Incidence of Severe Acute Respiratory Syndrome Coronavirus-2 (Sars-Cov-2) Antigen Presence in Adenovirus-Negative Conjunctivitis with Viral Infection Appearance
Hisataka Fujimoto*, Junichi Kiryu
Department of Ophthalmology, Kawasaki Medical School, Kurashiki, Okayama, Japan
*Corresponding author: Hisataka Fujimoto, Department of Ophthalmology, Kawasaki Medical School, Kurashiki, Okayama, Japan
Received: 24 August 2021; Accepted: 31 August 2021; Published: 02 September 2021
Article Information
Citation:
Hisataka Fujimoto, Junichi Kiryu. Incidence of Severe Acute Respiratory Syndrome Coronavirus-2 (Sars-Cov-2) Antigen Presence in Adenovirus-Negative Conjunctivitis with Viral Infection Appearance. Journal of Ophthalmology and Research 4 (2021): 258-262.
DOI: 10.26502/fjor.2644-00240044
View / Download Pdf Share at FacebookAbstract
This study aimed to investigate the incidence of the novel coronavirus (SARS-CoV-2) infection (COVID-19) in adenovirus-negative conjunctivitis in Japan. Twelve patients with unilateral or bilateral conjunctivitis with preauricular lymphadenopathy or serous discharge and an initial clinical diagnosis of viral conjunctivitis from April 1, 2020 to September 30, 2020, with negative adenovirus antigen test were included in this study. The residual smeared conjunctiva sample for the adenovirus antigen test was preserved by freezing at –70 °C in a completely anonymized manner. Seven to 180 days after preservation, the samples were tested using the SARS-CoV-2 antigen kit (ESPLINE® SARS-CoV-2 kit, Lot number, K4B00810; FUJIREBIO, Tokyo, Japan). One sample (8.8%) tested positive for the SARS-CoV-2 antigen. None of the patients exhibited systemic symptoms, including pneumonia. No medical staff related to these patients exhibited any infectious findings. These results suggest that the possibility of COVID-19 should be considered with care in daily medical examinations for conjunctivitis, including epidemic keratoconjunctivitis.
Keywords
<p>Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), COVID-19, Viral conjunctivitis</p>
Article Details
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is an enveloped, single-stranded RNA virus of the coronaviridae family [1]. SARS-CoV-2 is known to infect the respiratory tract and can result in severe respiratory distress, systemic inflammatory responses, and sometimes, mortality. Like most coronaviruses, SARS-CoV-2 symptoms are not restricted to respiratory disease, and many studies have reported broad symptomatology, including ocular involvement [2]. Multiple mechanisms have been proposed for the ocular SARS-CoV-2 infection. One accepted hypothesis is that the ocular surface represents a site of infection for this airborne virus via inoculation of the conjunctiva by droplets. Other plausible hypotheses include the possibility of virus migration from the nasolacrimal duct, hematogenous infection of the lacrimal gland, and inoculation of the mucosal immune system in the nasal cavity [3,4]. A meta-analysis of seven studies identified a large range of reported ocular manifestations of coronavirus disease 2019 (COVID-19) from 2% to 32% thus highlighting the need for further investigation into the true incidence of ocular manifestations in SARS-CoV-2 infection [5]. However, the incidence rate of COVID-19 in conjunctivitis with visits to ophthalmologists remains unclear. Thus, we investigated the COVID-19 antigen examination in adenovirus-negative conjunctivitis, with viral infection appearance including preauricular lymphadenopathy or serous discharge.
2. Materials and Methods
This retrospective observational study was completely anonymous. The approval for this anonymized observational study was confirmed to be unnecessary by the institutional review board in the guidelines from the Ministry of Health, Labour and Welfare of Japan [6]. The study adhered to the tenets of the Declaration of Helsinki. This study was not associated with patient or public involvement in its design, participant recruitment, or conduct. In total, 12 patients with unilateral or bilateral conjunctivitis in the Kawasaki Medical School Hospital from April 1, 2020 to September 30, 2020 were included in the study. All patients had preauricular lymphadenopathy or serous discharge, and the initial clinical diagnosis was viral conjunctivitis. All the results of the adenovirus antigen test (Capilia-Adeno-eye NEO®, Wakamoto Co., Ltd, Tokyo, Japan) were negative for smeared conjunctiva samples. The residual smeared conjunctiva sample for the adenovirus antigen test was preserved by freezing at –70 °C in a completely anonymized manner. Seven to 180 days after preservation, the samples were tested using the SARS-CoV-2 antigen kit (ESPLINE® SARS-CoV-2 kit, Lot number, K4B00810; FUJIREBIO, Tokyo, Japan). The false positive rate from product documentation was 0.9% (1/109) [7]. All samples were also tested using the herpes simplex virus antigen test (Checkmate Herpes Eye®, Lot number, 0308; Wakamoto Co., Ltd, Tokyo, Japan) and chlamydia antigen test (Rapid SP Chlamydia®, Lot number, 07014; SB Bioscience Co., Ltd., Hyogo, Japan). The exclusion criteria were as follows: history of viral infections, such as herpes simplex virus infection, varicella-zoster virus infection, cytomegalovirus (CMV)-related corneal endotheliitis, any active ocular disorders, such as ocular inflammatory disease, and/or nasolacrimal duct obstruction. Outcome measures of interest were the results of the SARS-CoV-2 antigen test.
3. Results
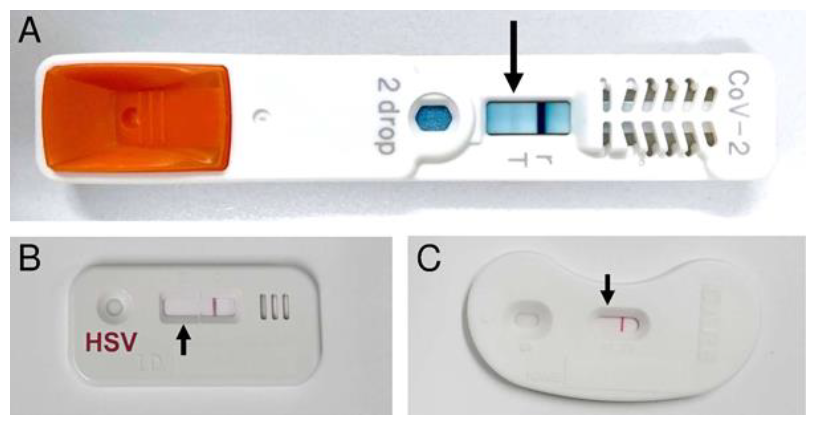
One of the 12 patients (8.8%) exhibited SARS-CoV-2 antigen positivity (Figure 1A). All of the samples were also tested by herpes simplex virus antigen test (Figure 1B) and chlamydia antigen test (Figure 1C). In other 11 cases, 7 cases were positive for the herpes simplex virus antigen tests, 1 case was positive for the chlamydia antigen test. 3 cases were negative for all of the performed antigen tests. None of the patients exhibited systemic symptoms, including pneumonia. In addition, no medical staff related to these patients exhibited any infectious findings.
The adenovirus antigen test was negative. The SARS-CoV-2 antigen test exhibited positivity (A), while both of the herpes simplex virus antigen test (B) and chlamydia antigen test (C) were negative.
5. Discussion
This study revealed that the SARS-CoV-2 antigen-positive case was present in conjunctivitis initially suspected to be epidemic keratoconjunctivitis. In these cases, antigen tests were negative for adenovirus, herpes simplex virus, and chlamydia, which are among the main causes of infectious non-bacterial conjunctivitis. All of the included cases were systemically asymptomatic, including pneumonia and digestive symptoms, which are regarded as severe clinical concerns in SARS-CoV-2 infection. One of the difficulties in SARS-CoV-2 infection management is that many of the infected cases are asymptomatic [8]. A previous meta-analysis on the ocular manifestations of COVID-19 included 14 studies, including a pooled analysis of polymerase chain reaction (PCR) positivity in tears. This provides unique insight into the true prevalence of ocular manifestations (5% in this report) and PCR positivity in individuals with ocular symptoms (38%) [9]. On the other hand, another large meta-analysis comprising 24,410 adults with COVID-19 reported that the prevalence of conjunctivitis in patients with COVID-19 was 2% or less [10]. Considering that our patient visited the ophthalmic hospital for conjunctivitis and was systemically asymptomatic but was SARS-CoV-2 antigen-positive, the possibility of COVID-19 infection should be considered in conjunctivitis patients initially presenting with viral conjunctivitis with preauricular lymphadenopathy or serous discharge. No medical staff related to the cases reported in this study exhibited SARS-CoV-2 infection. Prior to the start of the pandemic, ophthalmologists were sometimes relatively careless and did not use masks. Slit lamp shields were small and covered the area between the breadth of the physician and the patient. After the start of the pandemic, many practices have adopted masks and larger shields between physicians and patients. These shields may be awkward and represent another obstacle for a thorough clinical examination. A study by Ong et al. confirmed that regular face masks provided the most complete protection against both aerosol and droplet transmissions [11]. Careful protection should be considered when examining conjunctivitis cases, especially when yet to be diagnosed by antigen testing or microbial smear examination. One of the limitations of this study is the small sample size. Patients with viral conjunctivitis who do not show adenovirus antigens are relatively rare. In addition, vaccination is relatively delayed in Japan, even for medical staff, and further enrolment of cases was dangerous for the medical staff, including ophthalmologists. In addition, considering the sensitivity, we could not confirm the results of the antigen test by PCR because the amount of the smeared conjunctiva sample was very small and all the samples were used for antigen testing. The false-positive rates in antigen tests are typically extremely rare but not 0% in SARS-CoV-2 antigen kits. Hence, the obtained results need to be verified with a prospective, randomized, masked study including PCR in the future. The data presented herein suggest that examining a large cohort of patients with conjunctivitis that visit the ophthalmologic clinic will be important to confirm the incidence of SARS-CoV-2 in conjunctivitis.
5. Conclusions
In conclusion, our results suggest that the possibility of COVID-19 should be considered with care in daily medical examinations for conjunctivitis, including epidemic keratoconjunctivitis.
Acknowledgements
We thank Y. Setoguchi for discussions, advice, and criticism that benefited this project. This work was supported by the Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical’s Founder 2019 (to H.F.).
Conflicts of Interests
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors Contribution
H.F. conceived the project, wrote the main manuscript text, and prepared Figure 1. J.K. supervised the project. All authors reviewed the manuscript.
References
- Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocular Immunology and Inflammation 12 (2020): 391-395.
- Nora RL, Putera I, Khalisha DF, et al. Are eyes the windows to COVID-19? Systematic review and meta-analysis. BMJ Open Ophthalmology 22 (2020): 13-20.
- Chen X, Yu H, Mei T, et al. SARS-CoV-2 on the ocular surface: Is it truly a novel transmission route? British Journal of Ophthalmology 42 (2020): 1190-1195.
- Barnett BP, Wahlin K, Krawczyk M, et al. Potential of ocular transmission of SARS-CoV-2: A Review. Vision 11 (2020): 40-46.
- Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefe’s archive for clinical and experimental ophthalmology 8 (2020): 1351-1352.
- Ethical guidelines for medical and health research involving human subjects. Ministry of Health, Labour and Welfare of Japan.
- ESPLINE® SARS-CoV-2 kit product documentation. Fujirebio, Tokyo, Japan.
- Dawood FS, Varner M, Tita A, et al. Incidence, clinical characteristics, and risk factors of sars-cov-2 infection among pregnant individuals in the United States. Clinical Infectious Diseases 11 (2021): 713-720.
- Nora RL, Putera I, Khalisha DF, et al. Are eyes the windows to COVID-19? Systematic review and meta-analysis. BMJ Open Ophthalmology 4 (2020): 13-18.
- Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. Plos One 56 (2020): e0234765.
- Ong SC, Razali MAB, Shaffiee L, et al. Do slit-lamp shields and face masks protect ophthalmologists amidst COVID-19? Ophthalmology 19 (2020): 1427- 1429.


 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
