Development of Co-Processed Powders Containing Lactose, Mucuna flagellipes Seed Gum and Ipomoea batatas Tuber Starch
Kenneth Chinedu Ugoeze*, Maryam EJ Idris
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
*Corresponding Author: Kenneth Chinedu Ugoeze, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
Received: 20 October 2020; Accepted: 30 October 2020; Published: 05 November 2020
Article Information
Citation: Kenneth Chinedu Ugoeze, Maryam EJ Idris. Development of Co-Processed Powders Containing Lactose, Mucuna flagellipes Seed Gum and Ipomoea batatas Tuber Starch. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 256-275.
View / Download Pdf Share at FacebookAbstract
Abstract
Background: The search for new pharmaceutical excipients with multifunctional properties to meet the technical challenges inherent in the formulation of drug products containing special pharmacologically active compounds possessing complex physico-chemical characteristics and elaborate technologies in their production procedures is continuous.
Aim: The development of directly compressible (DC) co-processed powders (CPPs) containing lactose (LTS), Mucuna flagellipes seed gum (Mucuna gum) (MG) and Ipomoea batatas tuber starch (potato starch) (PS) was undertaken.
Materials and methods: Batches CPP-A: LTS (75%), MG (7.5%), PS (17.5%) and CPP-B: LTS (75%), MG (10%), PS (15%) were formulated. Homogenized constituents were transferred into a 1-litre beaker, blended with 80ml of deionized water, placed in a water-bath (100o C) and stirred till it began to gel. It was removed and stirred for 10 min, spread in an oven (50o C) for 1 h and sieved (1.7 mm), dried to constant weight (50o C) and sieved (250 µm). The CPPs were characterized, compressed into compacts and evaluated beside their constituent materials and MicroceLac®-100 (MC-100) as controls.
Results: The CPPs with improved flowability (CPP-B>CPP-A>MC-100) and compressibility were obtained compared to their starting materials, resulting to compacts with mechanical strength: MC-100 > CPP-B >> CPP-A with CPP-A and CPP-B not disintegrating after 30 min.
Conclusion: The CPP-A or CPP-B could be suitable as DC diluent for sustained-release or chewable tablets and in producing tablets with low dose drug of which a disintegrant may be incorporated extra-granularly.
Keywords
<p>Co-processed powder; Lactose; <em>Mucuna flagellipes</em>; <em>Ipomoea batatas</em></p>
Article Details
Introduction
Pharmaceutical excipients with multifunctional properties that could be applied in the formulation of drug products containing special pharmacologically active compounds or active ingredients (APIs) possessing complex physico-chemical characteristics coupled with elaborate technologies in their production procedures have consistently been explored [1]. The excipient is measured as any other constituent(s) contained in drug formula other than the API. An API is a substance engaged in a complete pharmaceutical product, expected to offer a therapeutic effect or else assume direct significance in the diagnosis, cure, mitigation, management or inhibition of disease, etc. [2,3]. Excipients facilitate the delivery of the API to the systemic system, taking into consideration, the projected route of drug administration to maintain improved medication compliance, dependability and regulation of drug bioavailability and superior drug product stability, etc. [4,5]. They are also designed to safeguard the firmness of the drug in its formulation media through its shelf life and also ensuring the solubility, permeation and ease of absorption of the API [3,6-9]. With the advancement in novel drug delivery systems, further improved excipients are essential to impart some features to the pharmaceutical finished formulary. The need for multifunctional excipients has been the principal motivation for the institution of co-processed excipients. Various procedures coupled with extensive use of particle-engineering and material sciences have been in use to generate a new class of powders noted as co-processed excipients (CE) [10,11]. Co-processing is an alternate approach of introducing new excipients without undertaking to go through the demand for safety testing of an entirely innovative chemical. In co-processing, two or more conventional excipients are merged by a suitable technology [12] to produce an excipient(s) with superior properties and value [13]. The Co-processed powders or excipients are applicable in the formulation of solid dosage forms such as tablets, capsules, powders, etc. due to their multi-functionality. Co-processing is adopted in producing new excipients to enhance powder flowability and compressibility [14]. These enhanced characteristics make co-processed excipients very useful in direct compression (DC) process of tableting since some of them serve as a filler-binder [15,16]. The limitation of a co-processed powder is that the proportion of each component in a mixture is constant and in bringing up another formula, a constant portion of the powder may not be an optimum choice for the API and dose per tablet under development [17]. Examples of co-processed excipients include microcrystalline cellulose, ludipress (lactose powder coated with polyvinylpyrrolidone and crospovidine), starlac (lactose monohydrate and maize starch produced by spray-drying). Avicel CE-15(microcrystalline cellulose and guar gum) and fizlent [18,19].
The objective of the current investigation was to come up with a directly compressible excipient by co-processing of lactose, starch obtained from the tubers of Ipomoea batatas and the polymers derived from the kernel of Mucuna flagellipes.
Starch has frequently been employed as a tablet excipient but, does not, in its native state, have the two characteristics needed to produce compacts with measurable mechanical strength, specifically, compressibility and flowability and most especially in the DC technique of tableting [20-22]. Though, lactose monohydrate in its fine state possesses enough surface area for the formation of a good tablet, however, it exhibits poor flow [23]. The co-processing of lactose, potato starch and the gum obtained from the kernel of Mucuna flagellipes may give rise to a new directly compressible powder that could possess better flow and compaction properties. The gum extracted from the kernel of Mucuna flagellipes has shown usefulness as an emulsifier in oil-in-water emulsions, a disintegrant, suspending and thickening agent [24-28] in foods and pharmaceutical dosage forms [24-28] and has been modified to serve as a direct compressible powder in tableting [29,30]. Ipomoea batatas is a rich source of starch and have been useful as raw materials in the food and pharmaceutical industries [31-34]. Depending on the application, specific starches are available as disintegrants, filler-binders. Though, unmodified starch does not compress well and leads to elevated tablet friability and capping, especially if added in high concentrations. As a diluent, starch is included in formulations to ease successive homogenization procedures in production processes [35-37].
Materials and Methods
Materials
The following materials were used: acetone, n-hexane, ethanol (96%), chloroform, hydrochloric acid (Sigma-Aldrich, USA), lactose (Surechem, England), sodium metabisulphite, calcium chloride anhydrous (Vickers Laboratories, England), MicroceLac-100 (Meggle, USA).
Methods
Procurement of Mucuna flagellipes kernel and Ipomoea batatas tubers
About 1 kg of Mucuna flagellipes kernel and 10 kg of Ipomoea batatas tubers were procured from Choba market, Port Harcourt, Nigeria.
Processing of gum from the seed of Mucuna flagellipes
The kernel of the Mucuna flagellipes was fragmented and dried at 100o C in an oven (Memmert, England) for 1h. They were pulverized with a homogenizer (Binatone, China) and soaked for 12 h in 0.1 % w/v solution of sodium metabisulphite to reduce oxidation [38]. The resulting slurry was homogenized and strained through a muslin cloth to remove particulate materials. The polymer was precipitated with acetone in 1:1 of acetone: slurry. The precipitated mass was left to dry in a desiccator loaded with fused calcium chloride (anhydrous) as a drying agent. The dried material was pulverized and passed through 150 µm stainless steel sieve (Endecott, England).
Processing of potato starch
The starch obtained from the Ipomoea batatas tubers was submerged in 0.1N hydrochloric acid for 18 h, then washed till its pH was neutral. It was kept at 50°C in a hot air oven to dry to a constant weight when it was pulverized and sieved through a 150 µm stainless sieve.
Preparation of co-processed powders (CPPs) based on lactose (LTS), mucuna gum (MG) and Ipomoea batatas tuber starch (Potato starch) (PS)
The CPPs were prepared using the formula presented in Table 1.
Table 1: Preparation of the CPPs.
|
Batch |
Lactose (LTS) (% w/w) |
Mucuna gum (MG) (% w/w) |
Potato starch (PS) (% w/w) |
|
CPP-A |
75.0 |
7.5 |
17.5 |
|
CPP-B |
75.0 |
10.0 |
15.0 |
Each batch of the CPPs was prepared by blending the respective quantities of the potato starch (PS), mucuna gum (MG) and lactose (LTS) (Table 1). The powder blend was transferred into a 1- litre glass beaker and homogenized with 80 ml of deionized water. It was immersed in a water-bath (100o C) and stirred until the slurry began to harden when it was removed from the water-bath, stirred for 10 min, spread out in an oven (50o C) for 1 h, sieved (1.7 mm) and left to dry further (50o C) to a constant weight and sieved (250 µm).
Characterization of LTS, MG, PS and CPPs
The physico-technical properties of the CPPs were assessed along with those of the LTS, MG and PS in comparison with the MicroceLac®-100 (MC-100), a commercially available CPP comprising 75% α-lactose monohydrate and 25% microcrystalline cellulose. The CPPs and the LTS, MG and PS were referred to as the powders in this report. Each test was carried out in triplicate.
Organoleptic, solubility and pH
The powders were verified organoleptically for colour, odour and texture. The pH of their 2% (w/v) aqueous dispersion was evaluated with a pH meter (PHS-25, China).
Densities
The bulk and tapped densities of a 15 g of each of the powders were evaluated with a Stampfvolumeter (STAV 2003JEF, Germany). Their particle density was evaluated by the displacement method using a 25.0 ml pycnometer and n-hexane as a non-solvent [39-41]. The densities of the powders were calculated from equations 1-3.

Flow properties
The funnel method [42] was used to assess the flow rate of 30 g of a particular powder. Their angle of repose (AR) was established by the fixed funnel method as reported by Zeleznik and Renak [43]. Using a fixed height of 3 cm from the funnel tip to a plane base, each powder was poured through a glass funnel of 60o C wall angle and efflux tube length of 5 cm and 1.1 cm orifice internal diameter till the apex of the heap of the powder obtained touched the tip of the funnel, thus, stopping the further discharge of the powder from the funnel. The width of the heaped powder was estimated. The AR of the samples that were not free-flowing was estimated by a modification of the methods of Jones and Pilpel, 1966 [44] as 50 g of powder was poured into a two open-ended tubular plastic pipe with a 3 cm internal diameter placed on a plane base. The pipe was slowly pulled up to let the powder to form a heap whose height and diameter was estimated. The flow rate and the AR were calculated from equations 4 and 5 respectively.
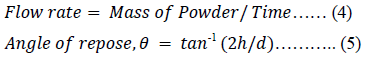
Where h = the altitude of the powder heap, d = diameter of the powder heap.
The Hausner ratio (HR) [45], Carr’s index (CI) [46] and porosity of the powders were calculated using equations 6, 7 and 8 respectively.
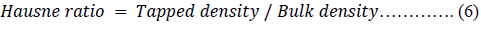
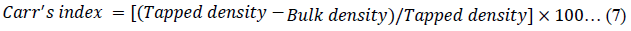
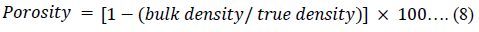
Moisture contents
The moisture contents of 2 g of the respective powders were evaluated with a digital moisture balance (Citizen, MB-50, China) at 105o C.
Hydration capacity
The hydration capacity of the respective powders was assessed by the method of Ring, 1985 [47] by weighing 1 g of each powder ( ), placing it in a 15 ml calibrated plastic centrifuge tube and adding 10 ml of water. The tube was shaken recurrently over 2 h period and left to stand for 1 h after which it was centrifuged for 10 min at 3000 revolutions per minute (rpm). The supernatant liquid was removed and the weight of the wet powder, was determined.
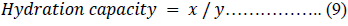
Where = the mass of wet powder after centrifugation, = the mass of the dry powder.
Scanning electron microscopy
The scanning electron micrographs of the LTS, PS, MG, CPP-A and CPP-B were obtained using a scanning electron microscope (SEM) instrument (Zeiss, Germany).
Differential scanning calorimetry (DSC)
The DSC thermograms of the LTS, PS, MG, CPP-A and CPP-B were obtained using a DSC equipment, DSC – 204F1 (NETZSCH, Germany).
Determination of compactability and dilution potential of the CPPs
Plain compacts targeted to weigh 400 mg were produced with CPP-A, CPP-B, MC-100, LTS, PS and MG powders at compression pressures of 0.25 tons using a 10.5 mm die and flat punches on a hydraulic hand press (Model C, Carver Laboratory Press, Menomonee Falls, WI. USA). The compacted samples were deposited in desiccators overnight before the assessment of their weights, thickness, hardness, tensile strength and disintegration. Their compactability and mechanical strength were evaluated and compared considering their tensile strength.
The tensile strength [48] of the compacts were calculated using equation 10.
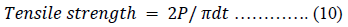
The dilution potential of the CPP-B and CPP-B were determined using paracetamol (PCM), metronidazole (MTZ) and ascorbic acid (ASBA). The choice of PCM among other drugs is due to its poor flowability and compressibility [49]. PCM, MTZ or ASBA were blended with either CPP-A or CPP-B in the proportions of 1:9-9:1 targeting a compact weight of 400 mg. Each drug-powder admixture was lubricated with 0.1% (w/w) magnesium stearate and compressed for 30 s at a compression pressure of 0.25 tons using a 10.5 mm die and flat punches fitted in the same hydraulic hand press described above. The compressed tablets were stored in a desiccator for 24 h before the determination of their diametrical hardness using a digital tablet hardness tester (Erweka, TBH 100, Germany).
Statistical Analysis
The figures were presented as a mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to assess the significance of the differences of the groups followed by the Fisher’s Least Significant Difference (LSD) post hoc test to determine the level of significance. Results with p < 0.05 were considered statistically significant.
Results
The results of the evaluations of the physico-technical properties of the powders were presented in Table 2. Figures 1-5 represent the scanning electron micrographs (SEM) for lactose, potato starch, mucuna gum, CPP-A and CPP-B respectively while Figures 6-10 denote the DSC thermograms for lactose, potato starch, mucuna gum, CPP-A and CPP-B respectively. Tables 3 and 4 display the properties of the compacts produced with the CPPs and the results of the dilution potentials for the CPPs respectively.
Table 2: Physico-technical properties of the powders
|
Parameter |
Sample |
|||||
|
LTS |
PS |
MG |
CPP- A |
CPP- B |
MC-100 |
|
|
Bulk density (g/ml) |
0.50±0.01 |
0.45±0.01 |
0.36±0.01 |
0.62±0.01 |
0.63±0.01 |
0.51±0.01 |
|
Tapped density (g/ml) |
0.70±0.02 |
0.70±0.02 |
0.39±0.01 |
0.73±0.003 |
0.74±0.01 |
0.63±0.01 |
|
Particle density (g/ml) |
1.51±0.21 |
1.42±0.25 |
1.01±0.22 |
1.25±0.01 |
1.24±0.01 |
1.50±0.04 |
|
Porosity (%) |
66.89±0.20 |
68.31±0.23 |
64.36±0.21 |
50.71±0.03 |
50.21±0.92 |
65.83±1.10 |
|
Carr’s index (%) |
28.57±0.21 |
35.71±0.22 |
7.69±0.12 |
16.14±0.06 |
13.94±0.08 |
20.21±1.24 |
|
Hausner ratio |
1.40±0.21 |
1.56±0.21 |
1.08±0.11 |
1.19±0.02 |
1.15±0.02 |
1.25±0.02 |
|
Angle of repose (deg.) |
41.57±0.87 |
39.82±0.78 |
28.07±0.32 |
37.21±0.12 |
36.08±0.36 |
26.11±0.78 |
|
Flow rate (g/s) |
No free flow |
No free flow |
4.56±0.24 |
14.28±0.10 |
15.08±0.24 |
9.78±0.92 |
|
pH (10%w/v) |
7.47±0.03 |
7.34±0.05 |
6.76±0.03 |
5.58±0.01 |
5.61±0.02 |
6.19±0.08 |
|
Moisture content (%) |
4.78±0.13 |
0.91±0.12 |
11.50±0.11 |
6.55±0.06 |
6.58±0.06 |
5.11±0.23 |
|
Hydration capacity |
1.72±0.12 |
2.33±0.13 |
4.33±0.11 |
1.70±0.02 |
1.74±0.05 |
1.14±0.05 |
Table 3: Properties of the compacts produced with the co-processed powders
|
Sample |
Weight (mg) |
Thickness (mm) |
Hardness (kgF) |
Tensile Strength (kg/m2) |
Disintegration (min) |
|
CPP- A |
395.50±4.93 |
2.69±0.01 |
3.86±0.04 |
87.08±0.58 |
Non-disintegrating |
|
CPP- B |
400.93±1.22 |
2.70±0.02 |
9.95±0.13 |
223.26±3.99 |
Non-disintegrating |
|
MC-100 |
400.13±0.67 |
2.61±0.02 |
10.57±0.16 |
245.86±3.91 |
< 10.0 |
|
PS |
389.52±2.12 |
2.46±0.32 |
1.94±0.52 |
47.81±2.15 |
< 1.0 |
|
LTS |
399.85±2.25 |
2.57±0.41 |
1.99±0.42 |
46.94±2.31 |
< 1.0 |
|
MG |
388.14±2.23 |
2.42±0.52 |
1.47±0.53 |
38.82±2.24 |
< 1.0 |
Table 4: The results of the dilution potentials for the CPPs
|
Co-processed powder |
Dilution potential (%) |
||
|
Paracetamol |
Metronidazole |
Ascorbic acid |
|
|
CPP-A |
≥ 80 |
≤ 60 |
≤ 50 |
|
CPP-B |
60-70 |
≤ 50 |
≤ 40 |
|
MC-100 |
≤ 50 |
≤ 40 |
≤ 40 |
Discussion
Densities and flow properties
The results of the physico-technical properties of the starting materials (LTS, PS and MG), the CPPs (CPP-A and CPP-B) were presented in Table 2. The bulk and tapped densities of the LTS, PS and MG indicated that these were bulk powders, though, MG exhibited lower bulk density and porosity compared to the LTS and PS (p < 0.05). The statistical contrast of the bulk and tapped densities of the LTS, PS and MG indicated a significant variation between them and suggests that the powders have poor flow characteristics, especially the LTS and PS. The values obtained for the Carr’s index, Hausner ratio and angle of repose for the LTS and PS confirm their poor flow properties. The corresponding values of the bulk and tapped densities of CPP-A and CPP-B were statistically similar and were higher than that of the MC-100 (p < 0.05). A statistical assessment of the bulk and tapped densities of the CPP-A, CPP-B and MC-100 showed a significant variation in their values (p < 0.05), indicating that, though the CPP-A and CPP-B may be compressible, they exhibit poor flowability. This inclination is possible since the interparticulate interfaces prompting the bulk characteristics of a pulverized solid material are similarly the same contacts that impede the flow of powdered solid substances as the contrast of the bulk and tapped densities offer a degree of the comparative significance of these interfaces in a set of powder. Such an assessment as Carr’s index (CI) or the Hausner ratio (HR) has often been used as a guide to estimate the flowability of a powder. These two factors define the inclination of a powder to be compressed and as such, they indicate the capability of powder to consolidate and they equally give room for the evaluation of the relative status of interparticulate interactions [50,51]. Powders that statistically display variations in the values of their respective bulk and tapped densities exhibit cohesiveness and may not have a very perfect flow properties [52].
A CI >25 % is predictable to display poor flow properties and the CI <15 % is expected to exhibit an enhanced flowability [46, 47]. The flowability of the CPPs could therefore be presented as CPP-B > CPP-A > MC-100 (p < 0.05) and could be confirmed considering the low value of HR for the CPP-B coupled with its high flow rate (Table 2) [45,46].
Moisture properties
The moisture content of the CPPs and the MC-100 were recorded as MC-100 < CPP-A < CPP-B (Table 2). The statistical breakdown indicated that there was no significant variation in the values of moisture contents for the CPP-A and CPP-B, hence, the results could be presented as (CPP-A = CPP-B) > MC-100 (p < 0.05). For the hydration capacity of the CPPs, a consistent increase in values was recorded between the CPP-A and CPP-B, with the MC-100 exhibiting the lowest value. The statistical examination of these results showed that there was no statistical disparity in the results for the CPP-A and CPP-B (p > 0.05), however, they were higher than that of the MC-100 (p < 0.05). The low hydration capacity and moisture content of the MC-100 may be due to its crystalline constituents, microcrystalline cellulose and α-lactose monohydrate as it has been documented that the crystalline component of solid materials does not get easily hydrated, but, the amorphous component does [53]. It has been noted that the native starch is semi-crystalline with a varying level of crystallinity that is associated with the amylopectin component [54], while the amorphous regions represent amylase. The MG on its own is a fully amorphous polymer. The composition of the CPP-A and CPP-B were not fully crystalline to the extent of the crystalline state of the constituents of the MC-100 and this could be the reason for their exhibition of higher values in hydration capacity and moisture content than MC-100.
Scanning electron microscopy (SEM)
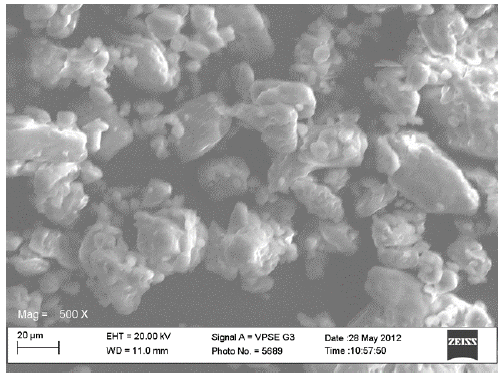
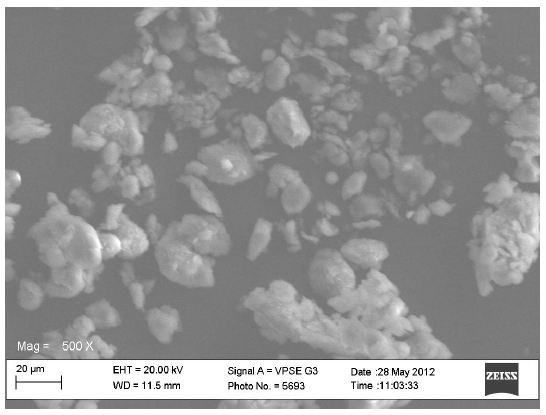
The scanning electron micrographs for the original constituents of the CPPs, the LTS, PS and MG were shown in Figures 1-3 respectively while Figures 4 and 5 represent the scanning electron micrographs for the CPP-A and CPP-B respectively. The particle size of the CPPs was in the neighbourhood of 20 µm. The CPP-A and CPP-B exhibited morphological structures and size distribution that were different from those of the LTS, PS and MG (Figures 1-3). The features demonstrated in the CPP- A (Figures 4) and the CPP- B (Figure 5) respectively were larger with a polyhedral shape, having a rough surface texture as compared to those in LTS (Figures 1), PS (Figure 2) and MG (Figure 3) which were smaller with smooth surface textures. The representations in the CPP-A (Figures 4) and CPP-B (Figure 5) depicted similar morphological characteristics in terms of size, shape, surface structure and texture, though, those of the CPP-A (Figure 4) has more particles in the larger particle size range than those in the CPP-B (Figure 5). These results indicate the possible development of a new powder from the base materials, LTS, PS and MG.
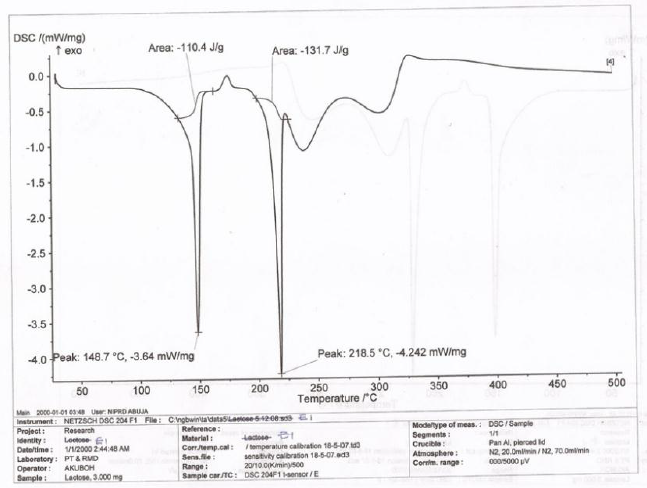
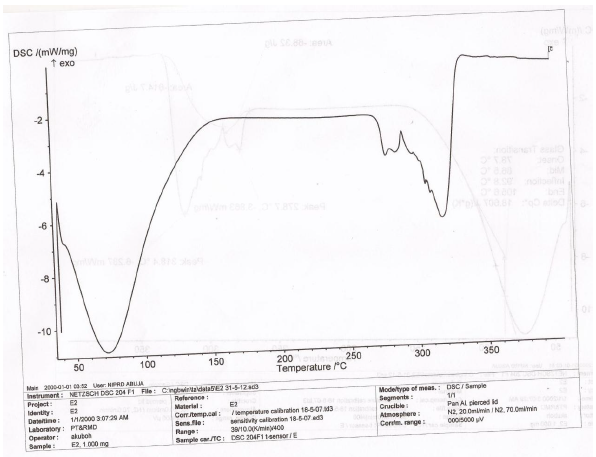
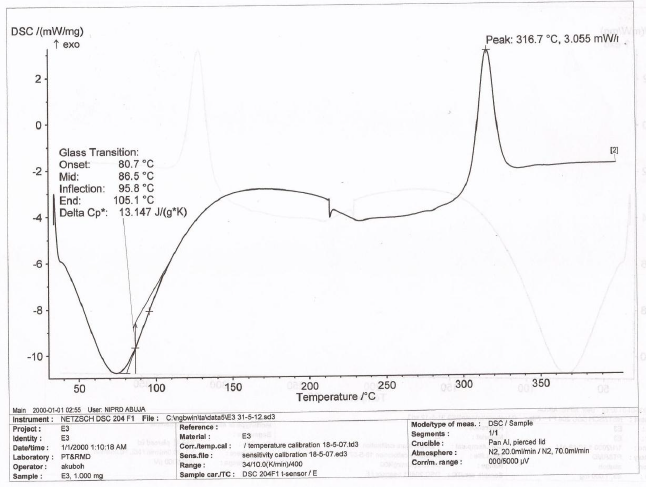
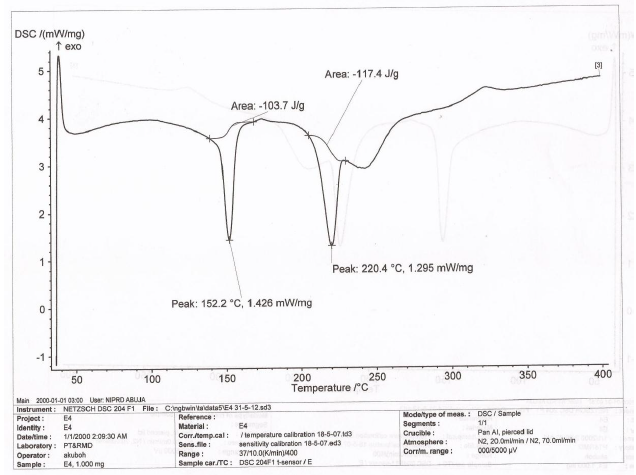
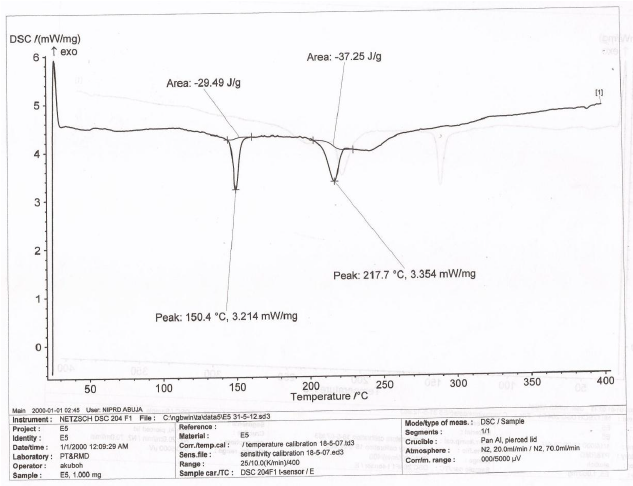
The DSC thermogram of LTS, PS, MG, the CPP-A and CPP-B were presented in Figures 6-10 respectively. The thermograms for LTS (Figure 6) shows two peaks, 146.7o C and 218.5o C; PS (Figure 7) displayed a glass transition with onset at 78.7o C and ended at 105.6o C, transiting into another phase that displayed two melting peaks at 278.7o C and 318.4o C respectively; MG (Figure 8) displayed a glass transition between the temperatures of 80.7o C to 105.o C, transiting into a crystalline material with melting peak at 316.7o C, an exothermic process. Figures 9 and 10 represent the DSC thermogram for the CPP-A and CPP-B. In Figure 9, two melting peaks were observed at 152.2o C and 220.4o C while Figure 10 exhibited two melting peaks at 150.4o C and 217.7o C. The peaks in Figures 9 and 10 were observed to vary from those of the original materials, LTS (Figure 6), PS (Figure 7) or MG (Figure 8). These observations illustrated a possible development of new materials through the co-processing of LTS, PS and MG in the respective proportions presented in Table 1.
Properties of the compacts
The properties of the compacted samples generated from the CPP-A, CPP-B and the MC-100 were presented in Table 3.
Compact mean weight
The results showed that the coefficient of variations of the compact mean weights was within the range of 0.17-1.25%. They were respectively lower than 5% which is the pharmacopoeia acceptable limit for tablets or compacts weighing up to 250 mg or more [55].
Compact mechanical strength
The LTS, PS and MG powders were fairly compactable as depicted by their low values of compact hardness and tensile strength (Table 3). The hardness of the compacts produced with the CPP-A was significantly less than those of the CPP-B and the MC-100 (p < 0.05). Oral tablets could possess the hardness of 4 -10 kg whereas hypodermic and chewable tablets are expected to be softer ( 3 kg) while some sustained-release tablets are anticipated to be harder ( 10-20 kg) [56, 57]. The tensile strength of the respective compacts produced with CPP-A, CPP-B and MC-100 appeared in a similar trend as in their respective hardness. The compacts produced with the CPP-A were mechanically weak and were statistically lower than those of the CPP-B and the MC-100 (p > 0.05). The CPP-B was therefore considered to be comparable to MC-100. The mechanical properties of the compacts could therefore be presented in the order: MC-100 > CPP-B > CPP-A > PS > LTS > MG (p < 0.05). It was recorded that the compacts prepared with the CPP- A and CPP-B failed to disintegrate after 30 min whereas those produced with MC-100 disintegrated in ≤ 10 min and < 1 min for LTS, PS and MG respectively. Improved compact mechanical properties of the CPPs against the original materials from which they were co-processed indicates that changes in the physical properties of LTS, PS and MG may have occurred resulting to the co-processed powders with improved compactability. Since compacts produced with CPP-A and CPP-B were non-disintegrating, they may be useful in the formulation of chewable or sustained-release tablets and could also serve as a directly compressible diluent in the tableting of immediate-release solid dosage formulas containing low dose drugs of which a disintegrant may be added extra-granularly. Scientific literature abounds with co-processed excipients with multiple benefits [58-60]. Menon, et al., 1996 presented a co-processed excipient made up of corn starch and polyvinylpyrrolidone which demonstrated high flowability and compressibility [61]. Ratnaraj and Reilly, 1997 manufactured a co-processed powder for a chewable tablet using microcrystalline cellulose and guar gum which exhibited enhanced compressibility and mouth feel [62]. Limwong et al., 2004 developed a composite powder containing rice starch and microcrystalline cellulose applicable as a directly compressible excipient and exhibited compressibility that was greater than the commercial spray-dried rice starch (Eratab), co-processed lactose and microcrystalline cellulose (Cellactose), and agglomerated lactose (Tablettose), but, lower than microcrystalline cellulose (Vivapur 101) [63].
Lang (1991) reported a blend of lactose, PVP and crospovidone which was produced through spray drying, spray granulation or wet granulation method. The novel direct tableting auxiliaries exhibited good flow, good compressibility under low pressure, excellent disintegration properties coupled with great hardness and low abrasion Lang (1991) reported a blend of lactose, PVP and crospovidone which was produced through spray drying, spray granulation or wet granulation method. The novel direct tableting auxiliaries exhibited good flow, good compressibility under low pressure, excellent disintegration properties coupled with great hardness and low abrasion Menon et al. (1996) described a co-processed excipient consisting of cornstarch and polyvinylpyrrolidone produced using fluid bed spray granulation method. The invention is free-flowing and exhibits good compressibility [60]. Ratnaraj and Reilly (1997) produced a co-processed excipient for the chewable tablet by thoroughly mixing an aqueous dispersion of MCC and guar gum under high shear conditions at room temperature. The homogenous dispersion was then spray dried to an aggregate powder having substantially spheroidal-shaped particles. The excipient has improved compressibility and mouth feel. It reduces tooth packing [62].
Dilution potentials of the CPPs
The dilution potential of the CPP-A, CPP-B and MC-100 are as shown in Table 4. The values presented depicts the level at which the compression of the powder blends yielded a tablet with fairly measurable hardness close to or above 3 kgf with the application of a uniform compression load of 0.25 tons.
Dilution potentials
The dilution potentials of the CPPs as determined are shown in Table 4.
Conclusion
The co-processing of LTS, PS and MG resulted to new CPPs coded as the CPP-A and CPP-B with varying properties from their starting materials as displayed in their scanning electron micrographs, DSC thermograms, compaction characteristics and other physico-technical properties of the original materials in contrast to the co-processed powders. Enhanced powder flowability was recorded with the CPP-A and CPP-B compared to the LTS, PS, MG and MC-100 with CPP-B exhibiting the best flow properties. Though the CPPs exhibited an improved compactability compared to LTS, PS and MG, higher tensile strength was recorded with the compacts of the CPP-B than CPP-A, though, MC-100 was the highest of all, hence, compactability could be presented as MC-100 > CPP-B > CPP-B (p < 0.05). The compacts produced with the CPP-A or CPP-B could not disintegrate after 30 min in contrast to the MC-100 which disintegrated in ≤ 10 min. Since the compacts produced with CPP-A or CPP-B were non-disintegrating, they may be suitable as directly compressible diluents for the compression of sustained-release or oral chewable tablets. It may also be useful as a directly compressible diluent in low dose drugs where a disintegrating agent may be incorporated extra-granularly.
References
- World Pharma Today. The rise and rise of Global Pharma Excipients Market. Available from: https://www.worldpharmatoday.com/techno-trends/the-rise-and-rise-of-global-pharma-excipients-market/. Retrieved on 5th September 2020.
- World Health Organization WHO Technical Report Series, No. 961, 2011. Available from: https://www.who.int/medicines/areas/quality_safety/quality_assurance/TRS961_Annex10.pdf. Retrieved on 6th September 2020.
- The Joint International Pharmaceutical Excipient Council- Pharmaceutical Quality Group (IPEC-PQG) Good Manufacturing Practices Guide for Pharmaceutical Excipients, 2017. Available from: http://academy.gmp- org/guidemgr/files/20170517-ipec-pqg-gmp-guide-final-1536242212.pdf. Retrieved on 5th September 2020.
- van der Merwe J, Steenekamp J, Steyn D, et al. The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics 12 (2020): 393.
- World Health Organization (WHO). Training Workshop on Pharmaceutical Development with a Focus on Paediatric Medicines. 15-19 October 2007. Available from: http://studylib.net/doc/9264523/guidelines---world-health-organization. Retrieved on 22nd June 2017.
- Ullmann P. Excipients selection for compounding pharmaceutical capsules: they are only fillers, right?. Australian Journal of Pharmacy 98 (2017): 78-83.
- European Pharmaceutical Review. The central role of excipients in drug formulation. Available from: https://www.europeanpharmaceuticalreview.com/article/18434/the-central-role-of-excipients-in-drug-formulation-2/. Retrieved on 6th September 2020.
- The European Pharmacopoeia (Ph. Eur.). Council of Europe; European Pharmacopoeia Commission, Strasbourg (2007).
- Abrantes CG, Duarte D, Reis CP. An overview of pharmaceutical excipients: Safe or not safe. Journal of pharmaceutical sciences 105 (2016): 2019–2026.
- Kanojia N, Kaur L, Nagpal M, et al. Modified excipients in novel drug delivery: Need of the day. Journal of Pharmaceutical Technology, Research and Management 1 (2013): 81–107.
- Block LH, Moreton RC, Apte SP, et al. Co-processed excipients. Pharm. Forum 35 (2009): 1026–1028.
- Reimerdes D. The near future of tablet excipients. Manufacturing Chemist 64 (1993): 14-15.
- Reimerdes D, Aufmuth KP. Tableting with co-processed lactose-cellulose excipients. Manufacturing Chemist 63 (1992): 21-24.
- York P. Crystal engineering and particle design for the powder compaction process. Drug Development and Industrial Pharmacy 18 (1992): 677-721.
- Belda PM, Mielck JB. The tabletting behaviour of Cellactose® compared with mixtures of celluloses with lactoses. European Journal of Pharmaceutics and Biopharmaceutics 42 (1996): 325-330.
- Schmidt PC, Rubensdörfer CJ. Evaluation of Ludipress as a “multipurpose excipeent” for direct compression: Part I: Powder characteristics and tableting properties. Drug Development and Industrial Pharmacy 20 (1994): 2899-2925.
- Bolhuis GK, Chowhan ZT. Materials for direct compression, pharmaceutical powder compaction technology, Vol-7, Marcel Dekker, USA (1996).
- Chougule AS, Dikpati A, Trimbake T. Formulation development techniques of co-processed excipients. Journal of Advanced Pharmaceutical Sciences 2 (2012): 231-249.
- Ugoeze KC, Nkoro VO. The Physico-technical properties of a multicomponent Lentinus tuberregium based co-processed excipient (Fizlent). American Journal of Pharmacy and Pharmacology 2 (2015): 13-20.
- Akhgari A, Sadeghi H, Dabbagh MA. Modification of flow and compressibility of corn starch using quasi-emulsion solvent diffusion method. Iranian Journal of Basic Medical Sciences 17 (2014): 553-559.
- Pifferi G, Santoro P, Pedrani M. Quality and functionality of excipients. II Farmaco 54 (1999):1-4.
- Odeku OA, Schmid W, Picker-Freyer KM. Material and tablet properties of pregelatinized (thermally modified) Dioscorea starches. European Journal of Pharmaceutics and Biopharmaceutics 70 (2008): 357-371.
- Gohel MC, Jogani PD. A review of co-processed directly compressible excipients. J Pharm Pharm Sci 8 (2005): 76-93.
- Onweluzo JC, Obanu ZA, Okwandu MC. Potentials of gum from Detarium microcarpum (DM) and Mucuna flagellipes (MF) seeds as raw beef burger stabilizers. Plant Foods for Human Nutrition 59 (2004): 137-141.
- Nwokocha LM, Williams PA. Isolation and rheological characterization of Mucuna flagellipes seed gum. Food Hydrocolloids 23 (2009): 1394-1397.
- Ene-Obong HN, Carnovale E. Nigerian soup condiments: traditional processing and potential as dietary fibre sources. Food Chemistry 43 (1992): 29-34.
- Versteeg MN, Amadji F, Eteka A, et al. Collaboration to increase the use of Mucuna in production systems in Benin. InCover crops in West Africa: contributing to sustainable agriculture= Plantes de couverture en Afrique de l'Ouest: une contribution à l'agriculture durable (1998).
- Udeala OK, Uwaga UN. Some emulsifying and suspending properties of a polysaccharide gum derived from Mucuna flagillepes, Papilionaceae. Journal of Pharmacy and Pharmacology 33 (1981): 75-78.
- Chukwu A. Evaluation of Mucuna flagellipes gum as a tablet disinterant B. Pharm Thesis of University of Nigeria, Nsukka, Nigeria 56 (1986).
- Udeala OK, Chukwu A. Tabletting properties of Musol; a new direct compression vehicle. Drug Development and Industrial Pharmacy 12 (1986): 1587-1612.
- Kitahara K, Nakamura Y, Otani M, et al. Carbohydrate components in sweetpotato storage roots: their diversities and genetic improvement. Breeding Science 67 (2017): 62-72.
- Howling D. The influence of the structure of starch on its rheological properties. Food Chemistry 6 (1980): 51-61.
- Singh N, Singh J, Kaur L, et al. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chemistry 81 (2003): 219-231.
- Singh J, Kaur L, McCarthy OJ. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocolloids 21 (2007): 1-22.
- Uhumwangho MU, Okor RS, Eichie FE, et al. Influence of some starch binders on the brittle fracture tendency of paracetamol tablets. African Journal of Biotechnology 5 (2006): 1950-1953.
- Rodrigues A, Emeje M. Recent applications of starch derivatives in nanodrug delivery. Carbohydrate Polymers 87 (2012): 987-994.
- Zia-ud-Din, Xiong H, Fei P. Physical and chemical modification of starches: A review. Critical Reviews in Food Science and Nutrition 57 (2017): 2691-2705.
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available from https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=3503. Retrieved on 14th September 2020.
- Ugoeze KC, Nwachukwu N, Okeke CE. The physico-chemical and filler-binder-disintegrant properties of improved hydrophilic powder derived from the fibre of Ipomoea batatas tuber in paracetamol tablet. Thai Journal of Pharmaceutical Sciences (TJPS) (2020) (In press).
- Odeku OA, Awe OO, Popoola B, et al. Compression and mechanical properties of tablet formulations: containing corn, sweet potato, and cocoyam starches as binders. Pharmaceutical Technology 29 (2003): 82-90.
- D854-92e1 Standard test method for specific gravity of soils, American Society for Testing and Materials (ASTM) (1997). Available from: https://www.micromeritics.com/Repository/Files/Volume_and_ Density_determinations_for_Particle_Technologists.pdf. Retrieved on 20th September 2020.
- Carstensen JT, Chan PC. Flow rates and repose angles of wet-processed granulations. Journal of Pharmaceutical Sciences 66 (1977): 1235-1238.
- Zeleznik JA, Renak JL. Flow and compaction properties of dibasic calcium phosphate blended with microcrystalline cellulose & silicified microcrystalline cellulose: A paper presented at the American Association of Pharmaceutical Scientists Annual Meeting & Exposition, 2001, Denver, Colorado.
- Jones TM, Pilpel N. The flow properties of granular magnesia. Journal of Pharmacy and Pharmacology 18 (1966): 81-93.
- Hausner HH. Friction conditions in a mass of metal powder. J. Powder Metal 3 (1967): 7-13.
- Carr R. Classifying flow of solids. Chemical Engineering 72(1965): 69-72.
- Ring SG. Some Studies on Gelatin. Starch 37 (1985): 80-87.
- Fell JT, Newton JM. Determination of tablet strength by the diametrical compression test, Pharm. Sci. 59 (1970): 688-691.
- Šimek M, Grünwaldová V, Kratochvíl B. Comparison of compression and material properties of differently shaped and sized paracetamols. KONA Powder and Particle Journal 34 (2017): 197-206.
- WHO (2012).Document QAS/11.450 Final. Bulk Density and Tapped Density of Powders: Final text for addition to The International Pharmacopoeia. Available from: https://www.who.int/medicines/publications/pharmacopoeia/Bulk-tapped densityQAS11_450FINAL_MODIFIEDMarch2012.pdf. Retrieved on 20th September 2020.
- United States Pharmacopoeia. Bulk density and tapped density. Available from: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c616.html. Retrieved on 20th September 2020.
- Abdullah EC, Geldart D. The use of bulk density measurements as flowability indicators. Powder Technology 102 (1999): 151-165.
- Stamm AF. Wood and Cellulose Science. New York: The Ronald Press Company, (1964).
- Stasiak M, Molenda M, Opali?ski I, et al. Mechanical properties of native maize, wheat, and potato starches. Czech Journal of Food Sciences 31 (2013): 347-354.
- British Pharmacopoeia. London: Her Majesty’s Stationery Office, (2012).
- Chauhan K, Parashar B, Chandel A, et al. Formulation and evaluation of fast dissolving tablets of telmisartan. International Journal of Pharmaceutical Sciences and Research 4 (2013): 1514.
- Martins E, Christiana I, Olobayo K. Effect of Grewia gum on the mechanical properties of Paracetamol tablet formulations. African Journal of Pharmacy and Pharmacology 2 (2008): 1-6.
- Kaur L, Singh I. Microwave grafted, composite and coprocessed materials: drug delivery applications. Therapeutic Delivery 7 (2016): 827-842.
- Thakur G, Singh A, Singh I. Chitosan-montmorillonite polymer composites: formulation and evaluation of sustained release tablets of aceclofenac. Scientia Pharmaceutica 84 (2016): 603-617.
- Kaur R, Sharma A, Puri V, et al. Preparation and characterization of biocomposite films of carrageenan/locust bean gum/montmorrillonite for transdermal delivery of curcumin. BioImpacts: BI 9 (2019): 37.
- Menon A, Gillece T, Chakrabarti S, inventors; ISP Investments LLC, assignee. Co-Processing method for making a free-flowing compressible powder and tablet therefrom. United States patent US 5,560,927 (1996).
- Ratnaraj S, Reilly Jr. WJ. Chewable pharmaceutical tablets. US5686107, (1997).
- Limwong V, Sutanthavibul N, Kulvanich P. Spherical composite particles of rice starch and microcrystalline cellulose: a new co-processed excipient for direct compression. AAPS PharmSciTech 5 (2004):40-49.





 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
