Different Categorizations of Synthetic Pesticides and Their Effects on Soil
A. El-Shabasy*,1, Hammad Ahmad Jan2, Ahmed Ali Mustafa3
1Department of Biology, College of Science, Jazan University, P.O. Box. 114, Jazan 45142, Saudi Arabia
2Department of Botany, University of Buner, Swari, Pakistan
3Department of Botany and Microbiology, Faculty of Science, University of Gezira, Sudan
*Corresponding author: A. El-Shabasy, Department of Biology, College of Science, Jazan University, P.O. Box. 114, Jazan 45142, Saudi Arabia.
Received: 28 August 2025; Accepted: 03 September 2025; Published: 09 September 2025
Article Information
Citation: A. El-Shabasy, Hammad Ahmad Jan, Ahmed Ali Mustafa. Different Categorizations of Synthetic Pesticides and Their Effects on Soil. International Journal of Applied Biology and Pharmaceutical Technology. 16 (2025): 12-29.
View / Download Pdf Share at FacebookAbstract
The present study illustrates the effect of all types of chemical pesticides on soil nature. It includes the classification of insecticides, herbicides, fungicides, bactericides and viricides. The study focuses on all types for each category explaining the degree of impact on soils in terms of soil persistence and accumulation, soil microbial impacts, soil enzyme inhibition, soil fertility, groundwater contamination and pesticide degradation. Diagrammatic schemes illustrate the pesticide mechanisms on soil structure. The study gives an overview on pesticide impact and discusses at conclusion section how to reduce harmful soil induction and show the suggested solutions.
Keywords
<p>organic matter, leaching, dehydrogenase, inhibition, Rhizobium</p>
Article Details
Introduction
Pesticides, insecticides and fungicides are the most common use in removal all types of pathogen presented externally and internally at crop plants to conserve them and increase output yield to improve life style of human beings after fulfill all their vital and main requirements. Unfortunately, they affect negatively on all basic abiotic global components like air, water and even soils (Adamski et al., 2009). Soil component includes physical, chemical and biological structures where they are destroyed within short or long-terms. Ecosystems can be influenced whether terrestrial or marine to give complete destruction on the planet. Precautions and thinking future plans should be direct to safe both agriculture and human life (Alford and Krupke, 2017). Different measurements can be accomplished to evaluate the state of soil health. Water management within soil profile after pesticide treatment will be calculated. The number of beneficial and non-beneficial microorganisms should be counted (Kronberg et al., 2018). Soil texture with chemical fluent are determined. Chemical components, minerals, chelation and mixtures are considered. Various procedures and analysis should be continuously executed to conserve soil sustainability (Tari et al., 2021). The aim of this study to give a complete picture of the effect of all types of insecticides, herbicides, fungicides, bactericides and viricides on soil nature in presence of soil persistence, soil microbes, soil fauna and risk of environment.
Main Chemical Types of insecticides
Organochlorines (OC)
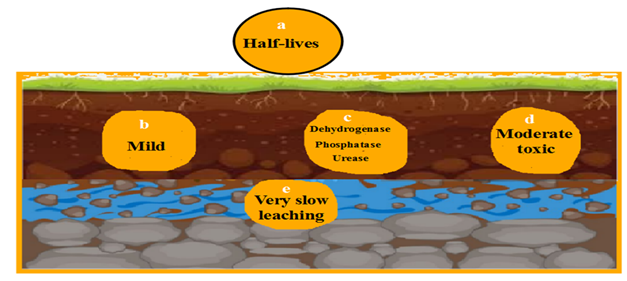
They have significant influence on soil nature because they last at soil profile for long time due to their stability and lipophilicity besides they are regarded as the main bioaccumulator at food chains. DDT, Lindane, Dieldrin, Heptachlor and Aldrin are examples of them. Due to their toxicity, some countries give some restrictions on dealing with them (Armitage and Gobas, 2007).
Persistence and Accumulation
They are fat soluble so they are able to stay from months to decades with soil particles binding to organic matter as well as clay particles. They are discovered at levels of food chains especially with successive treatments (Miglioranza et al., 2013).
Soil Microbial Impact
According to their toxicity, beneficial microbial communities are altered whether at nature, structure or diversity. They suppress all beneficial bacteria and mycorrhiza as well as dirupt the nutrient cycles occurred at soil profile as decomposition and nitrogen fixation (Mishra et al., 2012).
Soil Enzyme Inhibition
The vital microbial and phyto-enzymes are inhibited like urease, dehydrogenase and phosphatase so that organic matter and nutrient can’t be processed (Kottler et al., 2001).
Bioaccumulation and Biomagnification
Utilization of polluted remains are still present at food web by soil-dwelling organisms as earthworms. They are transferred through heterotrophs by predators (Weber et al., 2010).
Reduced Soil Fertility
Plant productivity is reduced by degradation of mineral availability induced with long-term exposure as well as alteration of chemical properties of soil texture like soil PH (Rodriguez-Campos et al., 2014).
Groundwater Contamination
Ground water is affected and contaminated especially small soil profile with sandy or disturbed soil particles (Simi´dova and Hofman, 2014).
Organophosphates (OP)
Unfortunately, they are wide spread at soil with high harmful impact compared with previous one due to highly degradation with bad health effects. Parathion, Chlorpyrifos, Parathion, Dimethoate, Diazinon and Malathion are good examples. They are able to inhibit nerve stimulator like acetylcholinesterase. They are vey toxic on all types of organism even humans (Monkiedje, 2002).
Soil Microbial Impact
Obviously, they suppress the most beneficial bacteria that involved in nitrogen fixation like Rhizobium and Azotobacter. Moreover, They reduce and decline microbial resistance and diversity respectively. They inhibit the organic matter decomposition with bad effects on nutrient cycling (Liu et al., 2005).
Enzyme Activity
The most important enzymes are altered and become inactive like dehydrogenase, phosphatase and urease that influence on soil fertility and nutrient availability (Mihajlovic et al., 2011).
Residue and Degradation
They can be degraded by hydrolysis mechanism, photo-degradation and microbial activities. They stay at soil from days to a few weeks according to abiotic factors like moisture, PH and temperature. Some metabolites are extracted from Organophosphates metabolism where are more or less toxic via biodegradation (Muñoz-Quezada et al., 2016).
Soil Fauna
Different types of living organisms can be affected by toxicity of Organophosphates like nematodes, earthworms and other beneficial invertebrates at aeration and structure (Pandey et al., 2019).
Carbamates
Aldicarb, Carbaryl (Sevin), Propoxur and Methomyl are categorized from Carbamates. They inhibit acetylcholinesterase with reversible mode of action. They are less persistent with moderate toxicity. They have a wide spectrum against variety of weeds, insects and fungi. They are less severe than previous ones but still notable especially with repeated use (Cai et al., 2015).
Soil Persistence
They stay from short to moderate half-lives in terms of a few days to a few weeks. They are degraded like Organophosphates in the form of microbial activities, photolysis and hydrolytic mechanisms. They can be accumulated with less level at heterotrophs presented at food webs while they can still be leached under certain conditions (Berman et al., 2017).
Effects on Soil Microorganisms
They can inhibit beneficial microbes but at high concentrations. Azospirillum and Rhizobium as nitrogen-fixing bacteria from one side besides actinomycetes and Bacilli as microbial decomposers from another side are affected negatively with long-term or excessive use (Devi and Iyer, 2017).
Soil Enzyme Activity
The same key enzymatic proteins are inhibited by disturbance of nitrogen cycle, denaturation of microbial communities and alteration of phosphate availabilities for urease, dehydrogenase and phosphatase respectively.
Soil Fauna Impact
Similarly, they impact on soil invertebrate fauna like earthworms, mites and nematodes and impair soil structure and aeration.
Pyrethroids
They are regarded as synthetic analogs of natural pyrethrins from Chrysanthemum sp. Cypermethrin, Deltamethrin, Lambda-cyhalothrin and Permethrin are common examples. They are distinguished with low toxicity to humans but have fast action and wide effects on soil ecosystems especially with frequent applications (Fareed et al., 2017).
Soil Persistence
Persistence and half-lives range from low to moderate as well as several days to a few months respectively. They are strongly adsorbed on soil particles especially in high organic matter or clay soils. Due to their low mobility, leaching to groundwater occur slowly (Hamada et al., 2015).
Effects on Soil Microorganisms
During repeated or high-dose applications of Pyrethroids treatments, they can suppress microbial activities and affect on beneficial bacteria like nitrogen fixers. They may cause temporary changes in microbial diversity with reversible action (Ishag et al., 2016).
Soil Enzyme Activity
They may reduce dehydrogenase, phosphatase and urease at microbial and plant combinations with delimitation of nutrient mineralization especially nitrogen and phosphorus cycles (Kulkarni and Kaliwal, 2018).
Soil Fauna Impact
They are highly toxic whether targeted or non-targeted invertebrates including earthworms, beetles and mites. They are able to change soil structure, aeration and organic matter composition (Omolo et al., 2012).
Environmental Considerations
They can bind tightly to soil particles besides accumulate around with repeated use. They are less toxic to mammals and birds but highly one to aquatic organisms if runoffs occur (Purushothaman and Kuttan, 2017).
Neonicotinoids
They affect insect nervous system on nicotinic receptors. They are systemic pesticides that used widely in seed treatments for complete removal of pests. Imidacloprid, Clothianidin, Acetamiprid, Dinotefuran and Thiamethoxam are common examples. They even affect beneficial insects like bees that decline the number and viability. They are called neonics because they are modeled from nicotine. They raise serious concerns for soil health and ecology due to their persistence and toxicity (Gabriela et al., 2024).
Soil Persistence
They are high persistence with different half-lives ranging from 100 to over 1,000 days depending on active compounds and soil conditions. For example, Imidacloprid is up to to 229–1,250 days while Thiamethoxam is similar to Clothianidin as long-lived duration. They moderately bind to soil particles however, they can still leach in sandy or low-organic soils (Zilli et al., 2020).
Soil Microorganisms
They are able to disturb soil microbial diversity particularly at activity level. They inhibit nitrogen-fixing bacteria, decomposers and fungi. They reduce soil respiration and nutrient cycling (Guimares et al., 2022).
Soil Enzyme Inhibition
All vital enzymes ar modified leading to slowly breakdown for organic matter and mineral availability (Chen et al., 2021).
Soil Fauna Impact
They are highly toxic to all types of minor organisms even Springtails and beneficial soil insects as well as they denature microbial population, soil structure and fertility (Onwona-Kwakye et al., 2020).
Environmental Risk
They are systemic that can be absorbed by whole plant tissues including pollen grains and nectar glands. They can leach into groundwater smoothly. They are toxic to pollinators, aquatic life and possibly human health (Zhang et al., 2021).
Highlight closure
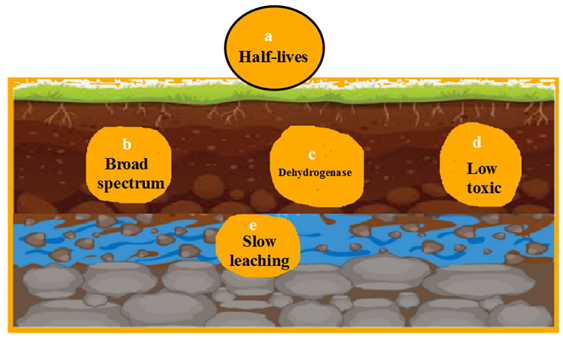
The insecticides have half-lives stability at soil structure. It influences on all types of microorganisms. The key enzymes are threated to be modified. Beneficial and non-beneficial invertebrates are affected. Leaching to groundwater is occurred (Fig:1).
Main Chemical Types of herbicides
Phenoxy Herbicides
They are categorized as herbicides such as Dicamba, 2,4-D (2,4-dichlorophenoxyacetic acid) and MCPA (2-methyl-4-chlorophenoxyacetic acid). They are selective commonly used to control broadleaf weeds in cereals and lawns by causing uncontrolled growth through mimic plant hormone stimulation. They have generally moderate soil impact. They cause environmental issues by improper use or long repetition (Hiller et al., 2012).
Soil Persistence
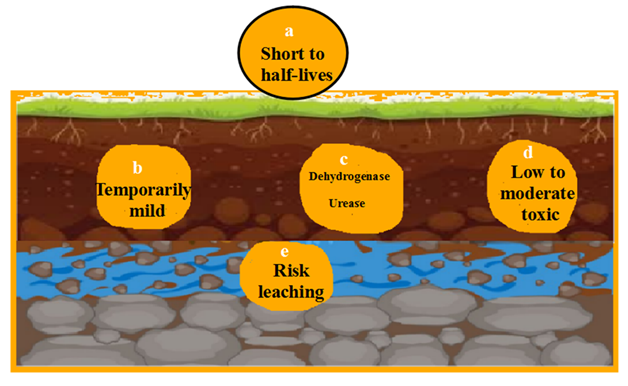
Half-life is between short to moderate typically 7 to 60 days depending on soil type, moisture, temperature and microbial activity. They are biodegradable by soil microbes that performed faster when conditions are warm and moist. They bind slowly to soil particles and penetrate downwards in light soils (Farenhorst et al., 2010).
Effects on Soil Microorganisms
At normal use levels, their effects are usually temporary and mild however, at high concentrations; they can reduce microbial activities, inhibit nitrogen-fixing bacteria and alter the balance of soil fungi and bacteria (Janniche et al., 2011).
Soil Enzyme Activity
They may inhibit dehydrogenase and urease briefly after applications however, enzyme activity can be recovered when herbicide degrades gradually (Gaultier et al., 2009).
Soil Fauna
Toxicity is low to moderate against earthworms and other soil invertebrates. They are usually non-lethal and short-lived unless misuse of application occurs (Xu et al., 2009).
Leaching Potential
Leaching risk is moderate especially in sandy or low-organic soils. They can contaminate groundwater if overused during heavy rains applies (David et al., 2013).
2.2. Triazines
They are Triazine herbicides such as Atrazine, Simazine, Propazine and Terbuthylazine that are widely used for controlling broadleaf and grassy weeds at Corn, sugarcane, and non-crop areas. They are known for their persistence and potential to contaminate soil and water. The main mode of action is inhibition of photosynthesis (Jasmin et al., 2011).
Soil Persistence
Half-life is long and ranges from 60 to over 300 days depending on atmospheric conditions. They bind moderately to soil particles especially in low-organic or sandy soils. There is a risk of accumulation with repeated use (Rocha et al., 2008).
Effects on Soil Microorganisms
They inhibit all beneficial and non-beneficial soil microbes whether bacteria or fungi besides alter microbial balance and reduce microbial biomass (Zhou et al., 2006).
Soil Enzyme Activity
Phosphatase, urease and dehydrogenase are inhibited as well as Triazine herbicides decrease the rate of nutrient cycling and breakdown the organic matter (Kolachi et al., 2010).
Soil Fauna Impact
They may harm earthworms and other soil invertebrates. In addition, the soil fauna impact may be generally occurred according to dose-dependent and become more severe with prolonged use (Afridi et al., 2006).
Leaching and Environmental Risk
High leaching is occurred and detected at groundwater especially when treatment of atrazine is executed. Persistent is distinguished at low-organic soils and areas with heavy rainfall (Katsumata et al., 2006).
Bipyridyls
Bipyridyls, mainly Paraquat and Diquat, are fast-acting, non-selective, quick burndown contract herbicides. They are widely used but recognized as high toxic to humans and animals with strong soil interaction (Nahim et al., 2021).
Soil Persistence
They bind tightly to soil particles especially clay ones besides organic matter. They are chemically inactive once bound but residues can remain for years. Hence, they don’t leach easily or even reach to groundwater (Rasheed et al., 2018).
Effects on Soil Microorganisms
Over time, microbial activity may recover after Bipyridyls treatments as the chemical compounds become immobilized but they are still harmful for all types of microorganisms (Kaur et al., 2016).
Soil Enzyme Activity
They can temporarily inhibit catalase enzyme in addition to urease and dehydrogenase. They are able to affect nutrient transformation and soil respiration (Brigante et al., 2013).
Soil Fauna
They are toxic to all forms of soil fauna like earthworms, insects and soil mites at application stages. Long-term impact is limited due to strong soil binding action (Fortenberry et al., 2016).
Environmental Considerations
They are highly toxic to humans and animals if ingestion or inhalation occur. Due to their immobilization, non-target plants can be injured and surface water may be contaminated if misapplied (Al-Ghouti and Da’ana, 2020).
Ureas and Substituted Ureas
Ureas and substituted ureas (e.g. Diuron, Monuron, Linuron) are herbicides that are used as pre-emergence control in agriculture and forestry. The main action is to inhibit photosynthesis (Ahmmed et al., 2024).
Soil Persistence
The persistence is moderate to high with half-lives that range from 60 to more than 180 days. Diuron is especially persistent, lasting months under dry or cold conditions. Slow degradation is occurred in low-organic or low microbial soils (Ni et al., 2023).
Soil Microbial Impact
Nitrogen-fixing bacteria and microbial decomposers can be inhibited leading to microbial reduction besides slowed nutrient cycling (Puga et al., 2020).
Soil Enzyme Activity
Phosphatase, urease and dehydrogenase are affected and enzyme recovery is slow due to long persistence (Ramalingappa et al., 2023).
Soil Fauna
They are toxic to soil invertebrates, especially at high concentrations. Long-term exposure can decline earthworm populations (Sangita et al., 2018).
Leaching and Runoff Risk
Leaching potential is moderate to high in sandy or low-organic soils. They may contaminate groundwater or surface runoff especially after Diuron treatment (Song et al., 2020).
Dinitroanilines
Dinitroanilines (e.g.Trifluralin, Pendimethalin) are pre-emergence herbicides that widely used to control grasses and some broadleaf weeds in row crops and turf through inhibition of cell division by assembly action of microtubules. Their influence on soil is generally less mobile but can affect microbial and biological activities over time (Jinyi et al., 2021).
Soil Persistence
They stay at soil with moderate to high persistence. Half-life ranges from 45 to more than 180 days. Their affinity to soil organic matter and clay particle is very high. Low mobility tends to remain near to soil surface layer (Soltani et al., 2020).
Soil Microbial Impact
Soil microbial impact is minimal to moderate level. Higher or repeated doses can reduce microbial biomass and nitrogen-fixing bacteria (Yu and Powles, 2014).
Soil Enzyme Activity
They can temporarily reduce dehydrogenase and phosphatase and impact negatively on microbial respiration and nutrient cycling (Lyons-Abbott et al., 2010).
Soil Fauna
They are toxic to earthworms and some beneficial insects located at soil surfaces. Long-term buildup may reduce soil biodiversity (Chen et al., 2019).
Leaching and Runoff Risk
Low leaching potential can be obtained due to strong absorption at soil textures. Runoff risk exists if they are applied before heavy rains on bare soil rocks (Délye, 2013).
Glyphosate (Organophosphonate)
Glyphosate is a widely used non-selective, systemic herbicide. Though it degrades relatively quickly, it can still influence on soil health. by inhibition of EPSP synthase (shikimic acid pathways) (Syrgabek and Alimzhanova, 2022).
Soil Persistence
Half-lives are short to moderate from 3 to 60 days. They bind strongly to soil particles, especially clay and organic matter. Low mobility present in moist soils but may persist longer in cold or low biological soils (Thind et al., 2018).
Microbial Impact
They can alter microbial communities by suppress nitrogenfixing bacteria and increase pathogenic fungi e.g. Fusarium sp. Some microbes can metabolize glyphosate as a phosphorus source (Suwardji and Made, 2021).
Soil Enzyme Activity
They may temporarily reduce dehydrogenase, phosphatase and urease. Enzyme activity can be recovered over time (Kanissery et al., 2019).
Soil Fauna
Low toxicity directs to earthworms and insects while they affect indirectly on food chains and microbial disruption occurs (Tarazona et al., 2017).
Leaching and Runoff Risk
Low leaching present due to strong soil particle affinity, however, runoff in sediments can be done after heavy rains (Wang et al., 2016).
Sulfonylureas
Sulfonylurea herbicides (e.g. metsulfuron-methyl, chlorsulfuron, tribenuron-methyl) are low-dose, selective post-emergence control that inhibit ALS enzyme for amino acid synthesis. They can significantly influence soil health under certain conditions (Timothy and Patrick, 2012).
Soil Persistence
Half-life is variable from 15 to more than 180 days. Longer persistence is remarkable in alkaline, dry or cold soils. They are more mobile in sandy or low-organic soils (Berisford et al., 2006).
Microbial Impact
- At normal use rates: minimal impact
At normal use rates, there is minimal impact on soil structure. However, high or repeated doses may inhibit soil fungi and bacteria besides affect nitrogen-fixing microbes and reduce microbial diversity (McCullough and Nutt, 2010).
Soil Enzyme Activity
They can suppress nitrate reductase, dehydrogenase and urease. They interfere with nitrogen and phosphorus cycling (Matocha and Senseman, 2007).
Soil Fauna
Generally, they are considered as low toxicity against earthworms and insects. There are indirect effects that may occur from altered microbial food base (Grey et al., 2007).
Leaching and Residual Effects
High mobility is found in high pH soils causing leaching risk to groundwater. They may injure sensitive crops in rotation if residues persist (Maheswari and Ramesh, 2007).
Imidazolinones
Imidazolinone herbicides (e.g. imazapyr, imazethapyr, imazamox) are systemic, broad-spectrum herbicides in crops like rice, soybeans that inhibit branched-chain amino acid synthesis by induction of ALS inhibitor. Their influence on soil is notable due to persistence and potential for mobility (Bundt et al., 2015).
Soil Persistence
Persistence is moderate to high with half-life ranges from 30 to more than 300 days. More persistent is present in alkaline soils with dry or low-organic conditions. They can remain active in soil for months. Negatively, they affect sensitive crops (Arbeli and Fuentes, 2007).
Microbial Impact
They can alter microbial communities by different ways; they suppress beneficial fungi and bacteria, reduce Rhizobium distribution. Some microbial species can degrade Imidazolinones slowly (Finlay, 2002).
Soil Enzyme Activity
They can inhibit dehydrogenase, urease and phosphatase and influence on diffusion of nutrient cycling and organic matter turnover (Mangels, 1991).
Soil Fauna
Low direct toxicity is detected; however, long-term residues may reduce food sources for earthworms and soil insects (Martinazzo, 2010).
Leaching and Carryover Risk
High mobility appears in sandy or alkaline soils. They can leach downwards to groundwater. Long residual activity may harm non-target crops in rotation time (Vischetti, 2000).
Glufosinate (Phosphinic Acid)
Glufosinate ammonium is a non-selective, contact herbicide used for post-emergence weed control for GM crops. Its mode of action is inhibition of glutamine synthetase (ammonia accumulation). Its influence on soil is generally limited compared to more persistent herbicides (Xiaoyun et al., 2023).
Soil Persistence
Low persistence is accomplished with typical half-life from 3 to 25 days. Glufosinate ammonium is degraded rapidly by soil microbes. It binds moderately to soil particles so that limiting mobility is present (Takano and Dayan, 2020).
Microbial Impact
At normal use, minimal effect on microbial populations is done. High doses may temporarily suppress nitrogen-fixing bacterial and fungal activity (Yang et al., 2019).
Soil Enzyme Activity
Minor and short-term inhibition on dehydrogenase and phosphatase are recognized by using Glufosinate ammonium. Enzyme activity can be recovered quickly after breakdown process (Bera and Ghosh, 2013).
Soil Fauna
Glufosinate ammonium has low toxicity against earthworms and beneficial insects. No significant long-term impact is occurred under recommended usage (Zhu et al., 2015).
Leaching and Environmental Risk
Low leaching potential can be carried out especially in medium to high organic matter soils. Unlikely, groundwater can be contaminated under typical field conditions (Shennan, 2008).
Benzoic and Carboxylic Acids
Benzoic and carboxylic acid herbicides (e.g. Dicamba, Picloram, 2,3,6-TBA) are synthetic auxins used to control broadleaf weeds as pasture management by inhibiting growth regulators. Their influence on soil depends on their specific chemical structure and environmental conditions (Tamara et al., 2022).
Soil Persistence
There is variable perisitence; Dicamba possess short to moderate from 1 to 60 days while Picloram is very persistent up to 1 year or more. As general, all benzoic and carboxylic acid herbicides have more persistent in dry, cold or low-organic soils (Ransom et al., 2014).
Microbial Impact
At normal rates, minimal microbial impact is occurred. Picloram can suppress microbial activity and degrade organic matter very slowly. Benzoic and carboxylic acid herbicides may slightly reduce bacterial diversity in some soils under different circumferences (Bish et al., 2019).
Soil Enzyme Activity
Temporary reduction at enzyme availability is occurred for dehydrogenase and urease. Enzyme levels can be recovered when the herbicide is broken down (Silva et al., 2020).
Soil Fauna
Earthworms and insects are toxin with low level. Moreover, Picloram may indirectly affect soil fauna by altering microbial balance (Tropaldi et al., 2021).
Leaching and Runoff Risk
Picloram possesses high leaching potential especially in sandy or alkaline soils. Moderate mobility of Dicamba can be executed at rain-prone areas (Carbonari et al., 2020).
Highlight closure
Soil persistence appears from short to half-lives while soil microbes are affected with medium level and reversible action may be done. Dehydrogenase and urease are denatured. Not all invertebrates are influenced however, leaching to groundwater is dangerous (Fig. 2).
Main Chemical Types of fungicides
Benzimidazoles
Benzimidazole fungicides are primarily systemic fungicides that disrupt fungal cell division by tubulin interference and are used to control a wide range of plant pathogens at fruits, vegetables, cereals. Common examples include Benomyl, Carbendazim and Thiophanate-methyl. Their influence on soil profiles varies by application rate and frequency (Radka, 2022).
Soil Persistence
Soil persistence is moderate where benomyl rapidly converts to carbendazim in soil structure. Half-lives range from 3 to 12 months, depending on soil type and conditions of irrigation (Syslova et al., 2019).
Effects on Soil Microorganisms
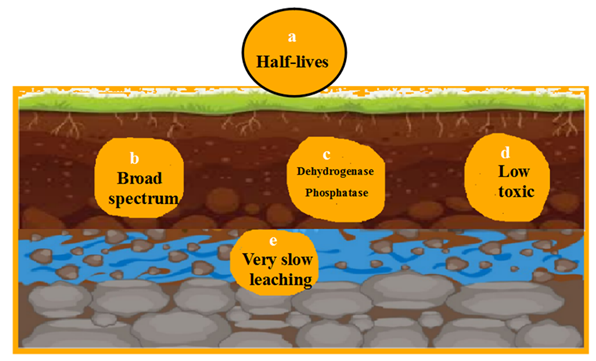
They are broad-spectrum antifungal agent that can suppress all types of fungi even beneficial ones like mycorrhizae besides soil bacterial communities. They may reduce fungal diversity and distort nutrient uptake and organic matter breakdown (Podlipna et al., 2021).
Soil Enzyme Activity
They inhibit major two key enzymes; dehydrogenase and phosphatase leading to temporary reduction in microbial activity and nutrient cycling (Navratilova et al., 2021).
Soil Fauna
Generally, low toxicity is recorded at Benzimidazole treatments against earthworms and non-target insects. Chronic use can cause subtle long-term effects on soil invertebrate distribution (Dogra et al., 2018).
Environmental Risk
Low leaching risk is remarkable due to moderate soil particle binding action. Breakdown products of Benzimidazole may still be bioactive or toxic (Bisognin et al., 2021).
Triazoles (DMI fungicides)
Triazoles (DMI fungicides) like tebuconazole, propiconazole and difenoconazole are systemic fungicides that inhibit fungal sterol biosynthesis in fungal membranes. They are broad-spectrum used at cereals, fruits and turf. Their influence on soil includes moderate persistence and impacts on microbial and enzymatic activity (Anastasia et al., 2023).
Soil Persistence
Soil persistence is moderate to high with half-life ranges from 30 to more than 400 days. It extends to be long period in cold, dry, alkaline or low-microbial soils. Triazoles combine to soil organic matter so that reducing mobility and increasing longevity are performed (Roman et al., 2021).
Microbial Impact
Triazoles can alter microbial community composition. They suppress beneficial fungi e.g. mycorrhizae. They may reduce bacterial activities at high doses treatment. Finally, overuse may lead to fungal resistance forming soil microbial imbalance (Han et al., 2019).
Soil Enzyme Activity
They inhibit all key enzymes; dehydrogenase, urease and phosphatase. They may impair nitrogen and phosphorus transformation (Mohiddin et al., 2021).
Soil Fauna
Low to moderate toxicity to earthworms and microarthropods is occurred. Indirect effects reduce microbial food sources (Wachowska et al., 2020).
Leaching and Environmental Risk
Low leaching potential is displayed due to soil binding action. Risk of residue buildup with repeated application can be done (Lloyd et al., 2021).
Strobilurins (QoI fungicides)
Strobilurins (e.g. Azoxystrobin, Pyraclostrobin, Trifloxystrobin) are broad-spectrum systemic fungicides that inhibit fungal mitochondrial respiration by action on cytochrome bc1 complex. Their influence on soil is important to consider due to persistence and microbial effects (Rasha and Mohamed, 2021).
Soil Persistence
Moderate to high persistence is significant. Half-life ranges 30 to 200+ days. Persistence increases in low-organic, dry, or cold soils. Strobilurins bind strongly to soil particles, especially organic matter (Feng et al., 2020).
Microbial Impact
Strobilurins can reduce beneficial fungi, including mycorrhizal associations and decomposer fungi. They may affect bacterial diversity at higher concentrations. They alter microbial balance, especially with repeated use (Hnátová et al., 2003).
Soil Enzyme Activity
Soil enzyme activity is suppressed. Dehydrogenase, urease and phosphatase are inhibited. Nutrient cycling and organic matter breakdown are controlled with negative impacts (Isamu and Makoto, 2005).
Soil Fauna
Low direct toxicity is exposed to earthworms and insects at field rates. Indirect impacts reduce microbial food base or enzyme shifts (Balba, 2007).
Leaching and Runoff Risk
Low leaching potential is done due to strong adsorption on soil particles. Risk of surface runoff is accomplished if applied before heavy rain (Rodrigues et al., 2013).
Dithiocarbamates
Dithiocarbamates (e.g. mancozeb, zineb, thiram) are broad-spectrum, contact fungicides used widely on fruits, vegetables, and field crops. The mode of action is denaturation of effective multi-active sites of vital enzymes. Their influence on soil can be significant, especially with repeated use (Claudia et al., 2023).
Soil Persistence
It is low to moderate persistence. Three to seventy days are half-life range time. Dithiocarbamates are degraded faster in warm, moist, and biologically active soils. Breakdown can release toxic byproducts like ethylene thiourea (ETU) (Catalina et al., 2014).
Microbial Impact
Dithiocarbamates can suppress soil microbial populations, including: nitrogen-fixing bacteria and decomposer fungi. Long-term use may reduce microbial diversity and biomass (Stadler et al., 2022).
Soil Enzyme Activity
Dehydrogenase, phosphatase and urease are inhibited. Dithiocarbamates slow nutrient cycling and organic matter decomposition (Öter and Zorer, 2021).
Soil Fauna
Dithiocarbamates are toxic to earthworms and nematodes, especially thiram. They can reduce soil invertebrate populations if overapplied (Losacco et al., 2022).
Leaching and Residue Risk
Generally low leaching is done. breakdown products like ETU are mobile and toxic. ETU is carcinogenic and can contaminate groundwater (Caiel et al., 2021).
Chloronitriles
Chloronitriles (mainly chlorothalonil) are broad-spectrum, protectant contact fungicides widely used in agriculture. They inhibit multiple metabolic pathways by deactivation of enzymes. Their influence on soil can be notable due to their chemical stability and impact on soil biology (Malgorzata et al., 2018).
Soil Persistence
It is moderate to high. Half-life is 30 - <120 days. There is strong binding between Chloronitriles and soil organic matter, especially in clay or humus-rich soils. Breakdown is slower in cool, dry, or low-microbial soils (Shahgholi, 2014).
Microbial Impact
Toxicity is dispread into many soil microorganisms like fungi and nitrogen-fixing bacteria. There is a reduction at microbial diversity and a disturbance occurs at nutrient cycling (Pimmata et al., 2013).
Soil Enzyme Activity
Inhibition occurs at dehydrogenase, urease and phosphatase. Soil enzyme activity may be suppressed for soil fertility functions for weeks (Oleszczuk et al., 2014).
Soil Fauna
Earthworms and other invertebrates have been eliminated due to toxicity. Chronic exposure can reduce soil biological activity (Srinivasulu and Rangaswamy, 2013).
Leaching and Environmental Risk
Low leaching is performed due to strong soil binding mechanism. However, toxic metabolites may accumulate in soil or enter runoff if misapplied (Lozowicka et al., 2016).
Phthalimides
Phthalimides, such as Captan and Folpet, are contact fungicides used mainly in fruit, seeds, ornamental and vegetable crops by interference with multi-active sites of major enzymes. Their influence on soil is generally short-term, but repeated or heavy use can impact soil biology (Bernard et al., 2022).
Soil Persistence
Low to moderate persistence and half-life of 3–30 days are occurred. Presence of rapid degradation is displayed by soil microbes and hydrolytic mechanical methods. Persistence increases in cold, dry, or low-biological activity soils (Li et al., 2022).
Microbial Impact
At normal application rates, minimal to moderate effects are present. High or repeated doses may suppress fungi more than bacteria as well as reduce temporarily microbial diversity (Al-Jaroudi et al., 2016).
Soil Enzyme Activity
Soil enzyme activity can be inhibited like dehydrogenase and phosphatase. These effects are usually short-lived (Patil et al., 2017).
Soil Fauna
Low toxicity to earthworms and soil insects is detected at standard doses. Some negative effects may occur at high concentrations or in sensitive soils (Castro-Castillo et al., 2013).
Leaching and Runoff Risk
Leaching potential is low while breakdown products generally bind to soil or degrade quickly (Dempster and Luzzio, 2011).
Sulfur Compounds
Sulphur-based fungicides (e.g. elemental sulphur, lime sulphur, wettable sulphur) are traditional, broad-spectrum contact fungicides against Powdery mildew. They disturb fungal respiratory mechanisms. Their influence on soil is generally mild, but can accumulate with frequent use (Ashish et al., 2020).
Soil Persistence
Sulphur oxidizes in soil to sulfates (SO42-) forming low persistence. Oxidation rate depends on moisture, temperature, and microbial activity (Arvind et al., 2018).
Microbial Impact
Mild microbial impact effects at normal doses are determined. High application rates can lower soil pH, suppress temporarily fungi and some bacteria and increase beneficial microbes like sulfur-oxidizing bacteria (Kumar et al., 2013).
Soil Enzyme Activity
Generally there is low soil enzyme impact that may slightly affect urease and phosphatase. On the other hand, Enzyme activity usually recovers quickly (Singh et al., 2015).
Soil Fauna
Low toxicity to earthworms and invertebrates is done while no significant long-term effects under standard use can be detected (Rai and Singh, 2018).
Leaching and Environmental Risk
Low leaching potential is present. Sulfates formed are generally stable and part of natural sulfur cycling (Girija et al., 2020).
Copper-based Compounds
Copper-based compounds (e.g. copper sulfate, copper oxychloride, Bordeaux mixture) are widely used as fungicides and bactericides, especially in organic farming at fruits, vegetables and vineyards. They pose risks to soil health when overused because they may be accumulated in soil over time (Martin et al., 2025).
Soil Persistence
They are very persistent because copper mixtures aren’t degraded. Accumulation at soil profile is observed with repeated applications. Long-term buildup can lead to copper toxic percentage (Tseng et al., 2021).
Effects on Soil Microorganisms
They are broad spectrum for all types of microorganisms whether beneficial or not that reduce microbial biomass and diversity over time (Vazquez-Blanco et al., 2020).
Soil Enzyme Activity
All key enzymes are inhibited with remarkable reduction of soil respiration and slower nutrient cycling (Wang et al., 2022).
Soil Fauna
High concentrations impair soil structure and aeration resulting from their high toxicity against all soil fauna members (Zhao et al., 2022).
Soil Properties
Copper mixtures bind strongly to clay particles and organic matter but excessive buildup may be occurred. Consequently, they alter soil PH and cation exchange capacilty. They interfere deeply with plant nutrient uptake especially iron and zinc (You et al., 2018).
Highlight closure
Fungicides stay medium period. They infect all types of microorganisms while not all key enzymes are affected. Similarly, not all invertebrates are killed. The rate of fungicide molecules penetration is not dangerous (Fig. 3).
Main Chemical Types of bactericides
Copper-Based Bactericides
Copper-based bactericides (e.g. copper hydroxide, copper sulfate, copper oxychloride and Bordeaux mixture) act similarly to copper fungicides by denaturing proteins and enzymes in bacteria using in fruits, vegetables, and vineyards. When used repeatedly, they accumulate in the soil and can significantly affect soil biology and quality (Yue et al., 2023).
Soil Persistence
Copper is a heavy metal and non-degradable creates very high persistence. It remains in soil for years to decades. It binds to organic matter and clay, limiting mobility but not toxicity (Marckmann et al., 2019).
Microbial Impact
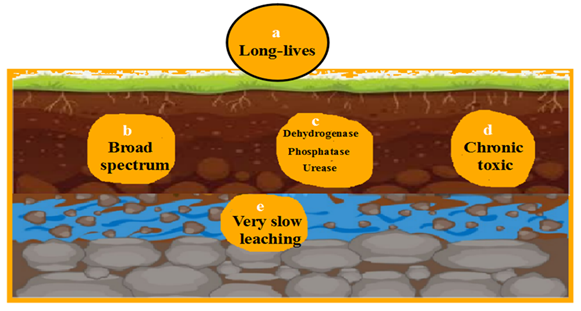
It is broad-spectrum toxicity to soil microbes as it inhibits nitrogen-fixing bacteria (e.g. Rhizobium). It reduces nitrifiers, decomposers, and overall microbial diversity. Repeated use can cause long-term shifts in microbial balance (Straw et al., 2018).
Soil Enzyme Activity
Strong inhibition of dehydrogenase, urease and phosphatase with slow rate of nutrient cycling and reduced soil fertility (Tarakanov et al., 2023).
Soil Fauna
It is toxic to earthworms, springtails, and beneficial nematodes. Accumulation may result in chronic toxicity and biodiversity loss (Sadek et al., 2022).
Leaching and Environmental Risk
Low leaching in most soils is found. Risk of runoff into waterways, where copper is toxic to aquatic life (Aien et al., 2023).
Quaternary Ammonium Compounds (QACs)
Quaternary ammonium compounds (QACs) e.g. benzalkonium chloride, didecyldimethylammonium chloride (DDAC) are disinfectants, surface sterilants bactericides often used in agriculture not typically applied directly to crops, greenhouses, and livestock settings. They disturb bacterial cell membranes. Their influence on soil depends on usage intensity and soil type (Ines et al., 2018).
Soil Persistence
It is moderate to high persistence that can last days to weeks. It strongly adsorbs to clay and organic matter, reducing mobility but potentially accumulating with repeated use (Ding et al., 2013).
Microbial Impact
It acquires with broad-spectrum antimicrobial action through killing Gram-positive and Gram-negative bacteria and suppressing beneficial microbes (e.g. Rhizobium, Pseudomonas). It can significantly reduce microbial biomass and diversity with overuse (Droge and Goss, 2013).
Soil Enzyme Activity
It may inhibit dehydrogenase and urease. It destroys nutrient cycling, especially nitrogen transformation (Ertekin et al., 2016).
Soil Fauna
It is toxic to soil invertebrates (e.g. earthworms, nematodes) at high doses. Indirect effects may be carried out from disrupted microbial food sources (Fo¨rster et al., 2008).
Leaching and Environmental Risk
Low leaching may be appeared due to strong soil binding action. Risk of runoff to water bodies is executed besides QACs are toxic to aquatic organisms (Fraise, 2011).
Silver-Based Compounds
Silver-based bactericides (e.g. silver nitrate, silver nanoparticles) are effective for experimental or high-value crops against a wide range of microbes by integrity within DNA replication and enzyme function, but their influence on soil can be significant due to their persistence and toxicity (Tsepina et al., 2022).
Soil Persistence
High persistence is translated into no degradation, especially in metallic or nanoparticle form. Silver components bind strongly to organic matter and clay, but remain biologically active (Neukum, 2018).
Microbial Impact
It is highly toxic and impact to soil microbes by enabling aseptic conditions against bacteria, fungi and actinomycetes. It inhibits nitrifiers, nitrogen-fixers, and decomposers as well as it reduces microbial diversity and enzyme production (Braun et al., 2015).
Soil Enzyme Activity
Strong inhibition is occurred of dehydrogenase, urease and phosphatase. It slows nutrient cycling and organic matter decomposition (Hedberg et al., 2015).
Soil Fauna
It is toxic to earthworms, nematodes, and microarthropods. It can bioaccumulate and cause long-term soil ecosystem imbalance (Savignan et al., 2023).
Leaching and Environmental Risk
Low mobility is present for silver molecules, but they may leach under certain conditions (e.g. low pH, high salt). It is very toxic to aquatic organisms if runoff occurs (Rehan et al., 2017).
Highlight closure
On contrary, bactericides have permanent persistence with severe aseptic against wide number of microbes. All types of soil enzymes are denatured. The degree of toxicity spread all types of invertebrates however, the rate of fungicide molecules penetration is limited (Fig. 4).
Main Chemical Types of viricides
Viricides (antiviral agents for plants) are fewer and less specific than fungicides or bactericides. They mainly work by inhibiting viral replication, inducing plant resistance, or inactivating viruses directly.
Ribavirin-Like Compounds
Ribavirin-like compounds like thiouracil derivatives (broad-spectrum antiviral agents) are not widely used as viricides in agriculture, but their analogs may be tested or studied for plant virus control by inhibiting viral RNA synthesis or replication. If applied to soil or released through treated plant residues. It is limited use in plants due to toxicity and cost (Xiaohui et al., 2024).
Soil Persistence
Soil persistence is moderate that may remain for days to weeks. Ribavirin-like compounds are subjected to degradation by soil microbes and hydrolysis. Persistence varies with temperature, pH, and microbial activity (Karjadi and Karjadi, 2022).
Microbial Impact
Ribavirin-like compounds can affect RNA-dependent processes in soil microbes that may suppress beneficial bacteria (e.g. Rhizobium, Pseudomonas), some fungi and actinomycetes. Broad-spectrum activity could alter microbial balance (Morales-Paredes et al., 2022).
Soil Enzyme Activity
Soil enzyme activity is potential to be inhibited nucleic acid-related enzymes in microbes. Ribavirin-like compounds may reduce dehydrogenase and urease activity temporarily (Shang et al., 2019).
Soil Fauna
There is limited data for soil fauna impact by Ribavirin-like compounds but they are potential for low to moderate toxicity to soil invertebrates. Risk increases with repeated exposure or high concentrations (Shi et al., 2018).
Leaching and Environmental Risk
Low to moderate leaching is obtained, depending on solubility and soil type. Possible contamination of water sources is occurred if applied in large volumes or poorly managed (Su et al., 2023).
Nanoparticles
Nanoparticle-based viricides (e.g. silver, zinc oxide, copper oxide, chitosan, silica nanoparticles) are emerging tools for plant virus control by interfering with virus particles or replication. Their influence on soil depends on the type, concentration, and frequency of use. It is a new approach that is experimental and increasingly studied in plant virology (Joanna et al., 2024).
Soil Persistence
High persistence is present especially for metal-based nanoparticles (Ag, Cu, Zn) that are considered as non-degradable and may accumulate in soil over time (Kulthong et al., 2010).
Microbial Impact
Nanoparticle-based viricides have broad-spectrum antimicrobial and antiviral effects by inhibiting virus-infected plant residue decomposition and kills bacteria, fungi, and actinomycetes. They reduce microbial diversity and interfere with nitrogen cycling (El Badawy et al., 2010).
Soil Enzyme Activity
Dehydrogenase, urease and phosphatase are inhibited after Nanoparticle-based viricides usage. They disrupt nutrient cycling and organic matter decomposition (Klitzke et al., 2015).
Soil Fauna
They are toxic to earthworms, nematodes, and microarthropods at high doses. Bioaccumulation risk in soil organisms with repeated application is done (Pachapur et al., 2016).
Leaching and Environmental Risk
Low mobility is the property of nanoparticle-based viricides but may be leached as ions (e.g. Ag+, Zn2+) under acidic or saline conditions. They are generally toxic to aquatic life if runoff occurs (Vance et al., 2015).
Highlight closure
The soil persistence is limited range period. Viricides have vigorous effect on all types of microorganisms besides virus and viroids. The key enzymes have limited effect. Although microorganisms are more affected, viricides are little effective on macroorganisms. Groundwater is affected with medium level (Fig. 5).
Conclusion
The chemical pesticides have negatively effects on soil structure whether physical impacts presenting at leaching property to groundwater, chemical impacts representing at different chemical structure and chelation abilities as well as biological impacts representing at microbial and fauna denaturation. Alternative pesticides should be used nowadays for conserving environment from more unhealthy impacts. Natural products or semi-synthetic presides are suggested solutions to carry out the target of pathogen elimination besides safety tools for all abiotic factors like air, water and soils.
References
- Al-Ghouti MA, and Da’ana D. Guidelines for the use and interpretation of adsorption isotherm models: a review. Hazard. Mater 393 (2020): 122383.
- Al-Jaroudi Z, Mohapatra PP, Jha A. Facile synthesis of 3-substituted isoindolinones. Tetrahedron Lett 57 (2016): 772–777.
- Adamski Z, Bloszyk J, Piosik K and et al. Effects of diflubenzuron and mancozeb on soil microarthropods: a long-term study. Lett 46 (2009): 3–13.
- Afridi HI, Kazi TG, Kazi GH. Analysis of heavy metals in scalp hair samples of hypertensive patients by conventional and microwave digestion methods. Spectrosc Lett 39 (2006): 1–12.
- Ahmmed MM, Abd Wahid S, Abba N, et al. Urea application in soil: processes, losses, and alternatives—a review. Discover Agric 2 (2024): 42.
- Aien J, Khan AA, Haq S, et al. Antibacterial, antioxidant and physicochemical properties of Piper nigrum-aided copper oxide nanoparticles. Crystals 13 (2023): 330.
- Alford A, Krupke CH. Translocation of the neonicotinoid seed treatment clothianidin in maize. PLoS One 12 (2017): e0173836.
- Vasilchenko AV, Poshvina DV, Semenov MV, et al. Triazoles and strobilurin mixture affects soil microbial community and incidences of wheat diseases. Plants 12 (2023): 660.
- Anderson JC, Dubetz C, Palace VP. Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci Total Environ 505 (2015): 409–22.
- Anjum SA, Ashraf U, Khan I, et al. Aluminum and aluminum oxide nanoparticles alter wheat productivity, traits, physiology, and biochemical processes. Environ Sci Pollut Res 28 (2021): 30581–94.
- Arora NK, Verma M. Modified atmosphere packaging for extending shelf life of fresh horticultural produce: a review. Int J Curr Microbiol Appl Sci 7 (2018): 170–83.
- Arora S, Rajwade JM, Paknikar KM. Nanotoxicology and in vitro studies: the need of the hour. Toxicol Appl Pharmacol 258 (2012): 151–65.
- Azeem F, Ahmad M, Qamar SA, et al. Effect of pesticides on soil microbial communities and enzymes: a review. Environ Chem Lett 22 (2024): 1–20.
- Balba H. Review of strobilurin fungicide chemicals. J Environ Sci Health B 42 (2007): 441–51.
- Banks KE, Hunter DL, Wachal DJ. Chlorpyrifos in surface waters before and after a federally mandated ban. Environ Int 37 (2011): 637–43.
- Bansal OP. Impact of pesticides on soil microbial communities and enzyme activities. J Pestic Sci 42 (2017): 1–6.
- Bargaz A, Lyamlouli K, Chtouki M, et al. Soil microbial inoculants for sustainable agriculture: review and perspectives. Agronomy 8 (2018): 241.
- Bass C, Denholm I, Williamson MS, Nauen R. The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol 121 (2015): 78–87.
- Battaglin WA, Sandstrom MW, Kuivila KM, et al. Occurrence of azoxystrobin, propiconazole, and selected other fungicides in US streams, 2005–2006. Water Air Soil Pollut 204 (2009): 275–84.
- Benbrook CM. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28 (2016): 3.
- Bennet AJ, Leifert C, Whipps JM. Effect of organic manures and composts on soil fertility and soil microbial activity. Biol Fertil Soils 25 (1997): 175–81.
- Berg G, Köberl M, Rybakova D, et al. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol Ecol 93 (2017): fix050.
- Bernhardt ES, Rosi EJ, Gessner MO. Synthetic chemicals as agents of global change. Front Ecol Environ 15 (2017): 84–90.
- Bhardwaj A, Sharma D, Jadhav K, et al. Toxic effects of pesticides on human health. J Entomol Zool Stud 8 (2020): 1950–55.
- Bhattacharyya A, Chakraborty S, Kumar R, et al. Effect of pesticide application on soil microorganisms. Int J Curr Microbiol Appl Sci 8 (2019): 2252–59.
- Bhushan B, Chauhan R, Sharma A, et al. Soil microbial diversity and its role in maintaining soil health. Int J Chem Stud 8 (2020): 217–22.
- Bhandari G, Zomer P, Atreya K, et al. Contamination of vegetables with pesticides in Nepal. J Agric Food Chem 71 (2023): 3735–42.
- Biddinger D, Rajotte EG. Integrated pest management: concept, tactics, strategies and case studies. Entomol Res 39 (2009): 89–101.
- Black RE, Levin C, Walker N, et al. Reproductive, maternal, newborn, and child health: key messages from disease control priorities, 3rd edition. Lancet 388 (2016): 2811–24.
- Blaser MJ. Antibiotic use and its consequences for the normal microbiota. Science 352 (2016): 544–45.
- Bollag JM, Myers CJ, Minard RD. Biological and chemical interactions of pesticides with soil organic matter. Sci Total Environ 123 (1992): 205–17.
- Bonmatin JM, Giorio C, Girolami V, et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22 (2015): 35–67.
- Borges RC, Gomes NC, Soares A, et al. Impact of pesticides on microbial diversity in soil and water environments. Ecotoxicol Environ Saf 206 (2020): 111282.
- Bottrell DG. Integrated pest management. Council Agric Sci Technol Rep 48 (1979): 1–10.
- Boucias DG, Stokes C, Storey G, Pendland JC. The effects of imidacloprid on the termite Reticulitermes flavipes and its gut microbial community. Soil Biol Biochem 33 (2001): 1401–07.
- Bove FJ. Birth outcomes following pesticide exposure during pregnancy. Environ Health Perspect 110 (2002): 1099–1104.
- Brar SK, Verma M, Tyagi RD, Surampalli RY. Engineered nanoparticles in wastewater and wastewater sludge—evidence and impacts. Waste Manag 30 (2010): 504–20.
- Brausch JM, Rand GM. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82 (2011): 1518–32.
- Brevik EC, Burgess LC. The influence of soil on human health. Sci Total Environ 733 (2020): 139397.
- Brookes G, Barfoot P. Economic impact of GM crops: the global income and production effects 1996–2006. AgBioForum 11 (2008): 21–38.
- Brown ME, Hazen TC. Microbial communities and processes in contaminated soils and sediments. Microb Ecol 65 (2013): 1–3.
- Bruinsma J. The resource outlook to 2050: by how much do land, water and crop yields need to increase by 2050? Expert Meeting on How to Feed the World in 2050. FAO, Rome (2009).
- Bryan NS, Loscalzo J. Nitrite and nitrate in human health and disease. Springer Science & Business Media (2011).
- Buchholz K, Schirmer M, Meuli RG, et al. Pesticide contamination in groundwater: a review. Water Res 175 (2020): 115678.
- Bünemann EK, Bongiorno G, Bai Z, et al. Soil quality – a critical review. Soil Biol Biochem 120 (2018): 105–25.
- Burke DJ, Weintraub MN, Hewins CR, et al. Effects of pesticide application on soil enzyme activities. Appl Soil Ecol 53 (2012): 44–50.
- Burns RG, Dick RP. Enzymes in the Environment: Activity, Ecology, and Applications. CRC Press, Boca Raton (2002).
- Businelli D, Gigliotti G, Giusquiani PL, et al. Long-term repeated applications of municipal waste compost: effects on soil quality. Soil Biol Biochem 31 (1999): 169–74.
- Calderón MJ, Posada-Baquero R, Peña A. Pesticides in soils: analytical methods and ecological risk assessment. Trends Environ Anal Chem 25 (2020): e00080.
- Calderón MJ, Villafranca-Sánchez M, Rodríguez-Liébana JA, Peña A. Impact of pesticides on soil and water. Appl Sci 10 (2020): 3934.
- Carvalho FP. Agriculture, pesticides, food security and food safety. Environ Sci Policy 9 (2006): 685–92.
- Castañeda M, Pérez-Rodríguez P, Pérez-Santín E, et al. Effects of pesticides on microbial biomass and enzyme activities in agricultural soils. Int J Environ Res Public Health 17 (2020): 7112.
- Cederlund H, Börjesson E, Stenström J. Effects of pesticides on microbial communities in agricultural soils. Soil Biol Biochem 42 (2010): 1164–73.
- Chagnon M, Kreutzweiser D, Mitchell EA, et al. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ Sci Pollut Res 22 (2015): 119–34.
- Chakraborty S, Newton AC. Climate change, plant diseases and food security: an overview. Plant Pathol 60 (2011): 2–14.
- Chandra R, Bharagava RN, Yadav S, et al. Impact of pesticides on soil microbiological health. Biorem J 22 (2018): 1–20.
- Chatterjee S, Poddar S, Ghosh S, et al. Nanoparticles in agro-environment: fate, transport, and toxicity. Environ Chem Lett 17 (2019): 1005–22.
- Chen M, Qiu L, Deng Z, et al. Impact of pesticides on soil enzyme activity: a review. Environ Sci Pollut Res 29 (2022): 44242–60.
- Chen S, Hu M, Liu J, et al. Soil microbial diversity loss caused by pesticides: a review. Environ Pollut 308 (2022): 119609.
- Cheng F, Cheng Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci 6 (2015): 1020.
- Chikere CB, Surridge AK, Okpokwasili GC, et al. Impacts of pesticide pollution on soil microbial communities. Appl Environ Soil Sci 2019 (2019): 1–15.
- Chowdhury A, Pradhan S, Saha M, et al. Impact of pesticides on soil microbial biomass and enzyme activities in agricultural soils. Environ Monit Assess 186 (2014): 6165–74.
- Cloyd RA. Pesticides and beneficial insects: limitations of the pesticide toxicity “red list” database. Insects 13 (2022): 719.
- Cloyd RA, Bethke JA. Impact of neonicotinoids on beneficial insects in greenhouse and nursery production. J Integr Pest Manag 2 (2011): I1–I9.
- Cobb GP, Norman DM, Kendall RJ, et al. Effects of agricultural chemicals on soil and aquatic ecosystems. Environ Toxicol Chem 14 (1995): 537–41.
- Coleman DC, Crossley DA, Hendrix PF. Fundamentals of Soil Ecology. Academic Press, San Diego (2004).
- Collins C, Fryer M, Grosso A. Plant uptake of non-ionic organic chemicals. Environ Sci Technol 40 (2006): 45–52.
- Colvin J, Omongo CA, Govindappa MR, et al. Pest and disease management in organic farming. Org Agric 4 (2014): 163–79.
- Cooper J, Dobson H. The benefits of pesticides to mankind and the environment. Crop Prot 26 (2007): 1337–48.
- Crouzet O, Batisson I, Besse-Hoggan P, et al. Response of soil microbial communities to the herbicide isoproturon. Environ Sci Pollut Res 17 (2010): 913–24.
- Cycon M, Piotrowska-Seget Z. Changes in bacterial diversity and community structure following pesticides addition to soil: a review. Int J Environ Res Public Health 13 (2016): 124.
- Cycon M, Markowicz A, Borymski S, Piotrowska-Seget Z. Imidacloprid induces changes in the structure and metabolic diversity of soil microbial communities. J Environ Manage 131 (2013): 55–65.
- Dai Y, Ning D, Huang X, et al. Long-term effects of pesticides on soil microbial communities: a review. Soil Ecol Lett 2 (2020): 1–17.
- Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health 8 (2011): 1402–19.
- Daniel R. The soil metagenome—a rich resource for the discovery of novel natural products. Curr Opin Biotechnol 15 (2004): 199–204.
- Dasgupta S, Meisner C, Wheeler D, et al. Pesticide use in India: trends, patterns, and determinants. World Bank Policy Research Working Paper (2007): 1–28.
- De A, Bose R, Kumar A, et al. Worldwide pesticide use. Environ Sci Pollut Res 29 (2022): 42804–15.
- DeLorenzo ME, Scott GI, Ross PE. Toxicity of pesticides to aquatic microorganisms: a review. Environ Toxicol Chem 20 (2001): 84–98.
- Demena BA, Worku T, Teshome B, et al. Effects of pesticides on soil microbial diversity: a review. Agric Res 12 (2023): 120–31.
- Denno RF, McClure MS, Ott JR. Interspecific interactions in phytophagous insects: competition reexamined and resurrected. Annu Rev Entomol 40 (1995): 297–331.
- Desneux N, Decourtye A, Delpuech JM. The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52 (2007): 81–106.
- Devine GJ, Furlong MJ. Insecticide use: contexts and ecological consequences. Agric Human Values 24 (2007): 281–306.
- Dhaliwal GS, Jindal V, Dhawan AK. Insect pest problems and crop losses: changing trends. Indian J Ecol 37 (2010): 1–7.
- Díaz-Chávez J, Rodríguez-Ruíz V, Ortega-Amaro MA, et al. Impact of pesticides on the rhizosphere microbiome: a review. Rhizosphere 21 (2022): 100457.
- Ding C, He J, Zhong Z, et al. Effects of pesticides on soil microbial community composition and function. Environ Sci Pollut Res 27 (2020): 43523–35.
- Divya B, Swetha V, Prasanthi K, et al. Effects of insecticides on soil enzymes. J Pharmacogn Phytochem 8 (2019): 512–15.
- Dores EFGC, Freire R. Contamination of groundwater and surface water by pesticides in cotton-growing areas of Mato Grosso, Brazil. Environ Sci Pollut Res 19 (2012): 4231–44.
- Doumbia M, Kwadjo KE. Pesticide practices and environmental risks in cotton-growing areas of Côte d’Ivoire. Agron Afr 23 (2011): 273–83.
- Dubey SK, Fulekar MH. Effect of pesticides on soil microbial community and enzyme activity. Int J Curr Microbiol Appl Sci 2 (2013): 108–14.
- Durán-Lara EF, Valderrama A, Marican A. Natural organic compounds for applications in crop protection. Molecules 25 (2020): 761.
- Eddleston M, Karalliedde L, Buckley N, et al. Pesticide poisoning in the developing world—a minimum pesticides list. Lancet 360 (2002): 1163–67.
- Edwards CA. The environmental impact of pesticides. In: Pimentel D (ed). CRC Handbook of Pest Management in Agriculture. CRC Press, Boca Raton (1991): 543–85.
- Edwards CA. Soil pollution by pesticides. Soil Sci 123 (1977): 284–91.
- Edwards CA, Bohlen PJ. Biology and Ecology of Earthworms. Springer, Dordrecht (1996).
- El-Demerdash FM. Oxidative stress and neurotoxicity of pesticides. Toxicol Lett 127 (2002): 55–63.
- Elgueta S, Valdés-Gómez H, Bordeu E, et al. Pesticide residues in wine grapes: effects on fermentation and wine quality. Food Control 59 (2016): 495–502.
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7 (1961): 88–95.
- Elsaesser D, Focks A, Voss S, et al. Modeling the environmental fate of pesticides in soil–plant systems. Environ Model Softw 27 (2012): 62–70.
- Ercisli S, Esitken A, Turan M, et al. Effects of plant growth-promoting rhizobacteria (PGPR) on growth and nutrient uptake of plants. Sci Hortic 111 (2007): 38–43.
- Ertli T, Marton A, Földényi R. Effect of pH and the role of organic matter in the adsorption of isoproturon on soils. Chemosphere 57 (2004): 771–79.
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and neurodevelopmental outcomes: a review. Environ Health Perspect 107 (1999): 409–19.
- Eskenazi B, Marks AR, Bradman A, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115 (2007): 792–98.
- European Food Safety Authority. The 2019 European Union report on pesticide residues in food. EFSA J 19 (2021): e06491.
- International Code of Conduct on Pesticide Management. Food and Agriculture Organization of the United Nations, Rome (2014).
- The future of food and agriculture – alternative pathways to 2050. Food and Agriculture Organization of the United Nations, Rome (2018).
- Pesticides use database. Food and Agriculture Organization of the United Nations (2022).
- Fantke P, Friedrich R, Jolliet O. Health impact and damage cost assessment of pesticides in Europe. Environ Int 49 (2012): 9–17.
- Fantke P, Juraske R, Antón A, et al. Pesticide emission modelling for life cycle impact assessment of crop production. Int J Life Cycle Assess 16 (2011): 575–86.
- Fantke P, Juraske R, Jolliet O. Global greenhouse gas emissions of pesticides during their life cycle. Resour Conserv Recycl 87 (2014): 137–44.
- Faria NMX, Fassa AG, Meucci RD. Occupational exposure to pesticides and respiratory health: a systematic review. Int J Environ Res Public Health 11 (2014): 4449–71.
- Felsot AS, Racke KD, Hamilton DJ. Disposal and degradation of pesticide waste. Rev Environ Contam Toxicol 177 (2003): 123–200.
- Fenner K, Canonica S, Wackett LP, Elsner M. Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science 341 (2013): 752–58.
- Fernández-Alba AR, García-Reyes JF. Large-scale multi-residue methods for pesticides and their transformation products in food and environmental samples by LC-MS. Trends Analyt Chem 27 (2008): 973–90.
- Fernández-Bayo JD, Achmon Y, Harrold DR, et al. Environmental fate and impact of pesticides in agricultural soils. J Environ Qual 48 (2019): 1186–200.
- Ferrer A, Cabral R, da Costa J, et al. Human exposure to pesticides: a global health problem. Rev Environ Contam Toxicol 252 (2020): 1–50.
- Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103 (2006): 626–31.
- Fierer N, Lauber CL, Ramirez KS, et al. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6 (2012): 1007–17.
- Filimon MN, Voia SO, Popescu S, et al. Effects of pesticides on soil microbial communities: a review. Rom Biotechnol Lett 22 (2017): 12926–36.
- Finlayson CM, Lowrance R, Zedler JB, et al. Wetland ecosystem services in agricultural landscapes. Ecol Eng 56 (2013): 1–3.
- Fließbach A, Oberholzer HR, Gunst L, et al. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric Ecosyst Environ 118 (2007): 273–84.
- Flores C, Wolters B, Varela E, et al. Pesticide residues in soil and their impact on microbial activity. Appl Soil Ecol 144 (2019): 123–32.
- Fogg P, Boxall ABA, Walker A. Degradation of pesticides in soil: kinetic and mechanistic studies. Pestic Sci 55 (1999): 448–56.
- Förster B, Schäfer RB, Liess M. Thresholds for the effects of pesticides on invertebrate communities in stream ecosystems. Environ Sci Technol 44 (2010): 4477–83.
- Fox JE, Gulledge J, Engelhaupt E, et al. Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc Natl Acad Sci USA 104 (2007): 10282–87.
- Freemark K, Boutin C. Impacts of agricultural herbicide use on terrestrial wildlife in temperate landscapes: a review. Environ Rev 3 (1995): 230–60.
- Freemark K, Kirk DA. Birds on organic and conventional farms in Ontario: partitioning effects of habitat and practices on species composition. Biol Conserv 80 (1997): 113–25.
- Gagic V, Kleijn D, Báldi A, et al. Combined effects of agrochemicals and landscape composition on pollinator diversity and crop yield. Ecol Lett 20 (2017): 1421–29.
- Gajendiran A, Krishnamurthy S, Abraham J. Microbial degradation of organophosphorus pesticides. Indian J Microbiol 55 (2015): 357–69.
- Gallai N, Salles JM, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68 (2009): 810–21.
- Gao J, Liu L, Liu Y, et al. Pesticide effects on soil respiration: a review. Environ Sci Pollut Res 26 (2019): 33978–90.
- Gao Y, Li Y, Zhang Y, et al. Microbial diversity in response to pesticide contamination. Environ Pollut 260 (2020): 114064.
- Garthwaite DG, Barker I, Parrish G, et al. Pesticide usage in the United Kingdom. Pesticide Usage Survey Reports, Defra, London (2000).
- Gavrilescu M. Fate of pesticides in the environment and its bioremediation. Eng Life Sci 5 (2005): 497–526.
- Geiger F, Bengtsson J, Berendse F, et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl Ecol 11 (2010): 97–105.
- Geiger F, de Snoo GR, Berendse F, et al. Agricultural intensification and farmland biodiversity: risks and opportunities. Ecol Lett 13 (2010): 858–71.
- Geiger F, Wäckers F, Bianchi F, et al. Pollinator diversity declines due to agricultural intensification and pesticide use. J Appl Ecol 51 (2014): 1123–31.
- George DR, Finn RD, Graham KM, et al. Molecular evidence for sublethal pesticide effects on honey bee immune function. J Invertebr Pathol 123 (2014): 20–25.
- Ghosh RK, Banerjee S, Chatterjee R, et al. Influence of pesticides on soil microbial ecology. J Environ Biol 39 (2018): 155–62.
- Gianessi LP, Reigner N. Pesticide use in U.S. crop production: 2002. CropLife Foundation (2003).
- Gianessi LP, Reigner N. The value of herbicides in U.S. crop production. Weed Technol 21 (2007): 559–66.
- Gilbert N. Rules tighten on use of pesticides. Nature 450 (2007): 1132–33.
- Gill RJ, Ramos-Rodriguez O, Raine NE. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491 (2012): 105–08.
- Gillund F, Myhr AI. Perspectives on risk assessment of GM plants: a review. Plant Sci 176 (2009): 500–06.
- Giovannucci D, Scherr S, Nierenberg D, et al. Food and Agriculture: The Future of Sustainability. UN Department of Economic and Social Affairs (2012).
- Giri S, Singh A, Singh RS. Soil microbial response to pesticides: a review. Environ Chem Lett 17 (2019): 125–40.
- Gomiero T, Pimentel D, Paoletti MG. Environmental impact of different agricultural management practices: conventional vs. organic agriculture. Crit Rev Plant Sci 30 (2011): 95–124.
- Gómez JD, Quintana M, Giraldo MC, et al. Effects of pesticides on soil microbial functional diversity. Appl Soil Ecol 74 (2014): 20–27.
- González-Chávez MC, Carrillo-González R, Wright SF, Nichols KA. The role of glomalin in sequestering soil carbon. Plant Soil 240 (2002): 325–33.
- Goulson D. An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50 (2013): 977–87.
- Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347 (2015): 1255957.
- Govindarajan M, Rajeswary M, Hoti SL, et al. Mosquito larvicidal properties of essential oils against Culex quinquefasciatus. Parasitol Res 109 (2011): 353–60.
- Graham DW, Olivares-Rieumont S, Knapp CW, et al. Antibiotic resistance genes in sewage and soils impacted by wastewater irrigation. Water Res 45 (2011): 701–10.
- Grewal SK, Grewal PS. Effects of compost amendments on soil quality and crop productivity. Appl Soil Ecol 44 (2010): 1–11.
- Grube A, Donaldson D, Kiely T, Wu L. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. US EPA, Washington, DC (2011).
- Grube A, Donaldson D, Kiely T, Wu L. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates. US EPA, Washington, DC (2016).
- Gupta PK. Toxicology of pesticides. In: Gupta RC (ed). Veterinary Toxicology. Academic Press, San Diego (2007): 525–48.
- Gupta PK. Pesticide exposure—Indian scene. Toxicology 198 (2004): 83–90.
- Gupta PK. Pesticide exposure: human hazards and common routes of exposure. Toxicology 198 (2004): 1–10.
- Gupta RC (ed). Veterinary Toxicology. 3rd ed. Academic Press, London (2018).
- Gupta S, Sharma A, Dewangan A, et al. Fate of pesticides in soil–water environment: a review. Curr World Environ 14 (2019): 110–20.
- Gurr GM, Wratten SD, Snyder WE, Read DMY. Biodiversity and Insect Pests: Key Issues for Sustainable Management. John Wiley & Sons, Oxford (2012).
- Gutierrez AP, Ponti L, Cossu QA. Prospects for integrated pest management of olive pests in Italy. Crop Prot 28 (2009): 115–21.
- Gyawali K. Pesticide uses and its effects on public health and environment. J Health Promot 6 (2018): 28–36.
- Hageman KJ, Simonich SL, Campbell DH, et al. Atmospheric transport of current-use and legacy pesticides to alpine ecosystems. Environ Sci Technol 40 (2006): 3174–80.
- Hahn M, Schotthöfer A, Schmitz J, et al. Effects of repeated pesticide exposure on aquatic invertebrate communities. Sci Total Environ 574 (2017): 1147–56.
- Handa SK, Agnihotri NP, Kulshrestha G. Pesticide Residues: Significance, Management and Analysis. Research Periodicals & Book Publishing House, Texas (1999).
- Hanson ML, Solomon KR. Haloacetic acids in the aquatic environment. Environ Pollut 106 (1999): 13–26.
- Hardell L, Eriksson M. A case–control study of non-Hodgkin lymphoma and pesticide exposure in Sweden. Cancer 85 (1999): 1353–60.
- Hariprasad P, Divakara ST, Niranjana SR. Isolation and characterization of chitinolytic bacteria for management of plant pathogens. Biol Control 54 (2010): 107–15.
- Harris CA, Renfrew MJ, Woolridge MW. Assessing the risk of pesticide residues to consumers: recent and future developments. Food Addit Contam 18 (2001): 1124–29.
- Harrison JF, Camazine S, Marden JH, et al. Effects of stress and nutrition on honey bee physiology. J Exp Biol 216 (2013): 3352–59.
- Hart A, Brown CD, Lewis KA, et al. Pesticide Properties Database (PPDB). Agriculture & Environment Research Unit, University of Hertfordshire (2021).


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
