Clinical Aspects of Patients with Dry Eye Disease according to Helicobacter Pylori Infection
Seunghyun Lee1, Sangmin Nam1, Helen Lew1*
*Corresponding Author:Helen Lew, Department of Ophthalmology, CHA Bundang Medical Center, CHA University, 59Yatap-ro, Bundang-gu, Seongnam 13496, Republic of Korea
Received: 20 March 2021; Accepted: 29 March 2021; Published: 09 April 2021
Article Information
Citation: Seunghyun Lee, Sangmin Nam, Helen Lew, Clinical Aspects of Patients with Dry Eye Disease according to Helicobacter Pylori Infection. Journal of Ophthalmology and Research 4 (2021): 104-113.
DOI: 10.26502/fjor.2644-00240030
View / Download Pdf Share at FacebookAbstract
Purpose: To compare the clinical aspects of patients with dry eye disease according to infection of Helicobacter pylori (H.pylori) using urea breath test which is non-invasive and find out the correlation of dry eye and H.pylori infection.
Methods: We detected the infection of H.pylori using urea breath test (BreathTek®, Otsuka America Pharmaceutical, Inc., USA) in 52 patients with dry eye disease from August 2018 to December 2019 and analyzed their medical records. Slit lamp examination, Ocular Surface Diseast Index (OSDI) questionnaire were performed. Tear meniscus height was measured by optical coherence tomography (Spectralis, Heidelberg Engineering, Heidelberg, Germany). Lipid layer thickness, total blinking rate, partial blinking rate and meiboscore were measured by ocular surface interferometer (Tear Science, Morrisville, NC, USA).
Results: The number of people who were positive for urea breath test was 17 (32.7%) and negative for test was 35 (67.3%). There were no differences in age, sex, tear meniscus height, lipid layer thickness, total blinking rate for one minute and partial blinking rate according to H.pylori infection. The OSDI scores (39.7, 28.5) and rate of severe loss of meibomian glands (23.5%, 4.3%) were significantly higher in the H.pylori positive group (p<0.05). The rate of patients diagnosed with Sjogren's syndrome (35.3%, 17.1%) was also significantly higher in the H.pylori positive group(p<0.05).
Conclusion: When urea breath test for H.pylori infection was positive in the dry eye patients, they presented mixed type of aqueous tear-deficient and evaporative type. Through detailed history taking for patients with dry eye, we could more actively suspect Sjogren's syndrome and conduct additional tests.
Keywords
<p>Dry eye disease; Helicobacter pylori; Sjogren's syndrome; Urea breath test</p>
Article Details
1. Introduction
Dry eye disease is a common condition that occurs when tears aren't able to provide adequate lubrication for eyes [1, 2]. Tears can be inadequate for many reasons such as decreased tear production, increased tear evaporation and an imbalance in tear composition. The tear film has three basic layers: oil, water and mucin. If a problem occurs even on one of layers, it can lead to dry eye disease. According to data from the Health Insurance Review and Assessment Service, the number of patients with dry eyes in Korea reached 2.33 million in 2017.The Definition and Classification Subcommittee of dry eye disease classified dry eye as aqueous tear-deficient dry eye (ADDE) and evaporative dry eye (EDE) [3]. Aqueous tear-deficient dry eye implies that dry eye is due to a failure of lacrimal tear secretion. It has two major groupings, Sjogren’s syndrome dry eye (SSDE) and non-Sjogren’s syndrome dry eye (NSSDE). Non-Sjogren’s syndrome dry eye is a form of ADDE due to lacrimal dysfunction, where the systemic autoimmune features characteristic of SSDE have been excluded. Sjogren’s syndrome is an exocrinopathy in which the lacrimal and salivary glands are targeted by an autoimmune process.The lacrimal and salivary glands are infiltrated by activated T-cells, which cause acinar and ductular cell death and hyposecretion of the tears or saliva [4]. Helicobacter pylori (H.pylori) is known to be potential trigger for many autoimmune diseases in addition to gastrointestinal diseases such as gastritis, gastric ulcers and stomach cancer. The increase in serum CRP (C-reactive protein) was reported in chronic H.pylori infections and it meant that the patient was in a persistent inflammatory state. There is a hypothesis that such chronic inflammation may continue to stimulate antigens and cause systemic inflammatory reactions, leading to autoimmune diseases other than gastrointestinal tract. Sjogren's syndrome, rheumatoid arthritis, lupus, vasculitis, idiopathic thrombocytopenia, autoimmune skin diseases are the examples [5-8]. The urea breath test is a rapid diagnostic procedure used to identify infections by H.pylori [9]. It is based upon the ability of H.pylori to convert urea to ammonia and carbon dioxide. Urea breath tests are recommended in leading society guidelines as a preferred non-invasive choice but highly accurate for detecting H.pylori before and after treatment. Studies of the relationship between H.pylori and Sjogren's syndrome have been steadily conducted overseas, but most of them were by serum test and no studies have been done by urea breath test which is non-invasive. To date, no studies have focused on the relationship between H.pylori infection and dry eye disease. Thus, in our study, a thorough assessment of differences in clinical aspects of patients with dry eye disease according to infection of H.pylori was performed, and the correlation of dry eye and H.pylori infection was found out.
2. Materials and Methods
The patients diagnosed as dry eye disease were recruited from the Department of Ophthalmology of Bundang CHA Hospital between August 2018 and December 2019. The urea breath test (BreathTek®, Otsuka America Pharmaceutical, Inc., USA) was performed to evaluate infection of H.pylori for fifty-two patients with dry eye disease. Subjects were excluded if they had a recent ocular infection, a history of ocular surgery, allergic disease or other diseases that may induce secondary dry eye, such as Steven–Johnson syndrome, vitamin A deficiency, Wegener’s granulomatosis, or any type of cancer. When patients suspected Sjogren's syndrome were referred to department of Rheumatology, the diagnosis of Sjogren's syndrome was made by 2016 ACR–EULAR Classification Criteria for Primary Sjogren’s Syndrome [4] (Table 1).
2.1 Questionnaire for ocular symptoms
The Ocular Surface Disease Index (OSDI) was used to identify eye discomfort in the patients. The OSDI questionnaire provides a method to assess dry eye symptoms, vision-related functions, and environmental triggers during the past week. The OSDI is assessed on a scale of 0 to 100, with higher scores representing greater disability [10, 11].
OSDI score = (sum of scores) x 25 / number of questions answered.
2.2 Measurement of tear meniscus height
Tear meniscus height (TMH) was assessed by one optometrist using spectral-domain optical coherence tomography (Spectralis; Heidelberg Engineering, Heidelberg, Germany). TMH was measured from the cornea-meniscus junction to the lower lid-meniscus junction with a scan line perpendicular to the mucocutaneous junction. The examination was performed three times for each patient [12].
2.3 Measurement of lipid layer thickness, blinking pattern, and meiboscores
The lipid layer thickness and blinking pattern were assessed using the LipiView II (TearScience, Morrisville, NC, USA) ocular surface interferometer by one optometrist. The lipid layer thickness information included the minimum, maximum, and average for 20 seconds. The blinking pattern was obtained by determining the total blinking and partial blinking rates in 20 seconds and then calculating the partial blinking/total blinking ratio. The meiboscores for lower eyelids were obtained by Lipiview II meibography. The meibographs were inspected for the presence of partial or absent meibomian glands and assigned a numerical score proportional to the area of involved eyelid [13]. Evaluation: (0): lid has no partial or missing glands; (1): involved lid area is <33%; (2): involved lid area is 33–66%; (3): involved lid area is >66%.
2.4 The urea breath test
The urea breath test was performed using BreathTek® (Otsuka America Pharmaceutical, Inc., USA). In fasting for more than four hours, patients breathe into a small collection bag to capture a baseline sample of breath. Then, they drink the Pranactin®-Citric drug solution (urea labelled with a non-radioactive carbon-13). In the subsequent 10 minutes, patients breathe into another collection bag. Then samples are sent to a reference laboratory for analysis in 24 hours. The detection of isotope-labelled carbon dioxide in exhaled breath indicates that the urea was split; this indicates that urease (the enzyme that H.pylori uses to metabolize urea) is present in the stomach, and hence that H.pylori bacteria are present.
Statistical analyses were performed using IBM SPSS Statistics ver. 20.0(IBM Corp., Armonk, NY, USA). Shapiro-Wilk test, Mann-Whitney U test, Chi-square test and fisher’s exact test were used. A p-value <0.05 was considered to indicate significance. This study was approved by the Research Ethics Committee of Bundang CHA Hospital, and informed consent was obtained from each subject according to the tenets of the Declaration of Helsinki. All assessments were performed by the same ophthalmologist.
Table 1: 2016 ACR (American College of Rheumatology)–EULAR (European League against Rheumatism) Classification Criteria for Primary Sjogren’s Syndrome.
|
Item |
Description |
Score |
|
Focus score of ≥1 |
A score determined by the number of mononuclear cell infiltrates containing≥50 inflammatory cells per 4mm² of minor labial salivary gland obtained on biopsy. |
3 |
|
Presence of anti-SSA antibodies |
Measured in serum; only anti-Ro 60 antibodies have to be considered; isolated anti-Ro 52 antibodies are not specific for Sjogren’s syndrome. |
3 |
|
SICCA ocular staining score of ≥5 |
A score determined by an ophthalmologist on the basis of examination with fluorescein and lissamine green staining; scores range from 0 to 12, with higher scores indicating greater severity. |
1 |
|
Schirmer test of ≤5mm per 5min. |
An assay for measuring tear production by inserting filter paper on conjunctiva in the lower eyelid and assessing the amount of moisture on the paper. |
1 |
|
Unstimulated whole salivary flow of ≤0.1ml per min. |
An assay for measuring the rate of salivary flow by collecting saliva in a tube for at least 5min after the patient has swallowed. |
1 |
|
Total score |
9 |
A score≥4 classifies a patient who meets the inclusion criteria and does not have any of the exclusion criteria: history of head and neck radiation, active HCV infection, AIDS, sarcoidosis, amyloidosis, graft-versus-host disease, IgG4-related disease.
3. Results
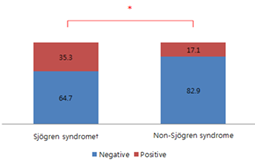
This study included 52 dry eye patients (104 eyes), including 35 male and 17 female patients. The average age was 60.2 ± 10.0 years. The patients who were positive for urea breath test were 17 dry eye patients (32.7%), including 4 males and 13 females and patients who are negative for the test were 35 dry eye patients (67.3%), including 8 males and 27 females. The average ages were 59.7 ± 10.0 years and 60.5 ± 10.1 years, respectively. For the sex and age, there were no significant differences. The average of OSDI score was significantly increased in infection group as 39.7 ± 21.1 and 28.5 ± 19.8 (p<0.05, Table 2). Tear meniscus height was 202.2 ± 109.3μm and 217.1 ± 131.4μm, resp-ectively. There was no significant difference. The lipid layer thickness assessed using the LipiView II were 77.8 ± 24.4nm, 77.3 ± 22.7nm. The numbers of total blinking events for one minute were 27.1 ± 4.0, 26.9 ± 4.9 respectively, and partial blinking rates in one minute were 46.5 ± 35.9%, 54.8 ± 34.6%. There were no significant differences. Meiboscores 0,1,2 were class-ified as mild to moderate loss of meibomian glands and meiboscore 3 was classified as severe loss. For the group who are positive urea breath test, the rate of severe type was significantly higher (p<0.05, Table 3). There was no significant difference in the lipid layer thickness, total blinking rate and partial blinking/total blinking ratio between the severity of loss of meibomian glands (Table 4). There were seventeen patients diagnosed with Sjogren's syndrome (32.7%) from total patients. Six of whom were positive (35.3%) for urea breath test and eleven were negative (64.7%). Six of the thirty-five patients with non-Sjogren's dry eye (17.1%) were positive and twenty-nine (82.9%) were negative. Thus, 35.3% of patients with Sjogren's syndrome and 17.1% of patients with non-Sjogren's dry eye showed positive in urea breath test, which showed statistically significant differences (p<0.05, figure 2). The odd ratio was 2.636 for Sjogren's syndrome if urea breath test was positive.
Table 2:Demographics and clinical characteristics of the patients with dry eye disease who tested positive versus negative for urea breath test.
|
Characteristics |
DED†: UBT (+) |
DED: UBT (-) |
Total |
p value |
|
Number of patients |
17 |
35 |
52 |
|
|
Sex (M: F) (%) |
4:13 (23.5:76.5) |
8:27 (22.9:77.1) |
12:40 (23.1:76.9) |
0.939 |
|
Age(years) |
59.7 ± 10.0 |
60.5 ± 10.1 |
60.2 ± 10.0 |
0.901 |
|
OSDI score |
39.7 ± 21.1 |
28.5 ± 19.8 |
31.9 ± 20.7 |
0.018* |
|
TMH (μm) |
202.2 ± 109.3 |
217.1 ± 131.4 |
212.2 ± 124.3 |
0.650 |
P-values are obtained by Mann-Whitney U test
* P<0.05
†Dry eye disease
Table 3:Clinical characteristics measured by LipiView® II in the patients with dry eye disease according to the result of urea breath test.
|
Characteristics |
DED†: UBT (+) |
DED: UBT (-) |
Total |
p value |
|
|
Lipid layer thickness (nm) |
77.8 ± 24.4 |
77.3 ± 22.7 |
77.5 ± 23.1 |
0.924 |
|
|
Total blinking rate(/1min) |
27.1 ± 4.0 |
26.9 ± 4.9 |
27.0 ± 4.6 |
0.94 |
|
|
Partial blinking ratio† (%) |
46.5 ± 35.9 |
54.8 ± 34.6 |
52.1 ± 35.1 |
0.271 |
|
|
Loss of meibomian glands (%) |
Mild to moderate |
76.5 |
95.7 |
0.003* |
|
|
Severe |
23.5 |
4.3 |
|||
P-values are obtained by Mann-Whitney U test (Lipid layer thickness, total blinking rate, partial blinking ratio) and by Chi-square test (Loss of meibomian glands)
† Partial blinking ratio= Partial blinking rate/total blinking rate
* P<0.05
Table 4: Association between loss of meibomian glands and lipid layer thickness.
|
Characteristics |
Loss of meibomian glands |
||
|
Mild to moderate |
Severe |
p value |
|
|
Lipid layer thickness (nm) |
77.0 ± 22.4 |
81.6 ± 29.2 |
0.27 |
|
Total blink rate (/1min) |
26.4 ± 4.5 |
33.0 ± 5.9 |
0.218 |
|
Partial blinking ratio (%) |
52.2 ± 34.6 |
58.6 ± 32.5 |
0.607 |
P-values are obtained by Mann-Whitney U test.
Values are presented as percentage (%).
†: Diagnosis meets the 2017 ACR–EULAR Classification Criteria for Primary Sjogren’s syndrome.
* P<0.05, odd ratio=2.636 by Chi-square test
4. Discussion
Recently researchers started to investigate the alterations in diversity of the oral, ocular, or intestinal microbiota in Sjogren's syndrome [14, 15]. The studies indicate that dysbiosis may play a significant role in pathogenesis of Sjogren's syndrome [16]. In some studies of non-obese diabetic (NOD) mice, they manifested not only type I diabetes (T1D) but Sjogren's syndrome-like autoimmune endocrinopathy as well and also presented a few hallmark autoantibodies like ANA, anti-SSA/Ro, anti-SSB/La, anti-a-fodrin, etc [17-19]. If human microbiome proves to be an important key to the pathogenesis and expression of Sjogren's syndrome, the next step could be new and promising therapeutic approaches such as probiotics or prebiotics.
P Aragona et al found that the prevalence of antibodies against H.pylori and HSP60 (heat-shock protein 60) was significantly higher in patients with primary Sjogren's syndrome (79.4%, 88.2%) than with various autoimmune diseases(18.2%, 27.3%) and healthy controls(48.8%, 37.2%) [20]. Yasser M.El Miedany et al studied four groups of patients with primary Sjogren's syndrome, secondary Sjogren's syndrome, various connective tissue diseases and healthy controls [21]. The prevalence and mean titer of H.pylori infection in patients with primary and secondary Sjogren's syndrome (80.6%, 71.0%) were significantly higher than in patients with connective tissue diseases (60.9%) and healthy controls (56.3%). Y Showji et al studied to assess the possibility that H.pylori might be an etiologic agent [22]. The titers of anti-H.pylori IgG in serum of patients with various connective tissue diseases including Sjogren's syndrome were compared with those of healthy volunteers by ELISA. The average titers in serum from patients with Sjogren's syndrome were much higher than the average of all serum sample, the age-matched controls. In our study, the odd ratio was 2.636 for Sjogren's syndrome if urea breath test was positive. This suggested a link between H.pylori and Sjogren's syndrome.
The OSDI was created in order to quickly assess the symptoms of ocular irritation in dry eye disease and how they affect functioning related to vision [10]. This 12-item questionnaire assesses dry eye symptoms and the effects it has on vision-related function in the past week of the patient’s life [11]. In our study, the average of OSDI score was significantly higher in positive group than negative group for urea breath test as 39.7 ± 21.1 and 28.5 ± 19.8. In other words, for dry eyes, patients with H.pylori infection tend to have more severe symptom than non-infectious patients.
Kim et al and Miz.oguchi S et al reported that inflammation on the ocular surface and inflammatory cytokines may affect the structure and function of meibomian gland [23, 24]. So, in dry eye patients with a systemic inflammatory status due to H.pylori infection, the degree of inflammation on the ocular surface is severe and this may lead to the loss of the meibomian glands. Sjogren's syndrome, a representative autoimmune disease in which H.pylori infection is being discussed as an etiology, is characterized by lymphocytic infiltration of exocrine glands and other organs and it result in gradual and progressive damage and dysfunction of organs like this study. Many studies have reported loss of meibomian glands in patients with Sjogren's syndrome, too [25]. But, there was no significant difference in the lipid layer thickness between the severity of loss of meibomian glands in our study.
The average lipid layer thickness of the subjects in this study is 72nm and 74nm, respectively, which is within the normal range. There are studies that there is no relationship between tear breakup time (TBUT) which reflect stability of tear film and lipid layer thickness and some studies have challenged the importance of lipid layer thickness [26]. King-Smith PE et al reported that the lipid would be a poor barrier to evaporation, perhaps because of deficiency in composition and/or structure [27]. For example, bacterial lipases may have broken down esters into component acids and alcohols, causing a defective tear film lipid layer structure with increased evaporation. Steven J Dell et al reported that in subjects with moderate to severe meibomian gland dysfunction, IPL (intense pulsed light) combined with meibomian gland expression reduced the number and severity of symptoms and signs of dry eye disease [28]. And all examined outcome measures such as tear breakup time, meibomian gland score significantly improved after treatment except for lipid layer thickness. Erich Knop et al reported that the change in the composition of the lipid layer weakens the adhesion between the lipid layer and the aqueous layer, causing instability of the entire tear film and make it vulnerable to evaporate [29]. That is, it can be considered that the quality, composition, or structure of the lipid layer is more closely related to the stabilization of the ocular surface in dry eye, rather than the thickness of the lipid layer. Therefore, even when the thickness of the lipid layer is sufficient, the lipid layer cannot serve as a stable protective film for the aqueous layer causing evaporation of tears as long as lipids and glycoproteins that are not qualitatively suitable are secreted [30].
In the TFOS DEWS (Tear film and Ocular Surface Society, Dry Eye Workshop, 2007), dry eye disease was categorized as aqueous tear-deficient type and evaporative type [3]. They proposed an algorithm that looked for Sjogren's syndrome as the first cause of the aqueous tear-deficient type and found other secondary factors if it is not Sjogren's syndrome. But a lot of studies revealed that both lack of lacrimal secretion and hyper evaporation due to dysfunction of the meibomian glands occurred in dry eye with Sjogren's syndrome. Regarding Sjogren's dry eye patients with H.pylori infection, it may be appropriate to classify as a mixed type because dry eye may occur due to a systemic inflammatory status caused by H.pylori infection. If ESR, CRP, MMP-9 (metalloproteinase 9) in tear and various cytokines were compared, it can strongly support this hypothesis. It may be considered in future research. In this study, the shirmer test had to be compared to measure lacrimal secretion, it was not done in all subjects. Through future prospective studies, we can find out that if clinical progress of Sjogren's dry eye patients with H.pylori infection is improved when H.pylori is treated .
Conclusion
Dry eye disease patients with Sjogren's syndrome presented higher incidence of H.pylori infection using urea breath test. In our study, the odd ratio was 2.636 for Sjogren's syndrome if urea breath test was positive. When urea breath test was positive, the dry eye patients presented mixed type of aqueous tear-deficient and evaporative type. Through detailed history taking for patients with dry eye, we can more actively suspect Sjogren's syndrome and conduct additional tests.
Conflicts of interest
There are no conflicts of interest.
References
- The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the International Dry Eye Workshop. The Ocular Surface 5 (2007): 75-92.
- Walt JG, Rowe MM, Stern KL. Evaluating the functional Impact of Dry Eye: The Ocular Surface Disease Index. Drug Information Journal 31 (1997): 1436.
- Craig JP, Nelson DJ, Azar DT, et al. TFOS DEWS II Report Executive Summary. The Ocular Surface Oct 15 (2017): 802-812.
- Franco Franceschini, Ilaria Cavazzana, Laura Andreoli, et al. The 2016 classification criteria for primary Sjogren’s syndrome: what’s new? BMC Medicine 15 (2017): 69.
- Daniel S Smyk, Andreas L Koutsoumpas, Maria G Mytilinaiou, et al. H.pylori and Autoimmune Disease: Cause or Bystander. World Journal of Gastroenterology 20 (2014): 613-629.
- Jackson, L., Britton, J., Lewis, S.A. A population-based epidemiologic study of H.pylori infection and its association with systemic inflammation. Helicobacter 14 (2009): 108-113.
- Eli Magen, Jorge-Shmuel Delgado., H.pylori and Skin Autoimmune Diseases. World Journal of Gastroenterology 20 (2014): 1510-1516.
- Jun-Ichi Kira, Noriko Isobe. H.pylori infection and Demyelinating Disease of the Central Nervous System. Journal of Neuroimmunology 329 (2019): 14-19.
- Cheal Wung Huh, Byung-Wook Kim. Diagnosis of H.pylori infection. The Korean Journal of Gastroenterology Vol. 72 No. 5, 229-236.
- Schiffman RM. Reliability and Validity of the Ocular Surface Disease Index. Archives of Ophthalmology 118 (2000): 615-621.
- Bottomley A, Jones D, Claassens L. Patient-reported outcomes: Assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency. European Journal of Cancer 45 (2009): 347-353.
- Jin Ha Kim, Kyu Ryong Choi, Roo Min Jun. Repeatability and Reproducibility of Tear Meniscus Evaluations Using Two Different Spectral Domain-optical Coherence Tomography. Journal of the Korean Ophthalmological Society 60 (2019): 929-934.
- Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 115 (2008): 911-915
- Jason Lloyd-Price, Galeb Abu-Ali, Curtis Huttenhower. The Healthy Human Microbiome. Genome Medicine 8 (2016): 51.
- Jack A Gilbert, Martin J Blaser, J Gregory Caporaso, et al. Current Understanding of the Human Microbiome. Nature Medicine 24 (2018): 392-400.
- Christina Tsigalou, Elisavet Stavropoulou, Eugenia Bezirtzoglou. Current insights in Microbiome Shifts in Sjogren’s Syndrome and Possible Therapeutic interventions. Frontiers in Immunology 9 (2018): 1106.
- Humphreys-Beher MG, Brinkley L, Purushotham KR, et al. Characterization of antinuclear autoantibodies present in the serum from non-obese diabetic (NOD) mice. Clinical Immunology and Immunopathology 68 (1993): 350–356.
- Humphreys-Beher MG, HuY, Nakagawa Y, et al. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjogren’s syndrome. Advances in Experimental Medicine and Biology 350 (1994): 631–636.
- Robinson CP, Yamamoto H, Peck AB, et al. Genetically programmed development of salivary gland abnormalities in the NOD (non-obese diabetic)-scid mouse in the absence of detectable lymphocytic infiltration: a potential trigger for sialoadenitis of NOD mice. Clinical Immunology and Immunopathology 79 (1996): 50–59.
- Aragona P, Magazzù G, Macchia G. Presence of antibodies against H.pylori and its heat-shock protein 60 in the serum of patients with Sjogren’s syndrome. The Journal of Rheumatology 26 (1999): 1306-1311.
- El Miedany YM, Baddour M, Ahmed I. Sjogren’s syndrome: concomitant H.pylori infection and possible correlation with clinical parameters. Joint Bone Spine 72 (2005): 135-141.
- Showji Y, Nozawa R, Sato K. Seroprevalence of H.pylori infection in patients with connective tissue diseases. Microbiology and Immunology 40 (1996): 499-503.
- Minjae Kim, Sungwon Yang, Jinhwan Park, et al. Risk Factors for Structural Changes in Meibomian Gland in Thyroid Eye Disease. Journal of the Korean Ophthalmological Society 59 (2018): 599-605.
- oguchi S, Iwanishi H, Arita R, et al. Ocular surface inflammation impairs structure and function of meibomian gland. Experimental Eye Research 163 (2017): 78-84.
- Chen X, UtheimØA, Xiao J, et al. Meibomian gland features in a Norwegian cohort of patients with primary Sjogren´s syndrome. PLoS One 12 (2017): e0184284.
- Fenner BJ, Tong L. More to stable tears than thickness of the tear film lipid layer. Investigative Ophthalmology & Visual Science 56(2015): 1601.
- King-Smith PE, Reuter KS, Braun RJ, et al. Tear film breakup and structure studied by simultaneous video recording of fluorescence and tear film lipid layer images. Investigative Ophthalmology & Visual Science 54 (2013): 4900–4999.
- Dell, S. J., Gaster, R. N., Barbarino, S. C., et al. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clinical Ophthalmology 11 (2017): 817-827.
- Knop, E., Knop, N., Millar, T., et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative Ophthalmology & Visual Science 52 (2011): 1938-1978.
- MJ Choi, SJ Han, SM Nam, et al. Meibum expressibility Improvement as a therapeutic target of Intense pulsed Light treatment in Meibomian Gland Dysfunction and Its Association with Tear Inflammatory Cytokines. Scientific reports 9 (2019): 7648.


 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
