Efficacy of the Rho-kinase Inhibitor Ripasudil for Fuchs’ Endothelial Corneal Dystrophy
Hisataka Fujimoto1*, Junichi Kiryu1
1Department of Ophthalmology, Kawasaki Medical School, Kurashiki, Okayama, Japan
*Corresponding author: Hisataka Fujimoto, Department of Ophthalmology, Kawasaki Medical School, Kurashiki, Okayama, Japan
Received: 17 July 2021; Accepted: 23 July 2021; Published: 12 August 2021
Article Information
Citation:
Hisataka Fujimoto, Junichi Kiryu. Efficacy of the Rho-kinase Inhibitor Ripasudil for Fuchs’ Endothelial Corneal Dystrophy. Journal of Ophthalmology and Research 4 (2021): 231-243.
DOI: 10.26502/fjor.2644-00240041
View / Download Pdf Share at FacebookAbstract
This study aimed to investigate the efficacy of the rho-kinase (ROCK) inhibitor ripasudil administered as ophthalmic solutions for chronic visual acuity impairment induced by Fuchs’ endothelial corneal dystrophy (FECD). Twelve patients with FECD and glaucoma (20 eyes) treated with eye drops (0.4% ripasudil) twice a day were investigated. The control group comprised 12 patients with FECD only (i.e., without glaucoma) (20 eyes). Ripasudil was used during the 18 months of the observation period. All participants had grade 6 dystrophy according to the modified Krachmer grading scale. The mean logMAR visual acuity was improved at 3, 6, 12, and 18 months after ripasudil administration. LogMAR changes 18 months after administration were 0.181 ± 0.170 in the ripasudil group and were significantly superior (P = 9.5 × 10−4) to those in the control group (−0.057 ± 0.106). Central corneal thickness (CCT) 18 months after ripasudil administration/CCT before administration were 0.945 ± 0.029 in the ripasudil group and significantly superior (P = 2.4 × 10−7) compared with those in the control group (1.040 ± 0.032). ROCK inhibitors can be effective against visual acuity impairment and corneal edema in FECD. They can be more effective when administered during the waiting period before corneal endothelium transplantation.
Keywords
<p>Rho-kinase (ROCK) inhibitor; Ripasudil; Fuchs endothelial corneal dystrophy; Corneal endothelium</p>
Article Details
1. Introduction
Rho-kinase (ROCK) is a protein kinase involved in the regulation of cell size and shape through the action of the cytoskeleton and in the calcium-independent regulation of smooth muscle contraction (Moshirfar et al. 2018). In ophthalmology, ROCK inhibitors are primarily used for glaucoma treatments (Novack 2013; Inoue & Tanihara 2013) and corneal endothelium disorders (Okumura 2017; Jafri & Colby 2019). Its applicability has also been examined for diabetic retinopathy and macular edema (Nourinia et al. 2017). The efficacy of ROCK inhibitors for the treatment of corneal endothelium disorders has been established in the field of regenerative medicine, wherein human corneal endothelial cell injection with ROCK inhibitor supplementation restored the cornea along with the preservation of normal corneal thickness and resolution of corneal edema (Kinoshita et al. 2018). The efficacy of ROCK inhibitors has also been reported in patients with acute surgical trauma (Okumura et al. 2015), as ROCK inhibitors can possibly treat acute corneal endothelial damage and prevent the progression of bullous keratopathy (BK) (Okumura et al. 2015).
Okumura et al. (Okumura et al. 2012) used a rabbit corneal endothelial dysfunction model to demonstrate that the transplantation of corneal endothelial cells with ROCK inhibitors helped recover corneal transparency and demonstrated hexagonal monolayer cells with normal expression of function-related markers in the reconstructed corneal endothelium. ROCK inhibitors have also been proven to improve corneal endothelial cell survival in vitro (Okumura et al. 2009). The protective and pro-proliferative effects of these inhibitors on the corneal endothelium play a critical role in regenerative medicine. One of these milestones is the use of the ROCK inhibitor Y27632 in cultured endothelium transplantation (Kinoshita et al. 2018).
The efficacy of ROCK inhibitors has been well established in corneal endothelial diseases (Okumura et al. 2017; 2020; 2016; Mimura et al. 2004). However, only few studies (Koizumi et al. 2013) have assessed the potential efficacy of single-use ROCK inhibitors for the treatment of BK, a disease characterized by edematous corneal stroma and the development of epithelial and sub-epithelial bullae. These occur owing to cell loss and de-compensation from the endothelium because of uveitis, endothelial dystrophy and surgical trauma during intraocular surgery (Pricopie et al. 2017). The ROCK inhibitor Y27632 developed by Koizumi et al. has been reported to be effective for the treatment of several corneal diseases (Koizumi et al. 2014). In contrast, other ROCK inhibitors, such as ripasudil that has been approved for clinical use for glaucoma, have not been examined as thoroughly as Y27632 for the treatment of corneal endothelial diseases. Furthermore, Okumura et al. has reported that ripasudil promotes corneal endothelial wound healing (Okumura et al. 2016), thus suggesting that it may be comparable with Y27632 in terms of efficacy. Thus, there is no consensus among the existing data.
Therefore, the potential efficacy of ripasudil for the treatment of chronic visual acuity impairment induced by corneal endothelial dysfunction should be clarified. This study aimed to investigate the efficacy of ripasudil administered in the form of ophthalmic solutions for the treatment of chronic visual acuity impairment induced by Fuchs’ endothelial corneal dystrophy (FECD).
2. Materials and Methods
This retrospective observational study was approved by the institutional review board of the Kawasaki Medical School Hospital (approval number 5182-00). Informed consent was obtained from all studied participants and the study adhered to the tenets of the Declaration of Helsinki. This study was not associated with patient or public involvement in its design, participant recruitment, or conduct.
In total, 24 patients with FECD were included. All of them had grade 6 dystrophy according to the modified Krachmer grading scale, based on the following criteria: a) confluent guttae >5 mm, b) presence of a corneal stroma, or c) presence of a corneal epithelial edema (Repp et al. 2013). Corneal specialists diagnosed FECD based on the presence of bilateral guttae in the eyes of patients without a history of contact lens wear or other corneal diseases. Patients with pseudo-guttae and pseudo-exfoliation syndromes were excluded. Inclusion criteria were as follows: absence of other endothelial dysfunction, such as any history of herpes simplex virus infection, varicella-zoster virus infection, cytomegalovirus (CMV)-related corneal endotheliitis, previous surgical trauma, or corneal surgery, laser iridotomy for closed-angle glaucoma and uveitis. In contrast, the exclusion criteria included a history of ocular surgery with the exception of cataract surgery, history of past ripasudil use, any active ocular disorders—such as epithelial erosions, infections, conjunctivitis, ocular inflammatory disease, nasolacrimal duct obstruction, and/or retinal disease—and any active systemic diseases, such as diabetes mellitus, cardiovascular disease, and/or renal disease. Only patients with systemic hypertension controlled through the administration of oral antihypertensive medications were included in the final cohort.
The ripasudil group included 12 patients (20 eyes) aged 51–92 years (mean: 75.8 years), who were diagnosed with controlled glaucoma. Glaucoma was diagnosed according to the presence of a typical localized glaucomatous retinal nerve fiber layer defect in red-free filter examinations or glaucomatous optic nerve head damage (cup-to-disc ratio asymmetry of >0.2 between fellow eyes, rim thinning, notching, or excavation). In 13 eyes, prostaglandin analogs were administered before and terminated after ripasudil administration was initiated. Approximately 0.4% of ripasudil hydrochloride hydrate (Kowa Company, Ltd., Nagoya, Japan) was prescribed (used twice a day) until the last examination day. The control group included 12 (20 eyes) patients without glaucoma aged 65–84 years (mean: 74.5 years), who were not prescribed 0.4% ripasudil or any other eye drops. The number of eyes was designed to be the same as those included in the ripasudil group. At the initial examination, all eyes had grade 6 dystrophy as determined using the modified Krachmer grading scale. Thus, specular microscopy was unavailable owing to the presence of confluent guttae with sizes >5 mm, corneal stromal, or epithelial edema. In both groups, no other eye drops were used within 6 months before and during the observation period.
Outcome measures of interest were logMAR visual acuity, central corneal thickness (CCT) and thinnest corneal thickness (TCT) before and at 1, 3, 6, 12, and 18 months after ripasudil administration. The sample size was planned for the examination of the null hypothesis (no logMAR visual acuity/CCT/TCT differences between the ripasudil and control groups) based on the specified probability.
Corneal tomographic measurements were obtained with anterior segment optical coherence tomography with the use of CASIA 2 (Tomey Corporation, Inc., Nagoya, Japan) with an infrared light wavelength of 1,310 nm. Measurements were obtained along the vertex normal and images were centered on the corneal vertex. The analysis software (Tomey Corporation, Inc., Nagoya, Japan) identified and digitized both the anterior and posterior corneal surfaces and aligned the reference axis of the measurements with the vertex normal. Tomographic and pachymetric maps were calculated from 16 radial cross-sectional images based on the use of the central 10 mm diameter of the cornea (total acquisition time: 0.34 s).
Swept-source optical coherent tomography (OCT) measurements have been shown to have adequate repeatability in healthy eyes and in eyes with keratoconus (Szalai et al. 2012). CCT and TCT were measured and defined as the pachymetric value at the thinnest location and the corneal vertex, respectively. CCT and TCT were calculated inside the central region of the cornea (diameter: 9 mm). Each measurement satisfied the following conditions: >96.5% of the data obtained within the central region (diameter: 6 mm) had adequate contrast to trace the corneal border; all 16 radial scans within the central region (diameter: 4 mm) of the topographic analysis region could be performed for both anterior and posterior corneal surfaces; the distance from the center of the image to the corneal vertex was <0.86 mm and >93% of scans inside the central topographic analysis region (diameter: 6 mm) could be obtained for both the anterior and posterior surfaces. To avoid the effects of same-day variation on corneal thickness, measurements were performed within 1 h of the scheduled measurement at the initial examination.
A non-contact type specular microscope (Cell Check 16, Konan Co., Ltd, Nagoya, Japan) was used for endothelial cell density (ECD) measurements. The captured images were 0.46 mm high and 0.24 mm wide. The imaging point was controlled by patient fixation, namely, locations were determined based on the patient’s primary line of sight and not the vertex normal. The area measured was confirmed by a monitor camera.
2.1 Statistical analysis
The data distribution was assessed with the use of the Shapiro–Wilk test. Following the assumption that all outcomes of interest followed a parametric distribution, two-sample independent t-test was used to compare logMAR changes at 1, 3, 6, 12, and 18 months after ripasudil administration between the ripasudil and control groups. The logMAR changes are presented as follows: −[logMAR value after administration − logMAR value before administration].
The same test was used to compare CCT and TCT changes at each interval (1, 3, 6, 12, and 18 months after ripasudil administration) with those at the baseline between the ripasudil and control groups. CCT (or TCT) changes are presented as follows: CCT (or TCT) after initiating ophthalmic solution administration/CCT (or TCT) before initiating ophthalmic solution administration.
All analyses were conducted with SPSS (version 25.0, IBM Corporation, Armonk, NY, USA) on a Windows platform.
3. Results
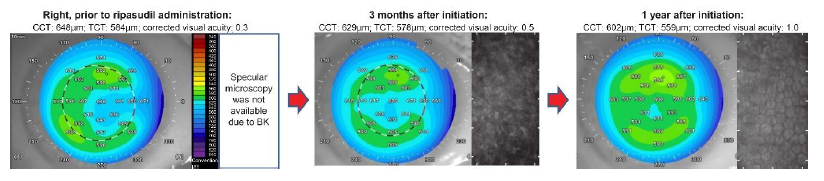
The study included 40 eyes from 24 patients with FECD (Figure 1). The mean age in both groups (ripasudil and control) was 75.14 ± 9.79 years.
Figure 1: An eye case with Fuchs’ endothelial corneal dystrophy (FECD) with normal tension glaucoma was prescribed ripasudil. Without keratoplasty, the logMAR value continually improved over a period of 1 year following the corneal edema improvement. Corneal topography included anterior segment optical coherent tomography (OCT) (left panel) and specular microscopy (right panel).
Table 1. LogMAR visual acuity, CCT, TCT, IOP, and background parameters before ripasudil administration.
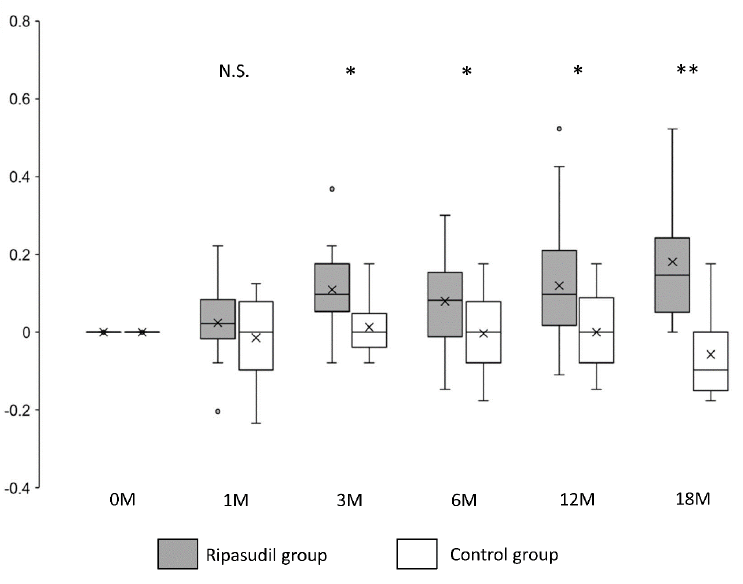
Figure 2: Changes in logMAR values in the ripasudil-treated and control groups. Shown are −[logMAR value after the administration − logMAR value at the initial examination]. Horizontal lines in the box and whisker plots represent the median values, and the bottom and top of the boxes represent lower and upper quartiles, respectively. The symbol x represents the mean, and bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles (*P < 0.05, two-sample independent t-test; LogMAR, logarithm of the minimum angle of resolution).
Background data examined, such as male/female ratio, age, history of cataract surgery, logMAR visual acuity, and CCT, TCT and intraocular pressure (IOP) before ripasudil administration, did not show significant differences between the ripasudil-treated and control groups (Table 1).
The mean logMAR visual acuity in the ripasudil-treated group was significantly improved (P < 0.05) at 3, 6, 12, and 18 months after ripasudil administration compared with the control group (Figure 2 and Table 2).
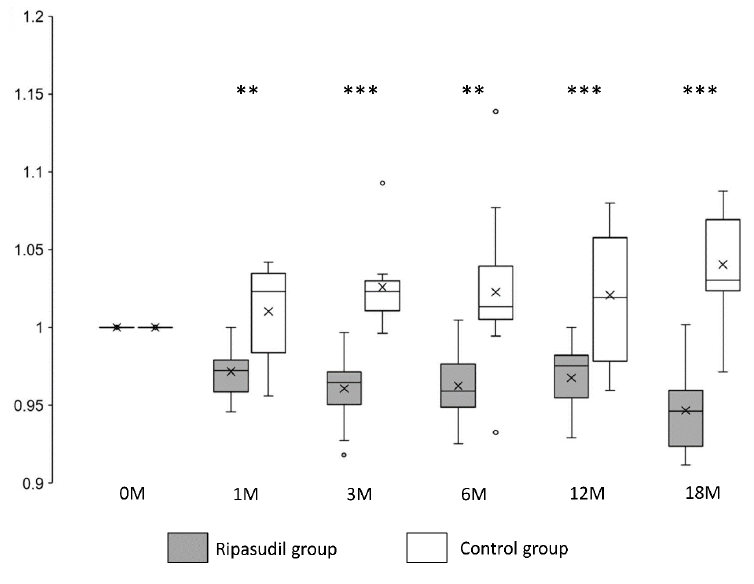
Corneal edema evaluated at the CCT was significantly (P < 0.01) reduced and improved at all observed points (1, 3, 6, 12, and 18 months after ripasudil administration) in the ripasudil-treated group compared with the control group (Figure 3 and Table 2).
|
Label |
Ripasudil-treated group |
Control group |
P-value between ripasudil-treated and control groups |
||
|
Mean ± SD |
95% confidence interval |
Mean ± SD |
95% confidence interval |
||
|
−{logMAR(1M)−logMAR(0M)} |
0.024 ± 0.095 |
−0.02 to 0.067 |
−0.014 ± 0.113 |
−0.081 to 0.052 |
0.366 |
|
−{logMAR(3M)−logMAR(0M)} |
0.109 ± 0.105 |
0.058 to 0.161 |
0.013 ± 0.080 |
−0.04 to 0.065 |
0.017 |
|
−{logMAR(6M)−logMAR(0M)} |
0.079 ± 0.129 |
0.02 to 0.139 |
−0.003 ± 0.094 |
−0.051 to 0.044 |
0.041 |
|
−{logMAR(12M)−logMAR(0M)} |
0.119 ± 0.159 |
0.049 to 0.189 |
0.000 ± 0.097 |
−0.053 to 0.052 |
0.012 |
|
−{logMAR(18M)−logMAR(0M)} |
0.181 ± 0.170 |
0.08 to 0.282 |
−0.057 ± 0.106 |
−0.115 to 0.000 |
9.5 × 10−4 |
|
CCT (1M)/CCT (0M) |
0.972 ± 0.013 |
0.965 to 0.978 |
1.010 ± 0.031 |
0.989 to 1.032 |
0.009 |
|
CCT (3M)/CCT (0M) |
0.961 ± 0.019 |
0.951 to 0.970 |
1.026 ± 0.027 |
1.009 to 1.042 |
6.9 × 10−6 |
|
CCT (6M)/CCT (0M) |
0.963 ± 0.021 |
0.953 to 0.972 |
1.023 ± 0.049 |
0.995 to 1.051 |
1.4 × 10−3 |
|
CCT (12M) CCT (0M) |
0.968 ± 0.020 |
0.959 to 0.976 |
1.021 ± 0.042 |
0.998 to 1.044 |
6.3 × 10−4 |
|
CCT (18M)/CCT (0M) |
0.945 ± 0.029 |
0.934 to 0.959 |
1.040 ± 0.032 |
1.023 to 1.058 |
2.4 × 10−7 |
|
TCT (1M)/TCT (0M) |
0.970 ± 0.009 |
0.965 to 0.974 |
1.015 ± 0.030 |
0.994 to 1.036 |
0.004 |
|
TCT (3M)/TCT (0M) |
0.964 ± 0.023 |
0.953 to 0.975 |
1.024 ± 0.024 |
1.01 to 1.038 |
1.5 × 10−6 |
|
TCT (6M)/TCT (0M) |
0.964 ± 0.022 |
0.954 to 0.973 |
1.031 ± 0.034 |
1.013 to 1.050 |
5.4 × 10−6 |
|
TCT (12M)/TCT (0M) |
0.971 ± 0.018 |
0.963 to 0.978 |
1.021 ± 0.039 |
1.001 to 1.042 |
3.3 × 10−4 |
|
TCT (18M)/TCT (0M) |
0.945 ± 0.026 |
0.934 to 0.958 |
1.019 ± 0.036 |
0.999 to 1.038 |
1.3 × 10−5 |
|
Number of specular microscopy available eyes after ripasudil administration |
14/20 |
0/20 |
|||
|
ECD |
727 ± 142 |
N/A |
N/A |
||
Table 2: Comparison of the endpoints (logMAR visual acuity, CCT, TCT) between ripasudil and control groups.
Figure 3: Changes in CCT in the ripasudil-treated and control groups. The CCT value divided by the CCT value at the initial examination is presented. Horizontal lines in the box and whisker plots represent median values, and the bottom and top parts of the boxes represent lower and upper quartiles, respectively. The symbol x represents the mean, and bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles (*P < 0.05, **P < 0.01 and ***P < 0.001, two-sample independent t-test; CCT, central corneal thickness).
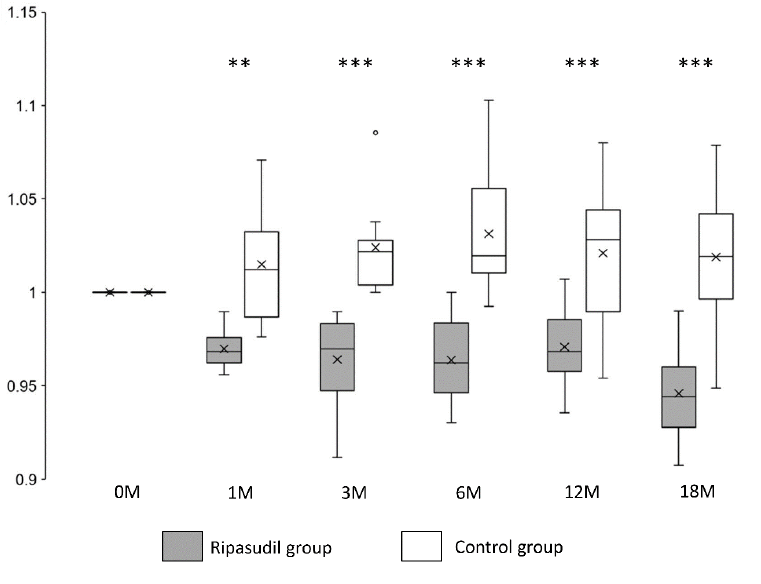
Corneal edema evaluated at the TCT was also significantly improved (P < 0.01) at all observed points in the ripasudil-treated group compared with the control group (Figure 4 and Table 2).
In all studied eyes, specular microscopy was unavailable before ripasudil administration owing to the presence of confluent guttae, corneal stromal, or epithelial edema. However, in the ripasudil group, specular microscopy was available at least once during the 18-month follow-up period in 14 eye cases. The observed mean ECD value in these 14 eyes was 727 ± 142 /mm2. Conversely, in the control group, specular microscopy values were unavailable during the 18-month follow-up period (Table 2).
Figure 4:Changes in TCT in the ripasudil-treated and control groups. The TCT value divided by the TCT value at the initial examination is presented. Horizontal lines in the box and whisker plots represent the median values, and the bottom and top parts of the boxes represent lower and upper quartiles, respectively. The symbol x represents the mean, and bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles (*P < 0.05, **P < 0.01 and ***P < 0.001, two-sample independent t-test; TCT, thinnest corneal thickness).
4. Discussion
This study revealed that after ripasudil administration, visual acuity was significantly improved in patients with moderate to severe FECD with grade 6 dystrophy according to the modified Krachmer grading scale. The improvement in visual acuity was maintained in the long term (1.5 years), with observable alleviation at 3 months after ripasudil administration. The improvement in corneal edema evaluated by both CCT and TCT was confirmed at least as early as 1 month after ripasudil administration and continued throughout the observation periods of 1.5 year.
Since 2014, ripasudil is available in Japan only for glaucoma treatments (Tanihara et al. 2020; Honjo & Tanihara 2018; Kusuhara & Nakamura 2020). It is expected to be used in other countries, such as the United States, because FECD is one of the major pathologies attributed to corneal transplantations. In addition, its safety has been proven for corneal endothelial diseases. The administration of the ROCK inhibitor ripasudil in the form of eye drops has been proven effective in the treatment of FECD visual impairment and corneal edema. Our study supports the idea that ROCK inhibitors should be formulated as eye drops for the treatment of acute corneal endothelial damage to prevent BK progression (Okumura et al. 2015).
ROCKs primarily mediated their effects by promoting endothelial cell migration (Okumura et al. 2015; Okumura et al. 2011; Koizumi et al. 2013). In patients with FECD, a marked endothelial reduction was observed at the central and paracentral regions of the cornea, whereas the endothelium in the surrounding region (periphery) remained relatively intact. In addition, the central cornea swelled more than the peripheral cornea in FECD (Fujimoto et al. 2014; Jun 2010; Maurice 1968; Naumann & Schlötzer-Schrehardt 2000) even though peripheral measurements were used to objectively grade the disease state (Repp 2013). The central cornea undergoes more severe degeneration owing to increased precursor cells in the endothelium of the peripheral cornea, which compensate for injuries. Alternatively, the peripheral corneal endothelium may contain migrative, if not proliferative, stem-like cells (He et al. 2012). Thus, the results observed in the present study may be attributed to the migration-promoting effects of ROCK inhibitors on healthy corneal endothelium rather than on the surrounding region.
The treatment of FECD by intentionally removing the Descemet’s membrane from the progressive loci in the central region and administering ripasudil eye drops has been reported previously (Moloney et al. 2017). A recent study revealed that ripasudil is associated with endothelial protective effects in intraocular surgery (Fujimoto et al. 2021). The findings of our study suggest that the irreversible and invasive procedure of Descemet’s membrane removal may not be necessary in some FECD cases, especially in the early stages. Ripasudil was reported to increase the expression of the Na+-K+ adenosine tri-phosphate pump in the rabbit corneal endothelium (Okumura 2016). Thus, ripasudil may increase the pump function of the endothelium in humans and could thus improve the corneal edema and visual acuity, as reported in our study.
The major strength of this study is that it demonstrated the efficacy of use of ripasudil for the treatment of FECD. In addition, our results suggest that both ripasudil and Y-27632 have similar effects. Koizumi et al. have reported that Y-27632 is effective for FECD treatments (Koizumi et al. 2014). However, ripasudil is the ROCK inhibitor formulated in the form of an eye drop (already approved for clinical use) that strengthens both the results of the current study and the potential application of the drug itself. However, in the systematic study of Koizumi et al., the effect of Y-27632 was examined over a period of only 1 week (Koizumi et al. 2014), which is relatively short compared with the 1.5 year period in our study.
One of the limitations of this study is its small sample size. Patients with FECD who did not undergo prior treatments and/or hospital visits are relatively rare. In addition, all included patients had glaucoma as ripasudil is currently only permitted for clinical use in these patients. If the drug was used for the treatment of patients without glaucoma, the results may have been different. Hence, the obtained results need to be verified with a prospective, randomized, masked study in the future. The data presented herein suggest that examining a large cohort of patients with and without glaucoma will be important to confirm that the use of ripasudil has a broad endothelial protective effect.
5. Conclusions
In conclusion, our results suggest that ROCK inhibitors are effective against chronic FECD. We demonstrated that the administration of these drugs during the waiting period before corneal endothelium transplantation can prolong the waiting period and improve vision quality among patients.
Acknowledgements
We thank Y. Setoguchi and H. Kamao for discussions, advice and criticisms that benefited this project. This work was supported by the Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical’s Founder 2019 (to H.F.); Research Project Grant 29B-009 from the Kawasaki Medical School (to H.F.); and JSPS KAKENHI [grants 25861630 and 16K18378 (to H.F.)].
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author Contributions
H.F. conceived the project, wrote the main manuscript text and prepared Figures 1–4. J.K. supervised the project. All authors reviewed the manuscript.
References
- Fujimoto H, Maeda N, Soma T, et al. Quantitative regional differences in corneal endothelial abnormalities in the central and peripheral zones in Fuchs’ endothelial corneal dystrophy. Investigative Ophthalmology & Visual Science 55 (2014): 5090-5098.
- Fujimoto H, Setoguchi Y, Kiryu J. The ROCK inhibitor ripasudil shows an endothelial protective effect in patients with low corneal endothelial cell density after cataract surgery. Translational Vision Science and Technology 10 (2021): 18.
- He Z, Campolmi N, Gain P, et al. Revisited microanatomy of the corneal endothelial periphery: new evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells 30 (2012): 2523-2534.
- Honjo M, Tanihara H. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Japanese Journal of Ophthalmology 62 (2018): 109-126.
- Inoue T, Tanihara H. Rho-associated kinase inhibitors: A novel glaucoma therapy. Progress in Retinal and Eye Research 37 (2013): 1-12.
- Jafri M, Colby K. New insights into corneal endothelial regeneration. Current Ophthalmology Reports 7 (2019): 37-44.
- Jun AS. One hundred years of Fuchs’ dystrophy. Ophthalmology 117 (2010): 859-860.e14.
- Kinoshita S, Koizumi N, Ueno M, Okumura N, Imai K, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. The New England Journal of Medicine 378 (2018): 995-1003.
- Koizumi N, Okumura N, Ueno M, Kinoshita S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea 33 (2014): S25-S31.
- Koizumi N, Okumura N, Ueno M, Nakagawa H, Hamuro J et al. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea 32 (2013): 1167-1170.
- Kusuhara S, Nakamura M. Ripasudil hydrochloride hydrate in the treatment of glaucoma: safety, efficacy, and patient selection. Clinical Ophthalmology 14 (2020): 1229-1236.
- Maurice DM. Cellular membrane activity in the corneal endothelium of the intact eye. Experientia 24 (1968): 1094-1095.
- Mimura T, Yamagami S, Yokoo S, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Investigative Ophthalmology & Visual Science 45 (2004): 2992-2997.
- Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis without grafting for Fuchs endothelial dystrophy-supplementation with topical ripasudil. Cornea 36 (2017): 642-648.
- Moshirfar M, Parker L, Birdsong OC, et al. Use of Rho kinase inhibitors in ophthalmology: a review of the literature. Medical Hypothesis, Discovery & Innovation in Ophthalmology 7 (2018): 101-111.
- Naumann GOH, Schlötzer-Schrehardt U. Keratopathy in pseudoexfoliation syndrome as a cause of corneal endothelial decompensation: A clinicopathologic study. Ophthalmology 107 (2000): 1111-1124.
- Nourinia R, Nakao S, Zandi S, Safi S, Hafezi-Moghadam A et al. ROCK inhibitors for the treatment of ocular diseases. British Journal of Ophthalmology 102 (2017): 1-5.
- Novack GD. Rho kinase inhibitors for the treatment of glaucoma. Drugs Future 38 (2013): 107-113.
- Okumura N, Koizumi N. Regeneration of the corneal endothelium. Current Eye Research 45 (2020): 303-312.
- Okumura N, Inoue R, Okazaki Y, et al. Effect of the Rho kinase inhibitor Y-27632 on corneal endothelial wound healing. Investigative Ophthalmology & Visual Science 56 (2015): 6067-6074.
- Okumura N, Kinoshita S, Koizumi N. Application of Rho kinase inhibitors for the treatment of corneal endothelial diseases. Journal of Ophthalmology 2017 (2017): 2646904.
- Okumura N, Koizumi N, Ueno M, et al. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. British Journal of Ophthalmology 95 (2011): 1006-1009.
- Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. The American Journal of Pathology 181 (2012): 268-277.
- Okumura N, Okazaki Y, Inoue R, et al. Effect of the rho-associated kinase inhibitor eye drop (ripasudil) on corneal endothelial wound healing. Investigative Ophthalmology & Visual Science 57 (2016): 1284-1292.
- Okumura N, Sakamoto Y, Fujii K, et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Scientific Reports 6 (2016): 26113.
- Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a rock inhibitor. Investigative Ophthalmology & Visual Science 50 (2009): 3680-3687.
- Pricopie S, Istrate S, Voinea L, et al. Pseudophakic bullous keratopathy. Romanian Journal of Ophthalmology 61 (2017): 90-94.
- Repp DJ, Hodge DO, Baratz KH, et al. Fuchs’ endothelial corneal dystrophy: subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology 120 (2013): 687-694.
- Szalai E, Berta A, Hassan Z, et al. Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus. Journal of Cataract & Refractive Surgery 38 (2012): 485-494.
- Tanihara H, Kakuda T, Sano T, et al. Safety and efficacy of ripasudil in Japanese patients with glaucoma or ocular hypertension: 12-month interim analysis of ROCK-J, a post-marketing surveillance study. BMC Ophthalmology 20 (2020): 275.


 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
