Association of Increased Risk of Atherosclerotic Cardiovascular (ASCVD) Event in Chronic Liver Disease Patients with and without Cirrhosis
Leila Hashemi1*, Cachet Magdolna Wenziger2, Tran Do3 , Arpan Patel4, Joseph R. Pisegna5, Tomas Ganz6, Mathew Budoff7, Jeffery Gornbein8, David Elashoff9, Elani Streja10
1Assistant Professor of Medicine, Department of General Internal Medicine, University of California, Los Angeles, VA Greater Los Angeles, USA
2Biostatistician, University of California, Irvine, Tibor Rubin VA Medical Center, Long Beach, California, USA
3MD/PhD Candidate, David Geffen School of Medicine, University of California, Los Angeles, California, USA
4Assistant Professor of Medicine, Division of Digestive Diseases, University of California, Los Angeles, California, USA; Division of Gastroenterology, Hepatology, and Nutrition, VA Greater Los Angeles Healthcare System
5Chief, Division of Gastroenterology, Hepatology and Parenteral Nutrition, Professor of Medicine and Human Genetics, David Geffen School of Medicine at UCLA, California, USA
6Distinguished Professor of Medicine and Pathology, Department of Medicine, David Geffen School of Medicine, University of California Los Angeles, California, USA
7Professor of Medicine, University of California, Los Angeles, California, USA. Endowed Chair of Preventive Cardiology, Lundquist Institute
8Principal Statistician, UCLA Dept of Medicine Statistic Core, Adjunct Professor in the Dept of Computational Medicine, Los Angeles, California, USA
9Professor of Medicine, Biostatistics and Computational Medicine, Director, Department of Medicine Statistics Core, University of California Los Angeles, California, USA
10 Assistant Professor in Residence, Health Science Specialist, University of California, Irvine, Tibor Rubin VA Medical Center, Long Beach, California, USA
*Corresponding author: Leila Hashemi, Assistant Professor of Medicine, Department of General Internal Medicine, University of California, Los Angeles, VA Greater Los Angeles, USA.
Received: 27 December 2021; Accepted: 05 January 2022; Published: 27 January 2022
Article Information
Citation: Leila Hashemi, Cachet Magdolna Wenziger, Tran Do, Arpan Patel, Joseph R Pisegna, Tomas Ganz, Mathew Budoff, Jeffery Gornbein, David Elashoff, Elani Streja. Association of Increased Risk of Atherosclerotic Cardiovascular (ASCVD) Event in Chronic Liver Disease Patients with and without Cirrhosis. Cardiology and Cardiovascular Medicine 6 (2022): 24-41.
View / Download Pdf Share at FacebookAbstract
Background: An area of debate in modern medicine is whether there is an association between cirrhosis and Atherosclerotic Cardiovascular Disease (ASCVD). To address this we conducted a retrospective cohort study composed of the 486,887 US Veterans with liver disease over the period of January 2000 to December 2019 to ascertain whether there is an association between cirrhosis and ASCVD. We further divided the cohort based on a diagnosis of cirrhosis. Cox-Regression, negative binomial and competing risk models were used to investigate the time interval between the first and recurrent ASCVD hospitalization with mortality as a competing event risk. The mean± SD age of the cohort was 58 ± 11 years, 4.6% were female, 63% White, 21% Black. 58% of the cohort had liver disease without a diagnosis of cirrhosis. The incidence of ASCVD hospitalization was much higher in liver patients with diagnosis of cirrhosis (11% vs 6%, p value<0.001). In a non-adjusted model with cirrhosis as the exposure the rate of first ASCVD hospitalization was 1.5 times higher than liver disease in patients without cirrhosis (HR: 1.49 (95%CI: 1.47- 1.50), p <0.001). In a fully adjusted model, the risk was attenuated but remained statistically significant (HR: 1.03 (95% CI:1.02-1.04, p <0.001)). The mean number of ASCVD hospitalizations in a count model was 30% lower in the cirrhosis group (mean count ratio 0.70 (95% CI: 0.68-.072)), due to higher competing risk of all-cause mortality with ASCVD events (0.77 (0.73-0.81)). Conclusion: We demonstrate in this retrospective cohort study that as liver disease progresses to cirrhosis, the risk of ASCVD events increases. We hypothesize that the pro-inflammatory states of liver disease could be a viable explanation for the increased risk of ASCVD events in cirrhosis patients. Further translational studies are needed to confirm this hypothesis.
Keywords
<p>Atherosclerotic Cardiovascular events; Cirrhosis; Cox-regression model; Liver Disease; Time to first ASCVD</p>
Article Details
1. Introduction
Cardiovascular Disease (CVD) is the leading cause of death globally [1], affecting up to 48% of the United States (U.S.) adult population [2]. Predicting and reducing CVD risk is therefore a top priority for clinical practice and improving public health. Cirrhosis is a morbid condition that is being increasingly recognized as a major public health concern. About 2% of adults have a diagnosis of liver disease and there were 41,473 deaths (12.8 deaths per 100,000 population) from chronic liver disease and cirrhosis in 2017 [3]. Understanding and reducing CVD risk factors in this population may slow this trend. CVD and cirrhosis share a number of common risk factors for progression, including obesity, insulin resistance, and diabetes. The progression of chronic liver disease to cirrhosis has been shown to correlate with concomitant heart involvement via development of insulin resistance, atherogenic dyslipidemia and up-regulation of pro-inflammatory cytokines [4]. Patients with liver disease and cirrhosis now live longer due to new advances in diagnosis and treatment; therefore, a lot of attention should be given to screening and prevention of Atherosclerotic Cardiovascular Disease (ASCVD) in this population. Currently the pooled cohort risk calculator (ASCVD risk calculator) is used in practice to estimate 10-year risk for a first ASCVD event in the general population. Whether the calculator could accurately predict the ASCVD risk in patients with chronic liver disease is unknown.
Current epidemiological evidence regarding the link between CVD and cirrhosis is conflicting. In the early 1960s, Howell et al reported that the incidence of myocardial infarction (MI), as determined by autopsy review, in patients with cirrhosis was 25% lower than the general adult population [5]. However, more recent studies suggest that CVD may be an important cause of morbidity and mortality in patients with cirrhosis. Carey et al [6] recognized that Coronary Artery Disease (CAD) is common in patients with cirrhosis undergoing liver transplant evaluation, and other studies have suggested that cardiovascular complications are the major cause of pre-and postoperative morbidity and mortality [7, 8]. Complications during the management of ASCVD in this population may also drive mortality. A higher rate of major complications and mortality following invasive treatments for acute MI has been observed. Hillerson et al found that patients with cirrhosis had lower rates of ST-Elevation MI (18.9% vs 26.7%) but also had lower rates of angiography, revascularization and coronary artery bypass graft following an acute MI. The rates of gastrointestinal hemorrhage and in hospital mortality was higher in patients with cirrhosis post cardiovascular procedures [9].
While some recent data has suggested an increased prevalence of CVD in patients with cirrhosis [10-14], others have shown a decreased rate or no difference [15-18]. Methodological differences, variation in study population and sample size, adaptation of different outcome measures as ASCVD surrogates (softer endpoints like percutaneous coronary intervention, coronary artery bypass graft and peripheral vascular disease or subclinical measurements like carotid intimal medial thickness, coronary calcium score), difference in statistical analysis and modeling make it difficult to draw a uniform conclusion from these studies. Definitive data from clinical trials is lacking. To address these issues, we analyzed a large cohort of patients with chronic liver disease and cirrhosis from the Veteran Health Administration electronic health record data to estimate the prevalence and incidence of ASCVD events. Our secondary aim was to identify risk factors associated with these events in patients with cirrhosis compared to individuals with chronic liver disease but without cirrhosis. We used the same model as used in the ASCVD calculator to test the accuracy of the model in patients with liver disease.
2. Methods
2.1. Study Population and Data Source
We conducted a retrospective cohort study composed of U.S. Veterans with liver disease over the period of January 1, 2000 to December 31, 2019. Our source population consisted of 2,658,969 patients from the U.S. Veterans Affairs (VA) databases with any International Classification of Diseases 9th and 10th revision (ICD-9, ICD-10) codes with liver disease. From this, a total of 486,990 Veterans were identified with a diagnosis for liver disease, determined by the presence of ICD-9 and ICD-10 codes for non-alcoholic fatty liver disease, alcoholic liver disease, viral hepatitis and corresponding ICD-9 and ICD-10 codes for cirrhosis (K74.6, K74.3, K75.8, K74.5, K78.8, K70, K70.3, K76.9, 571.2, 571.5, B18.2, B18.8, K73, 070, B18, B16, K70, K7581, 571.0) [19, 20]. Veterans were excluded from this study if they were under 18 years or if information on age was missing (N=3) and if there were errors in follow up time (N=100). The final cohort consisted of 486,887 adult Veterans (age ≥ 18 years) with a diagnosis of liver disease. Veterans were further divided into two subgroups based on liver disease with or without cirrhosis (Supplement Figure 1). Due to the nonintrusive and anonymous nature of this research and large sample size, the requirement for written informed consent was waived and the study was approved by the Institutional Review Boards of the VA Greater Los Angeles Health Care System.
2.2. Demographics and clinical measurements
Information on demographics and comorbidities were extracted from a combination of the VA and Centers for Medicare and Medicaid Services (CMS) databases. A combination of two outpatient or one inpatient ICD-9 or ICD-10 Diagnostic or Current Procedural Terminology codes were used to determine pre-existing comorbidity status and Charlson Comorbidity Index (CCI) prior to the first date of diagnosis for chronic liver disease or cirrhosis disease using the VA and CMS datasets [21, 22]. Laboratory measurements, including the lipid panel, were obtained from the VA Managerial Cost Accounting System Laboratory Results. Information on Body Mass Index (BMI) and Blood Pressure (BP) were obtained from the VA Central Data Warehouse (CDW) Vital Signs file. Laboratory and vital sign measurements were included if they were taken within 90 days of the first diagnosis date for liver or cirrhosis disease (either direction).
2.3. Exposure measurements
The main exposure variable of interest for this study was presence of chronic liver disease, with and without cirrhosis.
2.4. Outcome measurements
The co-primary outcomes of interest were 1) number of hospitalizations for ASCVD and 2) time to first ASCVD hospitalization after diagnosis of either chronic liver disease or cirrhosis. Information on mortality, ASCVD records and censoring events, were extracted from VA, National Death Index (NDI), and CMS data sources. Lost to follow-up status was determined by the last date of active use of VA or CMS services (inpatient, outpatient, laboratory, or pharmacy). Follow-up began at the first date of cirrhosis diagnosis and ended at the time of the first ASCVD event, death, lost to follow-up, or the end of the study period (December 31, 2019), whichever occurred first. The ASCVD outcomes were defined as a composite of non-fatal MI, unstable angina, ischemic stroke and cardiovascular mortality [23].
3. Statistical Analysis
Baseline demographic and clinical characteristics were reported using mean ± Standard Deviation (SD), or median (interquartile range [IQR]) for continuous data, or proportions (N (%)) for categorical data. In order to compare differences in covariates distribution between Veterans with and without cirrhosis, t-tests were used to compare means, the Mann-Whitney U test was used to compare medians, and Chi-square tests were used to compare proportions. Count models using negative binomial regression were used to examine differences in the number of recurrent ASCVD hospitalization events between Veterans with and without cirrhosis without time consideration. Cox proportional hazard regression was used to examine the relationship between cirrhosis vs no cirrhosiswith time to the first ASCVD hospitalization. Kaplan-Meier survival curves and negative log-log plots were used to check for violations in the proportional hazards assumptions. Fine and Gray competing risk analysis was used to examine the relationship between cirrhosis vs. no cirrhosis with time to the first ASCVD hospitalization, using mortality as a competing event. All analysis, including negative binomial regression, competing risk model and Cox proportional hazards regression were performed using an unadjusted model and models incrementally adjusted for age, sex, race, ethnicity, smoking status (current or past versus never), hypertension (HTN), diabetes mellitus (D), systolic blood pressure (SBP), diastolic blood pressure (DBP), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). We chose these variables from the pooled cohort risk prediction model which predicts the 10-year risk for a first ASCVD event in the general population. These variables were tested in the fully adjusted Cox proportional hazards regression in the cohort to find out whether these variables in patients with liver disease, regardless of cause, are predictive of 10-year risk of first ASCVD event [24].
Univariate analysis was used to explore the association between traditional cardiovascular covariates with ASCVD events for all models. Also, intermediate models adjusted for age, sex, ethnicity, race, smoking status, HTN and DM for all models were analyzed. Variables related to the severity of liver disease like albumin, bilirubin, international normalized ratio (INR) were not tested due to the a high percentage of missingness . In sensitivity analysis, we also examined associations for for first MI /unstable angina hospitalization and ischemic stroke following the diagnosis of liver disease/cirrhosis using all 3 models of adjustment. Missing values for BMI, HDL, LDL and blood pressure were imputed using mean imputation since data for these variables were only missing in less than 25% of the total cohort. Harrell’s C statistic was computed to assess the final Cox model accuracy. All analysis was performed using SAS Enterprise Guide version 7.1 (SAS Institute Inc, Cary, NC.).
4. Results
4.1. Demographics, risk factors, and clinical characteristics
As presented in Table 1, 58% of the cohort had liver disease without a diagnosis of cirrhosis. The mean±SD age of the cohort was 58± 11years, 4.6% were female, 63% were White, 21% were Black, and 7% Hispanic. Patients with cirrhosis had a higher prevalence of HTN compared to the liver disease patients without a diagnosis of cirrhosis (33% vs 17%, p<0.001) and DM (18% vs 9%, p <0.001). The overall prevalence of DM in the cohort was 12.5%. Rates of smoking were higher in patients with chronic liver disease without a diagnosis of cirrhosis (58% vs 42 %, p <0.001). Patients with a diagnosis of cirrhosis had lower LDL (89 ± 39 vs 103 ± 37, p <0.001), HDL (42 ± 18 vs 45 ± 17, p <0.001), total cholesterol (156 ± 48 vs 178 ± 46, p <0.001) and triglyceride (136 (76-159)vs 179 (90-204), p<0.001), and SBP (131 ± 20 vs 132 ± 18, P<0.001). High-sensitivity C-reactive protein (hsCRP) was available for 1.8% of the cohort. The average hsCRP was much higher in patients with cirrhosis (6.5 (2.3-9.6)vs 5.8 (2.1-7.9), p <0.001). The patients with cirrhosis had a lower BMI than those with chronic liver disease (28.9 ±6.3 vs 29.3 ±6.2, p <0.001). A higher number of patients with cirrhosis were on the combination of furosemide and spironolactone (11.5% vs 3.2%, p <0.001) and beta-blockers (15% vs 11%, p <0.001), however, more patients without cirrhosis were more likely to be on Angiotensin-converting-enzyme inhibitors (ACEi), Hydrochlorothiazide (HCTZ) and statin (Table 1).
|
Variable |
Cirrhosis |
No Cirrhosis |
P-value |
|
N (%) |
204926 |
281961 |
|
|
ASCVD events N (%) |
70,051(34%) |
101,715 (36%) |
<0.001 |
|
Mortality N (%) |
97,794(47.7%) |
57,472 (20.4%) |
< 0.001 |
|
Cohort/10000 patients/year |
63.1 |
172.3 |
< 0.001 |
|
ASCVD Incidence Rate (95% CI) |
11 (10.9-11.1) |
6 (5.8-5.9) |
< 0.001 |
|
Median Time to ASCVD in years (±IQR) |
3.0 (3.5- 4.4) |
6.0 (5.0-9.2) |
< 0.001 |
|
Age (years(±SD)) |
62.0 (±9.9) |
55.7(± 11.8) |
< 0.001 |
|
Sex (%male) |
97% |
94% |
|
|
Race (%) |
|||
|
White |
64% |
63% |
< 0.001 |
|
Black |
18.30% |
23.77% |
< 0.001 |
|
Others |
1.39% |
1.61% |
< 0.001 |
|
Hispanics |
7.37% |
7.32% |
0.51 |
|
Ever drinker |
39.27% |
39.19% |
0.56 |
|
Total cholesterol (±SD) mg/dL |
155.7(±48.0) |
178(±45.7) |
< 0.001 |
|
LDL(±SD) mg/dL |
88.7(±38.6) |
103.2(±37.4) |
< 0.001 |
|
HDL(±SD) mg/dL |
42.3(±17.8) |
44.7(±16.5) |
< 0.001 |
|
Triglyceride (IQR) mg/dL |
136 (76-159) |
179 (90-204) |
< 0.001 |
|
SBP(±SD) MM/HG |
130.6(±20.3) |
132.3(±18.2) |
< 0.001 |
|
DBP (±SD) MM/HG |
75.4(±12.6) |
79 (±11.8) |
< 0.001 |
|
HTN |
32.50% |
17.12% |
< 0.001 |
|
DM |
17.80% |
8.68% |
< 0.001 |
|
Smoking (Current and Past) |
42.10% |
57.90% |
< 0.001 |
|
BMI (±SD) kg/m2 |
28.9(±6.3) |
29.3(±6.2) |
< 0.001 |
|
Sodium (±SD) mEq/L |
137(±4.5) |
138(±3.5) |
< 0.001 |
|
ALT(IQR) units/L |
57(23-6) |
63(26-7) |
< 0.001 |
|
AST(IQR) units/L |
73.5 (29-8) |
50(24-5) |
< 0.001 |
|
Albumin (±SD) g/dL |
3.4(±0.7) |
4.05(±0.5) |
< 0.001 |
|
INR (±SD) |
1.3(±0.7) |
1.21(±0.7) |
< 0.001 |
|
hsCRP(IQR) mg/L |
6.5 (2.3-9.6) |
5.8 (2.1-7.9) |
< 0.001 |
|
HCT % |
38.7(6.6) |
42.6(4.9) |
< 0.001 |
|
Blood Sugar mg/dL |
128(62) |
119(54) |
< 0.001 |
|
ARB |
0.03% |
0.01% |
< 0.001 |
|
ACEI |
10.26% |
11.55 |
< 0.001 |
|
Statin |
11.17% |
16.30% |
< 0.001 |
|
Non-statin |
0.16% |
0.48% |
< 0.001 |
|
ASA |
6.15% |
5.85% |
< 0.001 |
|
Beta blocker |
14.50% |
11.1% |
< 0.001 |
|
HCTZ |
5.80% |
7.50% |
< 0.001 |
|
Lasix+ Sipronolactone (30% Aldactone) |
11.50% |
3.20% |
< 0.001 |
|
SD: standard deviation, IQR: inter quartile range, LDL: low density lipoprotein, HDL: high density lipoprotein, BMI: body mass index, SBP and DBP: systolic and diastolic blood pressure, HTN: hypertension, DM: diabetes mellitus, HC: hematocrit, hsCRP: high sensitivity- C-reactive protein, HCTZ: hydrochlorothiazide, ARB: angiotensin receptor blocker, ACEI: angiotensin enzyme inhibitor, ASA: aspirin, ALT: alanine transferase, AST: aspartate transferase, INR: International Normalized Ratio, mg/dL: milligram/deciliter, MM/HG: millimeter of mercury, g/dL: gram/deciliter, mEq/ L: milliequivalents per liter, kg/m2: kilogram per square meter, units/L: units per litter, CI: confidence interval |
|||
Table 1: Baseline characteristic stratified by cirrhosis diagnosis.
4.2. ASCVD hospitalizations comparing Veterans with and without cirrhosis
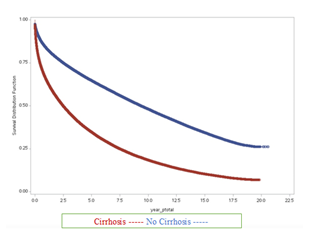
Although the overall prevalence of ASCVD hospitalizations in our cohort is higher in patients without cirrhosis (20.88% vs 14.38%), the incidence rate of ASCVD events was much higher in those with cirrhosis (11 vs 6 per 10,000 patient-years) (Table 1). Median time to the first ASCVD hospitalization in patients with cirrhosis was much shorter (3 years (IQR: 3.54- 4.38 years)) vs 6.02 years (IQR: 5.04-9.22 years)) in patients with chronic liver disease without cirrhosis (Table 2).
|
Variables |
Cox model Time - first ASCVD hospitalization |
|||
|
Univariate analysis |
Adjusted |
|||
|
Hazard Ratio (95%CI) |
P-Value |
Hazard Ratio (95%CI) |
P-Value |
|
|
Cirrhosis: No |
Reference |
Reference |
||
|
Cirrhosis: Yes |
1.52 (1.50-1.53) |
<0.001 |
1.03 (1.02-1.05) |
<0.001 |
|
Age (year) |
1.06(1.06-1.06) |
<0.001 |
1.05 (1.49-1.05) |
<0.001 |
|
Smoking |
1.09(1.08-1.10) |
<0.001 |
1.12 (1.11-1.13) |
<0.001 |
|
Sex (male) |
1.77(1.72-1.82) |
<0.001 |
1.25(1.22-1.30) |
<0.001 |
|
Race White |
Reference |
Reference |
Reference |
|
|
Race Black |
0.80(0.79-0.81) |
<0.001 |
0.92(0.90-0.92) |
<0.001 |
|
Race Others |
0.70(0.67-0.73) |
<0.001 |
0.85 (0.81-0.90) |
<0.001 |
|
Hispanic ethnicity |
0.73(0.71-0.74) |
<0.001 |
0.80 0.78-0.81) |
<0.001 |
|
HTN |
4.70(4.55-4.85) |
<0.001 |
1.90(1.87-1.91) |
<0.001 |
|
DM |
2.79(2.73-2.83) |
<0.001 |
1.53 (1.50-1.56) |
<0.001 |
|
HDL (mg/dL) |
0.990(0.990-0.991) |
<0.001 |
0.99 (0.992-0.993) |
<0.001 |
|
LDL (mg/dL) |
0.992(0.992-0.992) |
<0.001 |
0.99 (0.997-0.998) |
<0.001 |
|
SBP (MM/HG) |
1.004(1.004-1.004) |
<0.04 |
1.004 (1.003-1.004) |
<0.001 |
|
DBP (MM/HG) |
0.985(0.985-0.996) |
<0.001 |
0.99 (0.992-0.994) |
<0.001 |
|
HTN: Hypertension, LDL: low- density lipoprotein, HDL: high density lipoprotein, DM: diabetes mellitus, SBP: systolic blood pressure, DBP, Diastolic Blood pressure, CI: Confidence Interval, mg/dL: milligram/deciliter, MM/HG: millimeter of mercury, |
||||
Table 2: Cox model Time - first ASCVD hospitalization.
In univariate negative binomial exploratory analysis, male sex, HTN and DM were significant risk factors for recurrent ASCVD events throughout the study follow-up (Table 3). In an unadjusted count regression (negative binomial) model with cirrhosis versus liver disease without cirrhosis as the exposure, the mean number of repeated ASCVD hospitalization after the first event following the diagnosis of liver disease or cirrhosis was 30% lower in the cirrhosis group ((Mean Count Ratio=MCR:0.70, 95%CI (0.68-.072), p <0.001; Table 3). This risk in the fully adjusted model was further decreased (MCR: 0.40 (0.39-0.41), p <0.001) (Table 3). In our unadjusted competing risk model, the risk of ASCVD event rate was 23% lower in cirrhosis due to an increased risk of mortality (Sub-Hazard Ratio=SHR: 0.77(0.73-0.81), p <0.001), After adjusting for all the covariates, all-cause mortality was a strong competing risk for ASCVD events (SHR 0.76(0.72-0.80), p < 0.001) (Table 3). Therefore, the risk of higher mortality in patients with cirrhosis decreased the risk of recurrent ASCVD and therefore its incidence.
Table 3: Negative Binomial Model for Number of ASCVD Hospitalizations and Competing Risk Models for ASCVD Events:
In the univariate Cox regression exploratory analysis, age, sex, smoking, HTN and DM were the major risk factors for ASCVD events in addition to cirrhosis (Table2). In our unadjusted Cox regression model (Table 2), the patient with cirrhosis had a 1.5 times higher risk of ASCVD event rates (HR: 1.49 (95%CI: 1.47-1.50), p <0.001). After adjusting for covariates, the association was attenuated but remained statistically significant (HR: 1.03 (95% CI:1.02-1.04, p <0.001)). Harrell’s C statistic was calculated to evaluate the ability of the models to separate those who experienced the events from those who did not (accuracy/discrimination). The Harrell’s C Statistic for this model was 0.72 (standard error=0.0006).
5. Discussion
In our cohort of 486,887 adult Veterans with chronic liver disease and cirrhosis, we observed a higher incidence of ASCVD events, defined as the composite of unstable angina, MI, ischemic stroke and cardiovascular mortality, in patients with cirrhosis compared to those with liver disease subjects but without cirrhosis (11% vs. 6%, p <0.001). In an unadjusted and adjusted time-to-event model, cirrhosis was an independent risk factor for the first ASCVD hospitalization following diagnosis of chronic liver disease or cirrhosis. The discriminatory performance of the fully adjusted model is modest (Harrell’s C Statistic: 0.72). The risk of a recurrent ASCVD event was higher in patients with chronic liver disease without cirrhosis (Table 3, negative binomial model), due to the higher risk of mortality competing with ASCVD events in patients with cirrhosis (Table 3, compering risk model). Prevalence of ASCVD events in the entire cohort was 35%, which is far above the prior reported estimates of 7-10% seen in the general population in the United States [25, 26]. The prevalence of diabetes in this cohort was comparable to that seen in the US population (12.52% of our cohort vs 13% general population) [26]. However, statin use in this cohort was overall low, particularly in patients with cirrhosis (11.17%). Conversely, despite the large amount of evidence about the safety of statin use in patients with liver disease, prior studies have shown that statin use among patients with liver disease is underutilized by primary care physicians and cardiologists as we observed in our study [27-30]. Other cardio-protective medications like ACEI/ARB and beta-blockers are underutilized in our cohort as well (Table 1). Higher beta-blocker and spironolactone use seen in patients with cirrhosis was likely due to their use in treating complications of portal hypertension such as ascites.
Prior studies have used surrogate endpoints, such as utilization of procedures (percutaneous coronary intervention, coronary artery bypass graft) or subclinical measurements (carotid intimal medial thickness, coronary calcium score) to examine the association between cirrhosis and ASCVD outcomes. The present study used a composite of hard ASCVD outcomes, as well as predictors, used in a pooled cohort equation and ASCVD outcome clinical trials. Our main results showing an association between cirrhosis and ASCVD events were consistent across multiple sensitivity analyses analyzing MI and ischemic strke(CVA) outcomes separately. As it is shown in Table S1, the HR is much higher for CVA than for MI and composite of MI, CVA and cardiovascular mortality. This is in agreement with prior publications, which have shown higher risk of ischemic stroke in patients with cirrhosis and also hepatitis C [31, 32]. Determining risk factors associated with these events are a critical next step in understanding ways to curb ASCVD-associated mortality in this population. Previous data postulated that certain clinical factors are protective against ASCVD in patients with cirrhosis, such as lower SBP, lower plasma LDL level, elevated level of estrogen and vasodilatory peptides such as nitric oxide [33-36]. However, our study shows that with cirrhosis, the incidence of ASCVD events increases and the time to first ASCVD hospitalization shortens despite lower average SBP and plasma LDL, TG and total cholesterol concentration levels. In fully adjusted models, age, White race, male sex, SBP along with cirrhosis diagnosis are risk factors for ASCVD events while LDL, HDL and DBP were not. In fact, higher LDL in patients with cirrhosis was associated with lower risk of ASCVD events in our fully-adjusted survival analysis model, which may parallel trends seen with end stage renal disease (ESRD) and defined as reversed epidemiology [37]. Though cirrhosis is associated with alterations in lipid metabolism and increased atherogenicity due to inflammation and cytokine production, these factors may not be fully captured by routine blood level measurements [38-44].
Cirrhosis as an inflammatory state may also explain some of this association. In our cohort, serum hsCRP was only available in 1.8% of patients, but the average hsCRP level within 90 days of cirrhosis or liver disease diagnosis was much higher in cirrhosis than patients without cirrhosis (Table 1). Hepatic Stellate Cells (HSC) play a fundamental role in the inflammatory state of cirrhosis [45]. HSC would sense the altered hepatic tissue integrity (induced by injury or toxins) and activates the innate immune system response by hepatic macrophages. This will activate a cascade of pro-inflammatory and anti-inflammatory cytokines, which cause pathological remodeling of the liver architecture [45, 46]. Matyas et al [45] showed massive inflammation, oxidative stress, microvascular dysfunction in a bile-duct ligation-induced liver fibrosis mouse model. These pathological changes were accompanied by cardiac inflammation and oxidative stress and therefore there is a liver-heart inflammatory axis in advanced liver disease. Isaak et al [47] also showed the inflammatory liver-heart axis by cardiac magnetic resonance imaging with increased severity of myocardial edema and fibrosis as the severity of liver disease increased. Berzigotti et al [30] investigated 47 patients with cirrhosis by looking at the 10-year global cardiovascular Framingham risk score and found that almost 50% of these patients have moderately moderate to high cardiovascular risk, which in turn caused systemic endothelial dysfunction due to inflammation. They pointed out a high association between bilirubin, albumin and white cell count in patients with cirrhosis and endothelial dysfunction. Increased risk of ASCVD events has also been observed in autoimmune diseases such as rheumatoid arthritis. In recent years, hsCRP has gained importance as a novel risk factor in the development and progression of atherosclerosis [48-52]. Therefore, it is likely that the inflammatory state of liver disease leads to higher incidence of ASCVD events. Advanced liver disease could serve as a model to investigate the role of inflammation in the development and progression of atherosclerosis as well as the role of anti-inflammatory agents like IL-1 inhibitor (Canakinumab), and statin in both cirrhosis and ASCVD management [51-54].
6. Study Limitation
Despite a plethora of available data for this observational cohort study, we cannot rule out residual confounding nor make causal inferences. We were unable to adjust for potential confounders including other markers of nutrition or apolipoproteins, liver markers like albumin, INR and bilirubin and cardiac inflammatory markers like hsCRP, mostly due to missingness of data. Also, the severity of liver disease was only defined at one time-point as cirrhosis diagnosis versus no cirrhosis. Another limitation of this study is that our cohort mostly consists of white men, which makes the results harder to generalize to the general population.
6.1. Study Strengths
Highlighted strengths of this study include the large sample size, use of multiple sources of data, as well as long length of follow-up and the standard definition of ASCVD.
6.2. Future direction
We propose a further assessment of this cohort by creating a scoring system to quantify the severity of the liver fibrosis and then assess the association of the severity of liver disease with ASCVD outcomes. Also, we suggest for the next step of this study to use time-varying data for the variables used in the model as well as include markers of liver function, such as INR, albumin and bilirubin levels. Furthermore, clinical trial and animal model studies could play an important role in investigating the causal relationship of liver disease severity with development and progression of atherosclerosis and ASCVD outcomes with regards to inflammation. It is also crucial to study the role of anti-inflammatory agents like interleukin-1 (IL-1) inhibitors and statin drugs in regression of liver fibrosis and its association with ASCVD progression and outcomes in a double-blind control trial.
7. Conclusion
Our study showed that in a large cohort of Veterans with liver disease, diagnosis of cirrhosis was associated with higher incidence of of ASCVD events. The prevalence of ASCVD events was higher in patients with liver disease without a diagnosis of cirrhosis; however, this was because the competing all-cause mortality was higher in patients with cirrhosis. We hypothesized that as the liver disease advances, the activation of inflammatory pathways speeds up the progression of atherosclerosis.
Acknowledgements
The data reported her has been supplied by the US Veterans Administration. Support for VA/CMS data provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). Opinions expressed in this manuscript are those of the authors and do not represent the official opinion of the US Department of Veterans Affairs. LH, AP, JP and ES are employees of the US Department of Veteran Affairs.
Conflict of Interest
None of the paper’s content has been previously published. There is no relationship with industry and there are no potential financial conflicts of interest relevant to the submitted manuscript for any of the authors.
References
- Cardiovascular disease, world health organization.
- Benjamin EJ, Muntner P, Alonso A, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139 (2019): 526-528.
- Center for Disease Control and Prevention.
- Lonardo A, Sookoian S, Chonchol M, et al. Cardiovascular and systemic risk in nonalcoholic fatty liver disease-atherosclerosis as a major player in the natural course of NAFLD. Current pharmaceutical design 19 (2013): 5177-5192.
- Howell WL, Manion WC. The low incidence of myocardial infarction in patients with portal cirrhosis of the liver: A review of 639 cases of cirrhosis of the liver from 17,731 autopsies. Am Heart J 60 (1960): 341-344.
- Carey WD, Dumot JA, Pimentel RR, et al. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation 59 (1995): 859-864.
- Koshy AN, Gow PJ, Han HC, et al. Cardiovascular mortality following liver transplantation: predictors and temporal trends over 30 years. European Heart Journal-Quality of Care and Clinical Outcomes 6 (2020): 243-253.
- Van Wagner LB, Lapin B, Levitsky J, et al. High early cardiovascular mortality after liver transplantation. Liver Transpl 20 (2014): 1306-1316.
- Hillerson D, Ogunbayo GO, Salih M, et al. Outcomes and Characteristics of Myocardial Infarction in Patients With Cirrhosis. J Invasive Cardiol 31 (2019): 162-169.
- Danielsen KV, Wiese S, Hove J, et al. Pronounced Coronary Arteriosclerosis in Cirrhosis: Influence on Cardiac Function and Survival? Dig Dis Sci 63 (2018): 1355-1362.
- Hashemi L, Pisegna J, Budoff M, et al. Association of Cirrhosis and Increased Risk of Cardiovascular Events in a VA Patient Population, A Retrospective Cohort Study. Archives of Internal Medicine Research 3 (2020): 210-229.
- Patel SS, Nabi E, Guzman L, et al. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl 24 (2018): 333-342.
- Golabi P, Fukui N, Paik J, et al. Mortality risk detected by atherosclerotic cardiovascular disease score in patients with nonalcoholic fatty liver disease. Hepatology communications 3 (2019): 1050-1060.
- Lin SY, Lin CL, Lin CC, et al. Risk of acute coronary syndrome and peripheral arterial disease in chronic liver disease and cirrhosis: a nationwide population-based study. Atherosclerosis 270 (2018): 154-159.
- Tsai MC, Yang TW, Wang CC, et al. Favorable clinical outcome of nonalcoholic liver cirrhosis patients with coronary artery disease: A population-based study. World J Gastroenterol 24 (2018): 3547-3555.
- An J, Shim JH, Kim SO, et al. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: a registry-based matched case-control study. Circulation 130 (2014): 1353-1362.
- Berzigotti A, Bonfiglioli A, Muscari A, Bianchi G, Libassi S, Bernardi M, Zoli M. Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver Int 25 (2005): 331-336.
- Kalaitzakis E, Rosengren A, Skommevik T, et al. Coronary artery disease in patients with liver cirrhosis. Dig Dis Sci 55 (2010): 467-475.
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision Instruction manual. 2010 ed. Geneva: World Health Organization n.d. (2010)
- Nehra MS, Ma Y, Clark C, et al. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology 47 (2013): 50.
- Calculating a Comorbidity Index for Risk Adjustment Using VA or Medicare Data. Hines, IL: U.S. Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center (2014).
- Byrne MM, Kuebeler M, Pietz K, et al. Effect of using information from only one system for dually eligible health care users. Med Care 44 (2006): 768-773.
- Gómez G, Gómez-Mateu M, Dafni U. Informed choice of composite end points in cardiovascular trials. Circ Cardiovasc Qual Outcomes 7 (2014): 170-178.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 1 (2014): 632889-632934.
- Centers for disease control and prevention. National Diabetis statistics report 2020.
- Lewis JH, Mortensen ME, Zweig S, et al. and Pravastatin in Chronic Liver Disease Study Investigators, 2007. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 46 (2007): 1453-1463.
- Chalasani N, Aljadhey H, Kesterson J, et al. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 126 (2004): 1287-1292.
- Rzouq FS, Volk ML, Hatoum HH, Talluri SK, Mummadi RR, Sood GK. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. The American journal of the medical sciences 340 (2010): 89-93..
- Blais P, Lin M, Kramer JR, et al. Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Digestive diseases and sciences 61 (2016): 1714-1720.
- Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 144 (2013): 102-111.
- Amedeo Lonardo, Luigi E Adinolfi, Paola Loria, et al. Day, Steatosis and hepatitis C virus: Mechanisms and significance for hepatic and extrahepatic disease,Gastroenterology 2004 (126): 586-597.
- Gorcyca K, Khan I, Wadhera R, et al. Prevalence of atherosclerotic cardiovascular disease (ASCVD) and diabetes populations in the United States. Journal of Clinical Lipidology 9 (2015): 424.
- Clària J, Jiménez W, Ros J, et al. Increased nitric oxide—dependent vasorelaxation in aortic rings of cirrhotic rats with ascites. Hepatology 20 (1994): 1615-1621.
- Loria P, Marchesini G, Nascimbeni F, et al. Lonardo,Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology, Atherosclerosis 232 (2014): 99-109.
- Henriksen JH, Moller S. Liver cirrhosis and arterial hypertension. World Journal of Gastroenterology: WJG 12 (2006): 678.
- D'arienzo A, Manguso F, Scaglione G, et al. Prognostic value of progressive decrease in serum cholesterol in predicting survival in Child-Pugh C viral cirrhosis. Scandinavian journal of gastroenterology 33 (1998): 1213-1218.
- Kalantar-Zadeh K. Cardiovascular And Survival Paradoxes In Dialysis Patients: What Is So Bad about Reverse Epidemiology Anyway?. In Seminars in dialysis 20 (2007): 593-601.
- Cicognani C, Malavolti M, Morselli-Labate AM, et al. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Archives of internal medicine 157 (1997): 792-796.
- Trieb M, Horvath A, Birner-Gruenberger R, et al. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1861 (2016): 630-638.
- Loria P, Marchesini G, Nascimbeni F, et al. Lonardo,Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology, Atherosclerosis 232 (2014): 99-109.
- Moustafa T, Fickert P, Magnes C, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology 142 (2012): 140-151.
- Habib A, Mihas AA, Abou-Assi SG, et al. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clinical gastroenterology and hepatology 3 (2005): 286-291.
- Marsche G, Saemann MD, Heinemann A, et al. Inflammation alters HDL composition and function: implications for HDL-raising therapies. Pharmacology & therapeutics 137 (2013): 341-351.
- Pirillo A, Catapano AL, Norata GD. HDL in infectious diseases and sepsis. High Density Lipoproteins (2015): 483-508.
- Martínez-Esparza M, Tristán-Manzano M, Ruiz-Alcaraz AJ, García-Peñarrubia P. Inflammatory status in human hepatic cirrhosis. World journal of gastroenterology 21 (2015): 11522.
- Isaak A, Praktiknjo M, Jansen C, et al. Myocardial Fibrosis and Inflammation in Liver Cirrhosis: MRI Study of the Liver-Heart Axis. Radiology 297 (2020):51-61.
- Martinez BK, White CM. The Emerging Role of Inflammation in Cardiovascular Disease. Ann Pharmacother 52 (2018): 801-809.
- Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336 (1997): 973-979.
- Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347 (2002): 1557-1565.
- Ridker PM, Cannon CP, Morrow D, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 352 (2005): 20-28.
- Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev 24 (2006): 33-50.
- Tornai D, Szabo G. Emerging medical therapies for severe alcoholic hepatitis. Clin Mol Hepatol 26 (2020): 686-696.
- Wright AP, Adusumalli S, Corey KE. Statin therapy in patients with cirrhosis. Frontline Gastroenterol 6 (2015): 255-261.
- Parikh NS, Navi BB, Schneider Y, et al. Association between cirrhosis and stroke in a nationally representative cohort. JAMA neurology 74 (2017): 927-932.
- Centers for disease control and prevention. Heart Disease Facts.
- Matyas C, Erdelyi K, Trojnar E, et al. Interplay of Liver–Heart Inflammatory Axis and Cannabinoid 2 Receptor Signaling in an Experimental Model of Hepatic Cardiomyopathy. Hepatology 71 (2020): 1391-1407.
Supplementary Information
Table S1:
|
Time to First ischemic stroke Hospitalization |
Time to First MI Hospitalization |
||||
|
HR for cirrhosis vs no cirrhosis |
HR for cirrhosis vs no cirrhosis |
||||
|
Model |
Adjustment |
HR (95% CI) |
Model |
Adjustment |
HR (95% CI) |
|
1 |
unadjusted |
1.54(1.52-1.56) |
1 |
unadjusted |
1.49(1.47-1.50) |
|
2 |
Model 1 +age |
1.21(1.20-1.23) |
2 |
model1 +age |
1.16(1.15-1.17) |
|
3 |
Model 2 + race+ sex+ DM+ HTN |
1.135(1.12-1.15) |
3 |
Model2 + race+ sex+ DM+ HTN |
1.07(1.05-1.08) |
|
4 |
Model3+ SBP+DBP+LDL+HDL |
1.20(1.10-1.30) |
4 |
Model3+ SBP+DBP+LDL+HDL |
1.01(1.008-1.02) |
HTN: Hypertension, LDL: low- density lipoprotein, HDL: high density lipoprotein, DM: diabetes mellitus, SBP: systolic blood pressure, DBP, Diastolic Blood pressure, CI: Confidence Interval,
Figure 1: Cohort Description.
Table S2: Cox model for time to first ASCVD event, according to Cirrhosis + other demographics and comorbidities.
|
Variable |
HR (95% CI) |
P-value |
|
Cirrhosis |
1.085 (1.074-1.096) |
<.0001 |
|
Age |
1.053(1.052-1.052) |
<.0001 |
|
Smoking |
1.122(1.110-1.133) |
<.0001 |
|
Race-White |
Reference |
|
|
Race-Black |
0.886(0.875-0.896) |
<.0001 |
|
Race-Others |
0.845(0.809-0.883) |
<.0001 |
|
Ethnicity-Hispanic |
0.795(0.780-0.811) |
<.0001 |
|
HTN |
1.918(1.897-1.939) |
<.0001 |
|
DM |
1.606(1.576-1.636) |
<.0001 |
|
Sex |
1.302 (1.267-1.338) |
<.0001 |
HTN: Hypertension, LDL: low- density lipoprotein, HDL: high density lipoprotein, DM: diabetes mellitus, SBP: systolic blood pressure, DBP, Diastolic Blood pressure, CI: Confidence Interval,
Table S3: Negative Binomial model for ASCVD Hospitalizations Rates according to Cirrhosis and other demographics and Comorbidities MCR = mean count ratio
|
Variable |
MCR (95%CI) |
P-value |
|
Cirrhosis |
0.433 (0420-0.446) |
<.001 |
|
Age |
1.079(1.077-1.081) |
<.001 |
|
Smoking |
1.42 (1.38-1.47) |
<.001 |
|
Race-White |
reference |
|
|
Race-Black |
1.08 (1.04-1.12) |
<.001 |
|
Race-Hispanic |
0.67(0.63-0.71) |
<.001 |
|
Race-others |
0.66 (0.58-0.74) |
<.001 |
|
HTN |
4.62 (4.47-4.78) |
<.001 |
|
DM |
1.83 (1.71-1.96) |
<.001 |
|
Sex (male vs Female) |
3.69 (3.43-3.98) |
<.001 |
HTN: Hypertension, LDL: low- density lipoprotein, HDL: high density lipoprotein, DM: diabetes mellitus, SBP: systolic blood pressure, DBP, Diastolic Blood pressure, CI: Confidence Interval,
Table S4: Negative Binomial model for mean number of hospitalizations using Cirrhosis + Comorbidities + Labs
|
Variable |
MCR (95%CI) |
P-value |
|
Cirrhosis |
0.405(0.39-0.41) |
<.001 |
|
Age |
1.076(1.074-1.078) |
<.001 |
|
Smoking |
1.44 (1.39-1.48) |
<.001 |
|
Race-White |
reference |
|
|
Race-Black |
1.23 (1.18-1.27) |
<.001 |
|
Race-Hispanic |
0.68(0.64-0.72) |
<.001 |
|
Race-others |
0.67(0.60-0.76) |
<.001 |
|
HTN |
4.67(4.51-4.82) |
<.001 |
|
DM |
1.95(1.63-1.86) |
<.001 |
|
Sex (male vs female) |
3.62(3.36-3.90) |
<.001 |
|
LDL |
0.997(0.996-0.997) |
<.001 |
|
HDL |
0.981(0.980-0.983) |
<.001 |
|
SBP |
0.999(0.999-1.0) |
0.14 |
|
DBP |
0.991(0.989-992) |
<0.001 |
HTN: Hypertension, LDL: low- density lipoprotein, HDL: high density lipoprotein, DM: diabetes mellitus, SBP: systolic blood pressure, DBP, Diastolic Blood pressure, CI: Confidence Interval,


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 