humor Outflow and Lower Intraocular Pressure: A Proof of Concept for Glaucoma Treatment. J Ophthalmol Res 4 (2021): 114-127.
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20 (1999): 101-135.
- Baukrowitz T, Fakler B. KATP channels gated by intracellular nucleotides and phospholipids; Eur J Biochem. 267 (2000): 5842–5848.
- Sánchez JA, Gonoi T, Inagaki N, et al. Modulation of reconstituted ATP-sensitive K+-channel by GTP-binding proteins in a mammalian cell line. J Physiol 507 (1998): 315-324.
- Schulze D, Rapedius M, Krauter T, et al. Long chain Acyl-CoA esters and phosphatidylinositol phosphates modulate ATP inhibition of KATP channels by the same mechanism. J Physiol. 552 (2003): 337-367.
- Jovanovic P, Zoric L, Stefanovic I, et al. Lactate dehydrogenase and oxidative stress activity in primary open-angle glaucoma aqueous humour. Bosn J Basic Med Sci 10 (2010): 83-88.
- Kolko M, Vosborg F, Henriksen UL, et al. Lactate Transport and Receptor Actions in Retina: Potential Roles in Retinal Function and Disease. Neurochem Res 41 (2016): 1229-1236.
- Ola MS, LaNoue Molecular basis for increased lactate formation in the Müller glial cells of retina Brain Res Bull 144 (2019): 158-163.
- Vohra R, Aldana BI, Bulli G, et al. Lactate-Mediated Protection of Retinal Ganglion Cells. J Mol Biol. 431 (2019): 1878-1888.
- Vohra R, Kolko M. Lactate: More Than Merely a Metabolic Waste Product in the Inner Retina. Mol Neurobiol. 57 (2020): 2021-2037.
- Baltan S. Can Lactate Serve as an energy Substrate for Axons in Good and in Bad, in Sickness and in Health? Metabol Brain Dis 30 (2015): 25-30.
- Brooks GA. The Science and Translation of Lactate Shuttle theory. Cell Metabol 27 (2018): 757-585.
- Pellerin L, Bergersen LH, Halestrap AP, et al. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain, J Neurosci Res. 79 (2005): 55-74.
- Hashimoto T, Hussien R, Cho HS, et al. Evidence of mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PloS One 3 (2008): e2915.
- Mori S, Kurimoto T, Miki A, et al. Aqp9 Gene Deletion Enhances Retinal Ganglion Cell (RGC) Death and Dysfunction Induced by Optic Nerve Crush: Evidence that Aquaporin 9 Acts as an Astrocyte-to-Neuron Lactate Shuttle in Concert with Monocarboxylate Transporters to Support RGC Function and Survival. Mol Neurobiol 57 (2020): 4530-4548.
- Miki A, Kanamori A, Negi A, et al. Loss of Aquaporin 9 Expression Adversely Affects the Survival of Retinal Ganglion Cells. Amer J Pathol 182 (2013): 1727-1739.
- Akashi A, Miki A, Kanamori A, et al. Aquaporin 9 expression is required for l-lactate to maintain retinal neuronal survival. Neurosci Lett 589 (2015): 185-190.
- Fini ME. Another piece of the puzzle: MYOC and myocilin glaucoma. Invest Ophthalmol Vis Sci 58 (2017): 531.
- Rathi S, Danford I, Gudiseva HV, et al. Molecular Genetics and Functional Analysis Implicate CDKN2BAS1-CDKN2B Involvement in POAG Pathogenesis. Cells 9 (2020):
- Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295 (2002): 1077-1079.
- Bailey JN, Loomis SJ, Kang JH, et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat Genet 48 (2016):189-194.
- Traverso CE, Walt JG, Kelly SP, et al. Direct costs of glaucoma and severity of the disease: a multinational long-term study of resource utilisation in Eur. Br J Ophthalmol 89 (2005): 1245-1249.
- Li F, Huang W, Zhang X. Efficacy and safety of different regimens for primary open-angle glaucoma or ocular hypertension: a systematic review and network meta-analysis. Acta Ophthalmol 96 (2018): e277-e284.
- Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 114 (2007): 1965-1972.
- Bakhtiyari A, Karimeh, Haghani K, et al. Association between ABCC8 Ala1369Ser Polymorphism (rs757110 T/G) and Type 2 Diabetes risk in an Iranian population: A Case-Control Study. Endocr Metab Immune Disord Drug Targets Jul 12 (2020).
- Qin LJ, Lv Y, Huang QY. Meta-analysis of association of common variants in the KCNJ11-ABCC8 region with type 2 diabetes. Genet Mol Res 12 (2013): 2990-3002.
- Severino P, D'Amato A, Netti L, et al. Susceptibility to ischaemic heart disease: Focusing on genetic variants for ATP-sensitive potassium channel beyond traditional risk factors. Eur J Prev Cardiol 2 (2020): 2047487320926780. Online ahead of print.
- Sakamoto Y, Inoue H, Keshavarz P, et al. SNPs in the KCNJ11-ABCC8 gene locus are associated with type 2 diabetes and blood pressure levels in the Japanese population. J Hum Genet 52 (2007): 781-793.
- Fatehi M, Raja M, Carter C, et al. The ATP-sensitive K(+) channel ABCC8 S1369A type 2 diabetes risk variant increases MgATPase activity. Diabetes 61 (2012): 241-249.
- Fatehi M, Carter CC, Youssef N, et al.The mechano-sensitivity of cardiac ATP-sensitive potassium channels is mediated by intrinsic MgATPase activity. J Mol Cell Cardiol 108 (2017): 34-41.
- Schwanstecher C, Meyer U, Schwanstecher M. K(IR)6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic beta-cell ATP-sensitive K(+) channels. Diabetes 51 (2002): 875– 879.
- Schwanstecher C, Schwanstecher M. Nucleotide sensitivity of pancreatic ATP-sensitive potassium channels and type 2 diabetes. Diabetes51 (2002): S358– S362.
- Jones DP. Intracellular diffusion gradients of O2 and ATP. Am J Physiol 250 (1986): C663–C675.
- Menard L, Maughan D, Vigoreaux J. The structural and functional coordination of glycolytic enzymes in muscle: evidence of a metabolon? Biology (Basel) 3 (2014): 623-644.
- Saks V, Beraud N, Wallimann T. Metabolic Compartmentation - A System Level Property of Muscle Cells Int J Mol. Sci 9 (2008): 751-767.
- Hu H, Juvekar A, Lyssiotis CA et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton; Cell 164 (2016): 433-446.
- Alekseev AE, Reyes S, Selivanov VA, et al. Compartmentation of membrane processes and nucleotide dynamics in diffusion-restricted cardiac cell microenvironment. J Mol Cell Cardiol 52 (2012): 401-409.
- Selivanov VA, Krause S, Roca J, et al. Modeling of Spatial Metabolite Distributions in the Cardiac Sarcomere. Biophys J 92 (2007): 3492–3500.
- Neubauer S. The failing heart – an engine out of fuel. N Engl J Med 356 (2007): 1140-1151.
- Abraham MR, Selivanov VA, Hodgson DM, et al.Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem. 277 (2002): 24427-24434.
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 129 (2004): 1045-1056.
- Murakami S, Kurachi Y. Mechanisms of astrocytic K+ clearance and swelling under high extracellular K+ J Physiol Sci 66 (2016): 127-142.
- Danbolt NC. Glutamate uptake. Prog Neurobiol 65 (2001): 1-105.
- Takahashi S, Shibata M, Fukuuchi Y. Role of sodium ion influx in depolarization-induced neuronal cell death by high KCI or veratridine. Eur J Pharmacol 372 (1999): 297-304.
- Rimmele TS, Rocher AB, Wellbourne-Wood J, et al. Control of Glutamate Transport by Extracellular Potassium: Basis for a Negative Feedback on Synaptic Transmission. Cereb Cortex 27 (2017): 3272-3283.
- Toft-Kehler AK, Skytt DM, Paulsen KA, et al. Limited energy supply alters glutamate uptake. Neurochem Res 39 (2014): 941-949.
- Mawrin C, Pap T, Pallas M, et al. Changes in retinal glutamate transporter GLI-1mRNA levels following optic nerve damage. Mol Vis 9 (2003): 10-13.
- Hare WA, Wheeler L. Experimental glutamatergic excitotoxicity in rabbit retinal ganglion cells: block by memantine. Invest Ophthalmol Vis Sci 50 (2009): 2940-2948.
- Harada T, Harada C, Nakamura K, et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest 117 (2007): 1763-1777.
- Lowes DJ, Hevener KE, Peters Second-Generation Antidiabetic Sulfonylureas Inhibit Candida albicans and Candidalysin-Mediated Activation of the NLRP3 Inflammasome Antimicrob Agents Chemother 64 (2020): e01777-19.
- Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol 194 (2015): 3937-3952.
- Chen M, Simard JM. Cell swelling, and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. Neurosci 21 (2001): 6512-6521.
- Goyal MS, Vlassenko AG, Blazey TM, et al. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab 26 (2017): 353-360.
- Kou J, Klorig DC, Bloomquist JR.Potentiating effect of the ATP-sensitive potassium channel blocker glibenclamide on complex I inhibitor neurotoxicity in vitro and in vivo. Neurotoxicology 27 (2006): 826-834.
- Ishola IO, Akataobi OE, Alade AA, et al.Glimepiride prevents paraquat-induced Parkinsonism in mice: involvement of oxidative stress and neuroinflammation. Fundam Clin Pharmacol 33 (2019): 277-285.
- Obata T, Nakashima M.Opening of ATP-sensitive K(+) (KATP) channels enhance hydroxyl radical generation induced by MPP(+) in rat striatum. J Neurol Sci 15 (2016): 180-183.
- Obata T, Yamanaka Y. Block of cardiac ATP-sensitive K(+) channels reduces hydro-xyl radicals in the rat myocardium. Arch Biochem Biophys 378 (2000): 195-200.
- Simard JM, Tsymbalyuk N, Tsymbalyuk O, et al. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke 41 (2010): 531-537.
- Simard JM, Yurovsky V, Tsymbalyuk N, et al. Protective effect of delayed treatment with low dose glibenclamide in three models of ischemic stroke. Stroke 40 (2009): 604-609.
- Sebastiani P, Zhao Z, Abad-Grau MM, et al. A hierarchical and modular approach to the discovery of robust associations in genome-wide association studies from pooled DNA samples. BMC Genet 9 (2008): 6.
- Driss A, Asare KO, Hibbert JM, et al. Sickle Cell Disease in the Post Genomic Era: A Monogenic Disease with a Polygenic Phenotype. Genomics Insights 2 (2009): 23-48.


 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
