Central Serous Chorioretinopathy and COVID-19; A Case Series
Ghina El Chakik MD, Majd Haddam MD*, Charbel Wahab MD, Jad Ayash MD, Fadi Maalouf MD
Department of Ophthalmology and Visual Sciences, University of Balamand, Beirut, Lebanon
*Corresponding author: Majd S. Haddam, Department of Ophthalmology and Visual Sciences, University of Balamand, Beirut, Lebanon
Received: 22 May 2022; Accepted: 30 May 2022; Published: 23 June 2022
Article Information
Citation: Ghina El Chakik, Majd Haddam, Charbel Wahab, Jad Ayash, Fadi Maalouf. Central Serous Chorioretinopathy and COVID-19; A Case Series. Journal of Ophthalmology and Research 5 (2022): 97-107.
DOI: 10.26502/fjor.2644-00240064
View / Download Pdf Share at FacebookAbstract
Since the emergence of Coronavirus disease 2019 (COVID-19) and its declaration as a global pandemic till now, scientists and physicians are still in the process of discovering the health implications caused by the infection. As for the ocular manifestations of COVID-19, the most common findings are ocular dryness, conjunctivitis, refractive error exacerbation and retinal vasculature occlusion. Central serous chorio-retinopathy (CSCR) is a type of retinopathy characterized by neuroepithelium detachment with the accumulation of serous sub-retinal fluid. Risk factors include exogenous corticosteroid usage, type A personality, hypertension and others. No correlation has been found between COVID-19 and central serous chorioretinopathy so far except for the previously known correlation where steroids, commonly used to treat COVID-19 patients, can trigger CSCR. The objective of our case series is to demonstrate cases of CSCR post COVID-19 infection and propose a possible mechanism that would define COVID-19 as a potential risk factor of CSCR due to the sympathetic system activation correlated with this infection.
Keywords
<p>Central serous chorioretinopathy, CSCR, COVID-19, Sympathetic System</p>
Article Details
1. Intruduction
With the first reported case of COVID-19 from China in 2019, it rapidly accelerated to become a global health threat. The virus is believed to be of zoonotic source and mainly spreads through direct contact or through respiratory droplets. The virus mainly targets the respiratory system with symptoms ranging from fever, cough, anosmia and myalgia to severe respiratory failure [1]. This virus was found to target different body systems, such as the kidneys, leading to proteinuria, hematuria, elevated BUN and creatinine, the gastrointestinal tract; leading to anorexia, nausea and diarrhea, the central and peripheral nervous system; causing headaches, dizziness, acute cerebrovascular diseases, and the skin; causing inflammatory lesions or maculopapular rash [2]. As for the ocular manifestations of COVID-19, the most common reported symptoms include dry eye, foreign body sensation, redness, tearing, itching, eye pain and discharge [3]. No correlation has yet been established between COVID-19 and central serous chorioretinopathy (CSCR) except for the correlation formerly discovered between steroids, which are usually used in moderate to severe COVID-19 infections, and CSCR [4].
Central serous chorioretinopathy is a condition in which fluid accumulates under the retina, causing a serous, fluid filled, detachment. CSCR is most common in male patients aged 20-55 years with type A personality. It has been considered as the fourth most common nonsurgical retinal disease after age-related macular degeneration, diabetic retinopathy, and branch retinal vein occlusion [5]. The typical symptoms of this condition include central scotoma, metamorphopsia, micropsia, dyschromatopsia, and reduced contrast sensitivity. Visual acuity at presentation can vary, with best corrected acuity ranging from 6/6 to 6/60 [6].
Corticosteroids use was found to bring about or worsen CSCR. An association was also made between type a personality and the development of this condition. Other risk factors included alcohol use, untreated hypertension and several medications [7]. In our case series, we will present four observations who developed CSCR post COVID-19 without any known risk factors to develop the fore mentioned retinopathy and propose that these findings could be secondary to a mechanism related to sympathetic system activation as a result of the infection.
2. Materials and Methods
Out of the multiple patients who presented to the ophthalmology department in Saint Georges Hospital University Medical center, Beirut-Lebanon with CSCR after acquiring COVID-19 infection, only four patients met the inclusion and exclusion criteria. Inclusion criteria include a documented positive polymerase chain reaction (PCR) for COVID-19, recovery from COVID-19, a clinical presentation suggestive of CSCR (blurry central vision, blind spots, distorted vision) and positive OCT findings confirming CSCR. Patients with known risk factors for CSCR such as type A personality, hypertension and exogenous steroids use were excluded. The JAS questionnaire was used to screen for type A personality (Figure 1). The ethics committee at the University of Balamand approved this study. Patients participating in this study signed an informed consent to publish this paper.
3. Case Presentations
The study includes 2 men and 2 women. Age at diagnosis ranges from 38 to 55.
3.1 Patient 1:
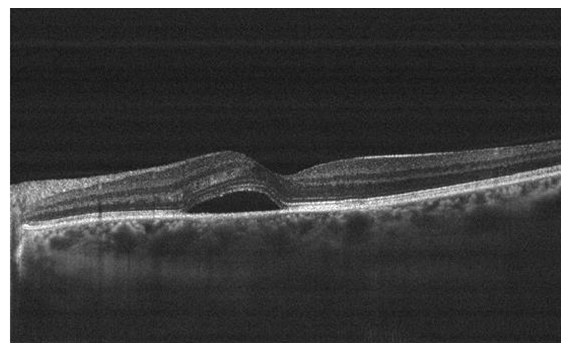
A 38-year-old male presented for sudden onset of blurry vision in the left eye (OS) of three days duration. The patient has no previous ocular history. Patient is only known to have gastroesophageal reflux disease (GERD) maintained on proton pump inhibitor. The patient tested COVID-19 positive 3 weeks prior to presentation, and reports uncomplicated course of illness and a smooth recovery. No headache, vision loss, diplopia, ocular pain, floaters, flashing lights or history of trauma were identified. Uncorrected visual acuity (UCVA) at presentation was 20/30 OS and 20/20 in the right eye OD, with best corrected visual acuity (BCVA) of 20/20 OS with a manifest refraction (MR) -0.50 +0.25x130. No pupillary abnormalities were noted with normal extraocular range of motion. Slit lamp exam (SLE) revealed a clear cornea, quite anterior chamber, no lens opacities, and normal intraocular pressure (IOP) (12 OD and 13 OS) measured using a Goldmann applanation tonometer. Dilated fundus exam revealed oval detachment of the retina at the macula with underlying sub-retinal fluid OS, along with several depigmented retinal pigment epithelium (RPE) foci within the detachment. Normal optic disc and vasculature were noted bilaterally. Optical coherence tomography (OCT) done OS confirmed the diagnosis of CSCR, showing neurosensory elevation of the retina at the macula with subretinal fluid (Figure 2). The patient was managed conservatively with observation. Upon follow up two months later, UCVA was 20/20 OS and a repeat OCT revealed resolution of the sub-retinal fluid.
3.2 Patient 2
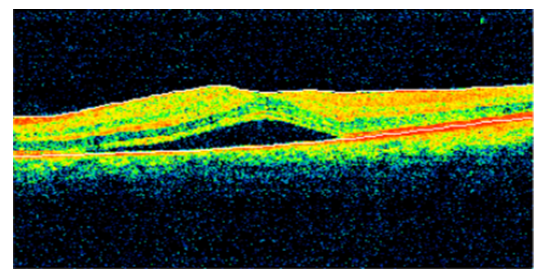
A 52-year-old female patient presented for one week history of decreased visual acuity OS. The patient has a medical history of type 2 diabetes mellitus (DM II) on oral hypoglycemic medications, last HbA1c 6.1%, dyslipidemia (DL) on statin therapy. The patient has no significant ophthalmological history. One month ago, the patient had a COVID-19 infection and suffered from fever, chills, diffuse myalgia. The patient was managed with symptomatic treatment and the infection resolved without any complications. Uncorrected visual acuity was 20/40 OS and 20/30 OD, BCVA was 20/25 OS using a +1.25 +0.50x150 lens (BCVA OS on the last visit 4 months ago was 20/20) and 20/30 OD. No pupillary abnormalities were noted with normal extraocular range of motion. Slit lamp exam (SLE) revealed a clear cornea, quite anterior chamber, no lens opacities OS with 1+ nuclear sclerotic cataract OD, and normal intraocular pressure (IOP) (11 OD and 12 OS) measured using a Goldmann applanation tonometer. Dilated fundus exam revealed blunt macula (OS) suggestive of macular edema, myopic fundus, no changes related to diabetic retinopathy and normal optic disc and vasculature OU. An OCT was done which revealed CSCR findings OS (Figure 3). The patient was managed conservatively without any intervention and was followed up 3 months later. Upon follow-up, her BCVA OS was 20/20 with a repeat OCT that revealed complete resolution of the previous CSCR findings OS.
3.3 Patient 3
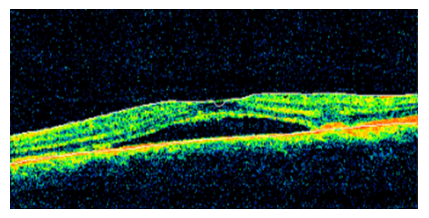
A 55 years old female patient presented to our clinic complaining of decreased visual acuity OS of 3 days duration. The patient is known to have type II diabetes mellitus with no previous ophthalmic history. The patient was diagnosed with COVID-19 two weeks prior to presentation where she developed cough, fever and myalgia. The patient was symptomatically managed with complete resolution of her infection without any complications. Her exam showed UCVA 20/50 OS and 20/20 OD. Her BCVA was 20/25 (OS) with a manifest refraction of -1.00 +0.25x115. No pupillary abnormalities were noted with normal extraocular range of motion. Slit lamp exam (SLE) revealed a clear cornea, quite anterior chamber, no lens opacities and normal intraocular pressure (IOP) (12 OD and 12 OS) measured using a Goldmann applanation tonometer. On dilated fundus exam, she was found to have a well delineated, shallow, serous macular detachment surrounded by a halo light reflex (OS) and normal vasculature and optic disc OU. OCT done showed sub-retinal fluid OS consistent with CSCR (Figure 4). The patient was managed without any medication or eye drops and was supposed to be followed up in three months, but she was lost to follow up.
3.4 Patient 4
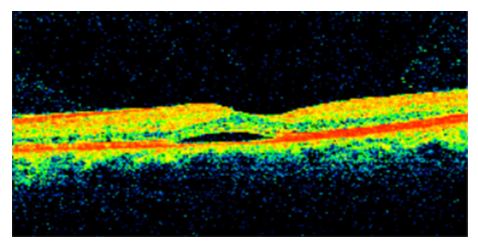
A 49 years old male patient presented to our clinic complaining of central blurry vision OS of two weeks duration. The patient has no prior medical or ophthalmic history. He was diagnosed with COVID-19 one month prior to presentation where his symptoms consisted of fever, cough, ageusia and anosmia. His infection resolved without the need for hospitalization and recovered completely after 10 days without any complications. His exam revealed UCVA 20/25 OD and 20/50 OS, with a BCVA 20/20 OD and 20/25 OS using a manifest refraction of -0.75 +0.50x60 and -1.00 +0.75x75 respectively. Amsler grid testing demonstrated metamorphopsia OS. No pupillary abnormalities were noted with normal extraocular range of motion. Slit lamp exam (SLE) revealed a clear cornea, quite anterior chamber, no lens opacities and normal intraocular pressure (IOP) (12 OD and 12 OS) measured using a Goldmann applanation tonometer. Dilated fundus examination showed blunting of the macula with elevation of the retina OS, normal vasculature and optic disk OU. OCT was done and demonstrated neurosensory detachment OS consistent with CSCR (Figure 5). The patient was disturbed by the decrease in the visual acuity and preferred to be managed by medication, therefore he was started on spironolactone 40 mg twice a day. The patient was supposed to be followed up within 10 days but he was lost to follow up.
4. Discussion
Since the emergence of COVID-19 and its declaration as a global pandemic till now, scientists and physicians are still in the process of discovering the health implications caused by the infection. Ophthalmic manifestations of COVID-19 have been observed and reported worldwide, which sheds lights on the importance of knowledge and insight in this field since it provides the tools that allow physicians to suspect, diagnose and treat such conditions adequately. Ocular manifestations of COVID-19 can be divided according to anatomic involvement. Findings involving the cornea and ocular surface such as follicular conjunctivitis, viral keratoconjunctivitis and episcleritis have been reported. Posterior segment manifestations can range from retinal vascular occlusions to vitritis and choroiditis. CSCR has only been described in the setting of exogenous steroid use, and no studies so far have proposed that COVID-19 infection can increase the risk of developing CSCR [32]. The objective of our case series is to present our observations of CSCR post COVID-19 infection and try to explain the relationship between the two events. It is important to understand the pathogenesis of CSCR as well as the risk factors predisposing to it in order to draw a proposed link between CSCR and COVID- 19 infection.
Several risk factors are directly linked to CSCR development, of which are the psychosomatic factors. The onset of CSCR has been linked with the incidence of psychosomatic stressors experienced by the patients with poor coping mechanisms, such as divorce, critical illness or bankruptcy [7, 8]. Type a personality is significantly linked with CSCR development [9] with a proposed mechanism related to the activation of the sympathetic system. Such individuals are found to have higher levels of circulating catecholamines (4 times) and corticosteroids (40 times) compared to individuals of type B personality [10]. Several studies have established this relationship, one of which utilizes the JAS questionnaire as a tool for assessing patients with type a behavior [11]. The JAS questionnaire demonstrated a good reliability in the prediction of type a behavior and thus can be considered a reliable tool in screening for this risk factor [11]. A high likelihood of having a type a personality can be deduced if the patient’s behavior agrees with the majority of the listed criteria. We used in our clinic the JAS questionnaire to screen our fore mentioned patients for type a personality, where none of our patients was identified as a type a personality.
Researches have also shown that all forms of exogenous corticosteroids, whether oral, topical, intravenous, intranasal, intraarticular or intravitreal, predispose to CSCR [12]. CSCR can occur in the time interval between one and several months after the administration of steroids, with no lower threshold related to the dose [13]. The pathogenesis of CSCR related to corticosteroid still remains ambiguous, with some studies reporting that corticosteroid could impact the choroidal circulation by increasing the vascular hyperpermeability or interfering with the action of cytokines responsible for autoregulation of blood vessels, the formation of Bruch membrane and the function of blood-retina barrier of retinal pigment epithelium [14]. Steroids are proposed to work synergistically with the sympathetic system and inhibit the parasympathetic system, which regulate the choroidal circulation directly [15, 16]. Through history taking, none of our patient was exposed to any form of steroids neither before nor during his/her COVID-19 infection, which eliminates this risk factor from consideration.
Other factors such as hypertension, H. pylori infection, sleeping disturbance, autoimmune disease, psychopharmacologic medication use, peptic ulcer, antihistamines usage, psychological stress, pregnancy, and alcohol use are risk factors relating to the occurrence of CSCR, with variable mechanisms of pathogenesis [6]. All of the patients involved have none of the risk factors mentioned so far based on medical history. As for the clinical course, CSCR can present in an acute or chronic form. Acute CSCR is the most common, with characteristic detachment of neurosensory retina and accumulation of serous fluid between RPE and photoreceptor outer segments.10 Fundus examination is remarkable for an oval elevation in macular area which corresponds to the underlying neurosensory detachment [10]. The foveal reflex is diminished or absent, and a central yellow spot is present due to increased visibility of xanthophyll [10]. These findings were observed in our patients, as all of them presented acutely, and are further described in the above sections. Other associated findings are pigment epithelial detachments (PEDs), evident in 5% to 63% of cases, which can be accompanied with overlying fine mottling and pigment clumping [17]. Whereas chronic CSCR represents approximately 5% of CSCR cases and is characterized by areas of widespread diffuse RPE pigmentary abnormalities, including RPE atrophy pigment clumping in the posterior pole and chronic shallow serous retinal detachment [7]. At presentation, patients are generally older and have more severely reduced presenting VA, and visual loss is often permanent [18].
Imaging modalities have provided insight on the pathophysiology behind CSCR, although complete understanding remains unclear. It is believed that the primary pathology originates in the RPE or choriocapillaries. Leaks in RPE have been detected by FFA, occurring often at extra-foveal sites; however, due to low thickness of the retina and the lower suction effect of RPE at the fovea, the serous neurosensory detachment occurs in the sub-foveal region [7]. This can be further explained by the damage to RPE cells causing a disruption of ion transfer, thus leading to a reversal in fluid movement into, rather from, the sub-retinal space [19].
Indocyanine green angiography (ICGA) and optical coherence tomography (OCT) provides further data that aid in the explanation of the pathophysiology of CSCR. ICGA and OCT shows active choroidal leakage from sites not seen with FFA indicating that the primary pathology is at the level of the choroid rather than RPE, along with an increase in the sub-foveal choroid thickness 80% greater than normal aged match controls [20, 23, 24]. This choroidal hyperpermeability will cause serous detachment of RPE which will decompensate allowing the diffusion of the serous fluid causing neurosensory detachment [6]. It has been hypothesized that the impaired autoregulation of choroidal circulation is the cause of choroidal hyperpermeability [21]. This can be supported by the findings of increased circulating catecholamines as well as choroidal ischemia in areas of ICGA leakage [22].
Further findings obtained with optical coherence tomography support the role of choroidal hyperpermeability by increased hydrostatic pressure in the pathogenesis of CSCR, in which the thickness of the subfoveal choroid was found to be up to 80% greater than normal aged match controls [23, 24]. It was also noticed that in individual eyes affected by clinical CSCR, mean choroidal thickness was greater in areas corresponding to leakage on FFA, and in areas corresponding to choroidal hyperpermeability and punctuate hyperfluorescent lesions on ICGA [25, 26], which further reinforces the link between choroidal pathology and serous retinal detachment [6].
In our case series, we propose that COVID-19 infection can be a risk factor for the development of acute CSCR through a mechanism related to sympathetic system activation. A large amount of studies has established a relationship between COVID-19 infection and sympathetic nervous system over-activation, particularly in explaining increased morbidity and mortality. It has been suggested that chronic intermittent hypoxia which characterizes respiratory dysfunction induced by COVID-19 increases sympathetic output through increased carotid body sensitivity [26]. Another proposed mechanism by which the sympathetic system may be activated is via increased production and release of angiotensin II (AngII), which increases sympathetic activity both centrally and peripherally [27, 28].
In a study conducted in Germany, SARS-CoV-2 RNA has been detected in autopsied retina of patients with COVID-19, and angiotensin converting enzyme 2 (ACE-2) have been also detected in the retina [29].
In a case control study from Spain, COVID-19 patients with moderate to severe disease were found to have decreased central vascular density on OCT-A compared to patients with mild disease or controls without viral infection [30]. It has been suggested that immune cells recruited by the virus in the vessel walls produce endothelial cellular edema leading to endothelial dysfunction and thus a decrease in central vascular density, which is considered a biomarker for several diseases like diabetes and may become a potential biomarker for micro vascular damage in COVID-19 patients [31, 32].
In our case series, CSCR was suspected by fundus exam findings and confirmed with OCT. Risk factors including exogenous steroid use, type a personality behavior, hypertension, H. Pylori infection and pregnancy, were identified by history taking. None of our patients was found to have any of the major confounding risk factors for the development of CSCR. The only common finding is that all of them had a documented history of COVID-19 infection with variable time interval between the date of positive PCR and the date of presentation to clinic. After a thorough literature review, no direct association has been established between COVID-19 infection and the development of CSCR, except in the setting of exogenous steroid use. From our observations, COVID-19 infection could be established as a risk factor for development of CSCR, although the relationship remains ambiguous. A proposed mechanism can be mediated by the proven link between COVID-19 and activation of sympathetic system leading to choroidal leak and predisposing to CSCR. However, sympathetic system over-action has been described and established in critically ill patients as it contributes to increased morbidity and mortality related to multi system failure. The action of the sympathetic system in clinically stable patient has not been well established, thus a direct link cannot be clearly drawn.
5. Conclusion
Further observations of CSCR patients with history of COVID-19 infection are needed, with more detailed comparative studies to draw definite intensive conclusions. Ophthalmologists should be aware of possible development of CSCR post COVID-19 infections.
References
- Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado LF, Dahal S, Kumar H, Kv D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J 96 (2020):753-758.
- Prasad A & Prasad M. Single Virus Targeting Multiple Organs: What We Know and Where We Are Heading? Frontiers in Medicine7.
- Nasiri N, Sharifi H, Bazrafshan A, Noori A, Karamouzian M, & Sharifi A. Ocular Manifestations of COVID-19: A Systematic Review and Meta-analysis. Journal of Ophthalmic and Vision Research (2021).
- Douglas K, Douglas V & Moschos M. Ocular Manifestations of COVID-19 (SARS-CoV-2): A Critical Review of Current Literature in Vivo34 ((2020): 1619-1628.
- Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol 86 (2008): 126-145.
- Central Serous Chorioretinopathy Treatment & Management: Medical Care, Surgical Care, Activity (2021).
- Spahn C, Wiek J, Burger T. Operationalized psychodynamic diagnostics (OPD) in patients with central serous chorioretinopathy. Psychother Psychosom Med Psychol 54 (2004): 52-7.
- Conrad R, Bodeewes I, Schilling G, Geiser F, Imbierowicz K, Liedtke R. Central serous chorioretinopathy and psychological stress. Ophthalmologe 97 (2000): 527-31.
- Liew G, Quin G, Gillies M, & Fraser-Bell S. (2013). Central serous chorioretinopathy: a review of epidemiology and pathophysiology Clinical & experimental ophthalmology 41 (2013): 201-214.
- Yannuzzi LA. Type a behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc 84 (1986): 799-845.
- Jenkins CD, Rosenman RH, Friedman M: Development of an objective psychological test for the determination of the coronary-prone behavior pattern in employed men. J Chronic Dis 20 (1967): 371-379.
- Carvalho-Recchia CA, Yannuzzi LA, Negrao S et al. Corticosteroids and central serous chorioretinopathy Ophthalmology 109 (2002): 1834-7.
- Song E, Wakakura M, Ishikawa S. Central serous chorioretinopathy induced by corticosteroids Nihon Ganka Gakkai Zasshi 101 (1997): 257-64.
- Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol 47 (2002): 431-448.
- Carvalho-Recchia CA, Yannuzzi LA, Negra S, et al. Corticosteroids and central serous chorioretinopathy Ophthalmology 109 (2002): 1834-1837.
- Mann RM, Riva CE, Stone RA, et al. Nitric oxide and choroidal blood flow regulation. Invest Ophthalmol Vis Sci 36 (1995): 925-930.
- Laatikainen L, Hoffren M. Long-term follow-up study of nonsenile detachment of the retinal pigment epithelium. Eur J Ophthalmol 1 (1991): 79-84.
- Spaide RF, Campeas L, Haas A etal. Central serous chorioretinopathy in younger and older adults. Ophthalmology 103 (1996): 2070-9.
- Negi A, Marmor MF. The resorption of subretinal fluid after diffuse damage to the retinal pigment epithelium. Invest Ophthalmol Vis Sci 24 (1983): 1475-9.
- Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy Arch Ophthalmol 112 (1994): 1057-62.
- Tittl M, Maar N, Polska E, Weigert G, Stur M, Schmetterer L. Choroidal hemodynamic changes during isometric exercise in patients with inactive central serous chorioretinopathy. Invest Ophthalmol Vis Sci 46 (2005): 4717-21.
- Sun J, Tan J, Wang Z, Yang H, Zhu X, Li L. Effect of catecholamine on central serous chorioretinopathy. J Huazhong Univ Sci Technolog Med Sci 23 (2003): 313-6.
- Regatieri CV, Branchini L, Fujimoto JG, Duker JS. Choroidal imaging using spectral-domain optical coherence tomography. Retina 32 (2012): 865-76.
- Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 29 (2009): 1469-73.
- Jirarattanasopa P, Ooto S, Tsujikawa a et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology (2012).
- Porzionato A, Emmi A, Barbon S, Boscolo-Berto R, Stecco C, Stocco E, Macchi V & De Caro R. Sympathetic activation: a potential link between comorbidities and COVID-19. The FEBS journal 287 (2020): 3681-3688.
- Iturriaga R & Castillo-Galan S. Potential contribution of carotid body-induced sympathetic and renin-angiotensin system overflow to pulmonary hypertension in intermittent Curr Hypertens Rep 21(2019): 89.
- Fung ML. The role of local renin-angiotensin system in arterial chemoreceptors in sleep-breathing disorders. Front Physiol 5 (2014):
- Casagrande M, Fitzek A, Püschel K, Aleshcheva G, Schultheiss HP, Berneking L, et Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm 28 (2020): 721-5.
- Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P,et SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med 173 (2020): 242-3.
- Zapata MÁ, García SB, Sánchez A, Falcó A, Otero-Romero S, Arcos G,et Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br J Ophthalmol (2020): 317953.
- Honavar SG, Sen M, Sharma N & Sachdev MS. Covid-19 and eye: A review of ophthalmic manifestations of covid-19.Indian Journal of Ophthalmology 69 (2021): 488.



 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
