Evaluation of Aqueous Creams Containing Ethanolic Extract of Curcuma longa (Turmeric) as Bioactive Ingredient for the Management of Wounds
Kenneth Chinedu Ugoeze1*, Majiroghene Uyoyoumaero Okpa1, Nkemakolam Nwachukwu1, Bruno Chukwuemeka Chinko2, Kennedy Emeka Oluigbo3
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
2Department of Human Physiology, Faculty of Basic Medical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
3Department of Clinical Pharmacy and Biopharmaceutics, Faculty of Pharmaceutical Sciences, Enugu State University of Science and Technology, Agbani City, Enugu, Nigeria.
*Corresponding Author: Kenneth Chinedu Ugoeze, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Nigeria
Received: 28 January 2021; Accepted: 06 February 2021; Published: 11 February 2021
Article Information
Citation: Kenneth Chinedu Ugoeze, Majiroghene Uyoyoumaero Okpa, Nkemakolam Nwachukwu, Bruno Chukwuemeka Chinko, Kennedy Emeka Oluigbo. Evaluation of Aqueous Creams Containing Ethanolic Extract of Curcuma longa (Turmeric) as Bioactive Ingredient for the Management of Wounds. International Journal of Applied Biology and Pharmaceutical Technology 12 (2021): 322-337.
View / Download Pdf Share at FacebookAbstract
Abstract Background: Wounds are considered health indispositions with detrimental socio-economic influences on the afflicted and their kin. Curcuma longa or turmeric has been used in the treatment of wounds. Employing the extracts of turmeric as a bioactive ingredient in an aqueous cream could enhance its value in wound treatment.
Aim: This study aimed to formulate aqueous creams containing concentrations of ethanolic extract of turmeric (EET) as bioactive ingredients, evaluate their stability and wound healing activities in male Wistar rats using hydroxyproline (HXP) as a biochemical marker.
Materials and methods: Solutions of 1.5, 3.0 and 5.0 % w/v of crude EET were prepared and also incorporated as bioactive ingredients in aqueous creams. The stability of the creams was evaluated and their wound healing effects were studied using distilled water, dimethyl sulphoxide (DMSO) and cholesterol as controls in male Wistar rats.
Results: The creams were stable in colour, pH, viscosity, etc. and exhibited wound healing activities. The animals treated with the crude 1.5 % w/v EET and its cream demonstrated the highest tissue HXP level showing significant percentage differences in tissue HXP levels from the control groups (p < 0.05).
Conclusion: The crude EET has been confirmed to possess wound healing properties with an optimal effective concentration for wound healing in male Wistar rats determined as 1.5 % w/v which when incorporated as a bioactive ingredient in an aqueous cream retained its efficacy in wound healing and could therefore be beneficial in the treatment of body injuries.
Keywords
<p>Wound healing; Aqueous cream; <em>Curcuma longa</em>; Turmeric; Bioactive ingredient; Hydroxyproline; Wistar rats</p>
Article Details
Introduction
An injury or wound is a harm to the superficial cover of the body which interferes with the other soft skin. It is a universal term that discusses the hurt triggered by accidents, falls, hits, weapons, surgery, sutures or stitches, etc. Injuries range from minor to life-threatening types which could occur at work or play, indoors or outdoors, driving a car or walking across the street. Wounds are damages that disrupt the skin or other body tissues and includes cuts, scrapes, scratches and punctured skin [1,2]. The existence of wounds brings about the socio-economic burden to the victim and their relations [3]. Body injuries may be caused by physical, chemical, thermal, microbial or immunological manipulation to the body tissue [4,5] and may be described based on their degree, the time-lapse between its initiation and restoration, the advance of restoration, the existing disease state in the victim, the accompanying risk of mortality and the effect on the worth of life of the victim [6,7]. When a body is broken or torn by any sharp object, it is considered as an open wound. On the other hand, when an unsharpened object or forceful distress causes a bump on the body, the result is described as a closed wound. The types of hurtful damages considered as a burn could be triggered by fire, heat, radiation, chemicals, electricity or sunlight [4,5,8]. Wound healing takes some time to have the original flesh fully reestablished as the procedure to achieve that is somewhat complicated with some transforming stages of cellular and biochemical reactions which must take place consistently and at the due period to harness the reestablishment of the natural flesh. The actualization of these implicates continuous cell-cell as well as cell-matrix interactions giving room for the process to advance in sundry conforming sectors and ways encompassing inflammation, wound contraction, re-epithelialization, tissue re-modelling and development of granulation tissue with angiogenesis. The extent of progress a damaged part of the body could be repaired is time- dependent. In other words, if the expected degree of restoration fails to take place at the right time frame, it is possible that the expected healing may fail to be attained and of course, may lead to such injuries that take a long time to heal [9-12]. The paramount stage of restoring an injury attempts to control bleeding [13,14]. The processes that bring about the constriction of the blood vessels, platelet migration and realization of coagulated fibrin reinstates haemostasis soon after vascular damage, giving room for an extracellular setup for cell migration. Following these, mediators of wound healing draw inflammatory cells to the area of injury permitting the successive phase of inflammation [15]. This additional stage intersects with the previous stage comprising haemostasis and clotting and is commenced in a few hours following the injury. This point is predominantly distinctive from the accumulation of leukocytes and macrophages [14,16]. At this time macrophages migrate into the distressed area, discharge growth factors like a platelet, easing the improvement of fresh connective tissue or granular tissue [14]. Macrophages likewise aid in the resolution of inflammation and ignite tissue regeneration, supporting the modification from the inflammatory to reparative steps of proliferation and remodelling, taking place in the time frame of about 21 days from the day the injury occurred. In the third stage of wound healing, proliferation is illustrated by the development of granulation tissue, re-epithelialization of the surface of the wound and receding of the margins of the injury [13,16]. Granulation tissue encompasses macrophages, fibroblasts and immature collagen. These are all agreed to encourage the formation of granulation tissue. Concurrently, blood vessels will motivate capillary growth. Fibroblasts about the surface of the injury ignite the production of collagen, one of the key constituents of the extracellular matrix. The last phase of restoration of a wound may well linger to an extent of time and involve the reorganization of collagen fibres to produce new skin [13,16]. This might progress less than a quarter of its eventual strength within three weeks from the day of the injury and oftentimes, it may be difficult to achieve restoration of the original skin [14].
Numerous plant-based formulations have severally been engaged in the handling of wounds and have similarly been used to support blood clotting, fight infection and fast-track the curing of wounds. These invaluable benefits resulting from plant extracts are a pointer to the great need to screen as much plant extracts as are available for their phytoconstituents and screening them for their wound healing activities [17-19]. Phytoconstituents derived from plants include the alkaloids, essential oils, flavonoids, tannins, terpenoids, saponins and phenolic compounds [20,21]. The medicinal value of plant extracts is influenced by the bioactive phytochemical constituents providing positive physiological activity in the human body [21,22]. The in vitro assays using these plant extracts are convenient, rapid and reasonably cheap. Small animals offer a multitude of model varieties for several human wound conditions [17].
Curcuma longa, ordinarily known as turmeric is of the family, Zingiberaceae. The dried pulverized rhizome of it serves as a spice or colourant in foods. Turmeric has a long history of medicinal use since over 4000 years [23-25] and has been useful in Ayurvedic medicine in the handling of inflammatory health disorders. The following constituents have been identified in Curcuma longa: the three curcuminoids: curcumin (diferuloylmethane - responsible for its yellow colour), demethoxycurcumin and bisdemethoxycurcumin, volatile oils (turmerone, atlantone and zingiberone), sugars, proteins and resins. The multiple pharmacological activities, antioxidant and antimicrobial benefits of turmeric have been accredited to curcumin [26]. Turmeric paste has been used to restore wounded bodies or to guard against contamination. In certain parts of Bangladesh, turmeric has been the commonest application to the postpartum excised umbilical cord [27] and has also been found useful in the healing of peptic ulcers [28]. In old Hindu medicine, turmeric is widely used for the management of sprains and swelling caused by injuries. Traditionally, Indian medicine uses turmeric powder for the treatment of biliary disorders, anorexia, coryza, cough, diabetic wounds, hepatic disorders, rheumatism and sinusitis [29]. In China, Curcuma longa has been in use for ailments related to intestinal discomforts [30] and has also demonstrated anti-inflammatory, anticarcinogenic, anti-ageing, antioxidant activity, as well as efficacy in wound healing [31]. The contemporary works on the wound healing properties of curcumin indicated that it can improve granulation tissue formation, collagen deposition, tissue remodeling and wound contraction [32].
Remarkably, curcumin is a hormetic agent (hormetin), being stimulatory at low doses (i.e. antioxidant effects) and inhibitory at high doses (i.e. induction of autophagy and cell death). This prompts the biphasic dose-response of curcumin on cells, with low doses having stronger effects than high doses. By this, it has been documented that curcumin modulates wound healing in vitro in a biphasic dose-response manner, being stimulatory at low doses and inhibitory at higher doses [31]. Curcumin has also been reported to expedite fibrinolysis and cellular migration in the healing of injury through the modification of urokinase plasminogen activator expression [33]. Up-to-date studies have shown that compared to its oral administration, topical application of curcumin has more noticeable effects on wound healing due to the greater accessibility of the drug at the wound site [34].
This study was undertaken to appraise the stability and wound healing dimensions of ethanolic extract of turmeric (EET) as a bioactive ingredient in an aqueous topical cream formulated for the management of wounds using male Wistar rats as model and hydroxyproline (HXP) as a biochemical marker. The extent of wound healing activity of the EET as a bioactive ingredient in the topical creams will be based on the level of wound contraction, however, especially on the level of tissue HXP detectable in the portion of the healed wound in male Wistar rats. HXP is a non-essential amino acid derivative formed during post-translational protein modification through hydroxylation of the amino acid, proline by the enzyme prolyl hydroxylase which requires vitamin C as a co-factor. HXP is a major component of the protein, collagen and plays a key role in the stability of the collagen triple helix [35].
Materials and methods
MaterialsThe following materials were used for the studies as procured and include hydroxyproline assay kit (Elabscience, China), dimethyl sulphoxide (DMSO) (Sigma-Aldrich, USA), cholesterol (Molychem, India), emulsifying wax, liquid paraffin and soft paraffin (Kerax, UK).
Methods
Collection and extraction of the sample of turmeric
The sample of rhizomes of turmeric used was identified by a Taxonomist, assigned an e-herbarium identification no. EH/P/069; EH/C/016 and deposited in the Ecoland Herbarium, University of Port Harcourt.
The rhizomes were air-dried and pulverized. A 100.0 g of the powder was extracted in a Soxhlet apparatus at 78o C using ethanol. The ethanol was evaporated in a rotary evaporator to obtain the ethanolic extract of turmeric (EET).
Adaptation of animals for studies
Forty-five adult male Wistar rats weighing 200-250 g were sourced from the animal house of the Faculty of Pharmaceutical Sciences, University of Port Harcourt and kept in separate cages to acclimatize for two weeks with free access to standard feed and water all through and in standard conditions of temperature (25-29 ), relative humidity (55-66 %) and natural dark/light cycle. Each rat was anaesthetized by 50 mg/kg ketamine intramuscularly. Animal maintenance was generally conducted in firm observance of the ethical requirements of the University of Port Harcourt. All the investigations were conducted in line with the procedures for ethical conduct in the care and use of non-human animals in research [36]. The experimental protocols for the studies involving animals were verified and approved by the Research Ethics Committee of the University of Port Harcourt with approval reference no. UPH/CEREMAD/REC/MM71/044.
Preliminary verification of the wound healing potential and the optimum effective concentration of the EET for wound healing in male Wister rats.
Table 1: Verification of the wound healing potential and determination of the optimum effective concentration of EET for wound healing in male Wistar rats
|
Ingredient |
Batch |
||||||||
|
A |
B |
C |
D |
E |
F |
G |
H |
I |
|
|
Concentration (%) |
|||||||||
|
Solution of EET |
Aqueous cream of EET |
Controls |
|||||||
|
EET |
1.5 |
3.0 |
5.0 |
1.5 |
3.0 |
5.0 |
- |
- |
- |
|
DMSO |
- |
- |
- |
5.0 |
5.0 |
5.0 |
5.0 |
- |
- |
|
Cholesterol |
- |
- |
- |
10.0 |
10.0 |
10.0 |
- |
10.0 |
- |
|
Glycerol |
- |
- |
- |
5.0 |
5.0 |
5.0 |
- |
- |
- |
|
Emulsifying ointment |
- |
- |
- |
20.0 |
20.0 |
20.0 |
- |
- |
- |
|
Water, q. s. |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
Formulation of the aqueous creams
Aqueous topical creams containing 1.5, 3.0 and 5.0 % w/w respectively of EET were prepared based on the formula in Table 1 for batches D-F respectively. In preparing the respective batches of creams, a separate mixture of DMSO, glycerol and emulsifying ointment was prepared and another separate mixture of EET, cholesterol and water was prepared. Both mixtures were separately raised to a similar temperature and later added together in a glass beaker and homogenized to obtain an aqueous cream which was made up to the required weight using distilled water at the same temperature. It was stirred and kept to cool to room temperature.
Evaluation of the stability of creams
The physical appearance and colour of the cool cream were considered. The consistency was evaluated by rubbing a little portion of it amid the fore and first fingers. Its homogeneity was measured in terms of creaming and phase separation. An amount of the cream was introduced in a wide-mouthed plastic bottle in triplicate and stored at -5°, ambient temperature and 40°C individually. They were examined daily for any changes in colour, appearance, consistency and homogeneity for 14 days.
The pH of the cream was assessed with a pH meter (Ultrameter II, 6PFC E; Myron L, UK). The cream was left at ambient temperature while its pH was studied every other day for 14 days [40]. It was also considered for its spreadability, a procedure determined by putting a noted quantity of it between two slides while a 100.0 g weight was positioned on it for 10 s and recording the distance shifted by the slides.
Viscosity
The viscosity of the creams was evaluated in alternate days for 14 days using a Brookfield viscometer (Brookfield DV2TLVTJO, USA) fitted with spindle no. 62 at ambient temperature.
Evaluation of the wound healing potentials of the pure EET and its aqueous creams
Forty-five male Wistar rats were placed into 9 groups (A-I) (n=5). Each rat was anaesthetized with 50 mg/kg ketamine intramuscularly. Shaving, cleaning of the dorsal area of each rat and excision of a 1.5 cm × 1.0 cm full-thickness open wound was made [37]. Groups A-C were treated with topical application of solutions containing 1.5, 3.0 and 5.0% w/v of EET in water. Groups D-F were treated with topical aqueous cream containing 1.5, 3.0 and 5.0% w/w of EET respectively. Groups G-I were treated with 5.0% v/v of DMSO, 10.0% w/v of cholesterol and distilled water respectively serving as controls. DMSO and cholesterol were employed as controls to evaluate their effect in the evaluation of the wound healing influence of the creams in which they were constituent. Wounds were treated daily, measuring wound contraction each day before cleaning and application of the various wound treatment interventions. The entire treatment was carried out for 21 days. The percentage of wound contraction was calculated using equation 1 [38].

Tissue bioassay of hydroxyproline (HXP)
At the end of the wound treatment, the rats were sacrificed. Tissue bioassay was conducted using 100.0 mg of the tissues collected from the site of the healed wound of the respective rats. It was added to 1 ml of 6.0 M hydrochloric acid, boiled for 6 h and cooled. The pH was adjusted to 6.8 while the volume was made up to 10.0 ml using distilled water. Each sample was centrifuged and 1ml of its supernatant was used for the assay of HXP level using the HXP kits (Elabscience, China) and conducting the experiment based on the protocols outlined in the manufacturer’s manual which is in line with the methods described by Bergman and Loxley [39] after the principle that the oxidation product produced by HXP under the action of an oxidant reacts with dimethylaminobenzaldehyde (DMAB; Ehrlich’s reagent) showing a purplish red colour. The HXP was calculated by measuring the absorbance at 550 nm using a UV-VIS spectrophotometer (Jenway 6405, UK). The values were reported as µg/g dry weight of tissue.
2.2.6 Statistical analysis
The figures were presented as a mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed followed by Fisher’s Least Significant Difference (LSD) post hoc test to determine the level of significance.
Results and discussion
Evaluation of the aqueous creams
A homogeneous yellow smooth and steadily stable creams were produced. Medicinal constituents used in the healing of skin disorders are expected to access the target cells in the skin to exert its therapeutic influence. With this, topical management of skin ailments becomes attractive since local drug delivery offers a robust straight contact to the target cells with minor unwanted effects in contrast to systemic drug administration [41].
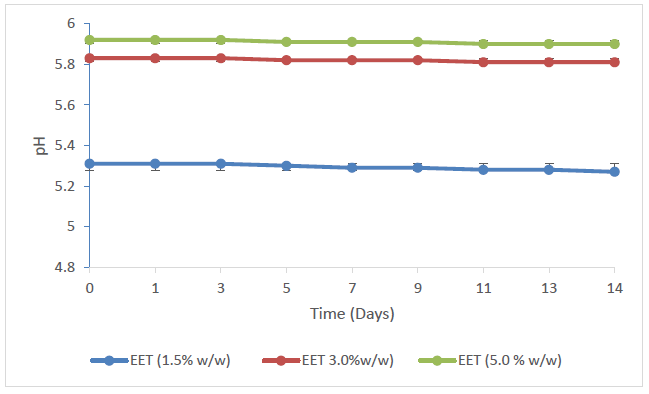
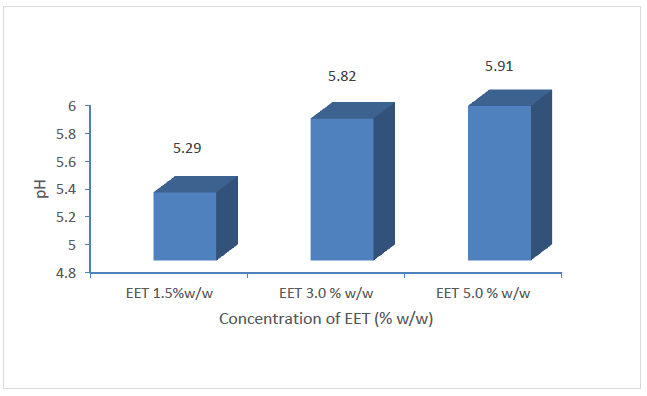
It is vital to look at an appropriate medicinal agent that boosts the peak distribution of the active component bringing about the effectiveness of the therapeutic agent as such agents are considered as applicable for the managing of open wounds. Such topical agents are expected to be smeared without difficulty as well as providing emollient features. The EET was conveyed as an aqueous cream using the techniques of oil-in-water emulsions [42] as shown in Table 1. The addition of emulsifying ointment in the formulation is to improve the firmness of the oil-in-water cream and bring about the hydrophilic part. This amid water and glycerol affords emollient and moisturizing influence and supports the decrease of dryness and irritation around the injured outer body cover and creates an occlusive barrier on the skin which obstruct the escape of moisture from the outer cover of the body. Moisturizing of the stratum corneum allows the opening up of the intra and intercellular channels for easy infiltration of EET into the cells and hurt tissues [43]. Both the DMSO and cholesterol incorporated in the design of the creams function as amphipathic surfactants which provide additional stability to the product particularly imparting hydrophobic and hydrophilic characteristics to the creams to stabilize the EET to retain its activity in the environment of other adjuvants. In addition to its actions as an amphipathic surfactant, DMSO also acts as a penetration enhancer [44,45] and would be predictable to increase the permeation of the EET into the body tissue, thereby improving the effectiveness of the formulation. Additional characteristics of the oil-in-water based cream consider ease of washability and high skin pore occlusion efficiency. Generally, occlusion of wounds has been acknowledged to vividly decrease inflammation and reducing pains, scaring and fast-tracking healing [46-48]. The stability of cream is critical to its efficacy and safety. The form and consistency of the preparations were factors considered in the evaluation of the stability of the creams since obvious instability may seem as an alteration in colour and/or consistency. Making an allowance for these sorts, there were no suggestions of coalescence, alteration in colour or inconsistency in the creams in the several stress conditions they were open to. Degeneration in certain creams could follow as changes in pH giving rise to undesirable experiences like skin irritation [49]. The pH of the respective batches of creams exhibited a very minor decrease in value as the storage time increased (Figure 1) while their mean pH varied significantly with the increasing concentration of the EET (p < 0.05) (Figure 2) which, however, are within the normal range of pH for the skin. Variable skin pH values are being reported in the literature, all in the acidic range but with a broad range from pH 4.0 to 7.0 [50,51].
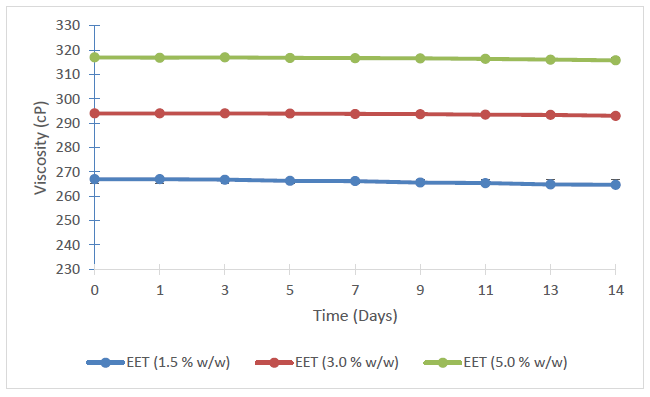
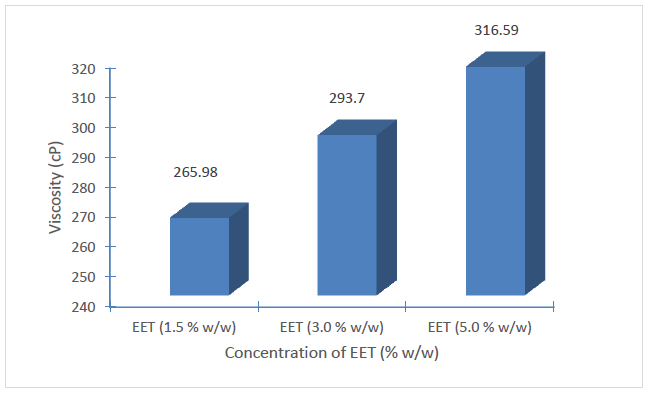
The viscosities of the respective batches of creams exhibited a very minor decrease with storage (Figure 3). However, statistically incremented level of viscosity was observed with increasing concentration of EET in the creams (p < 0.05) (Figure 4).
The seemly consistency in the pH and viscosities of the creams following storage points to the consistency and stability of the batches of the topical aqueous creams.
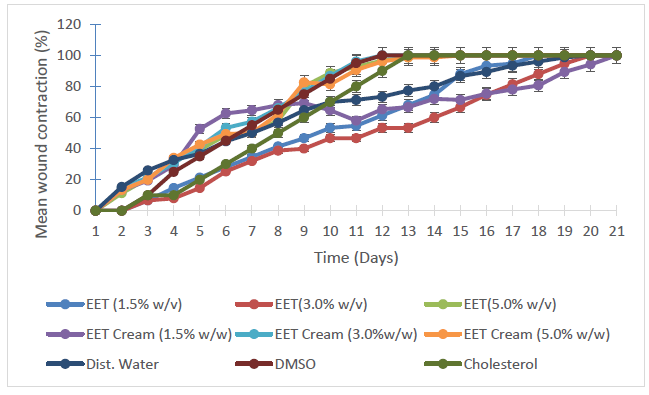
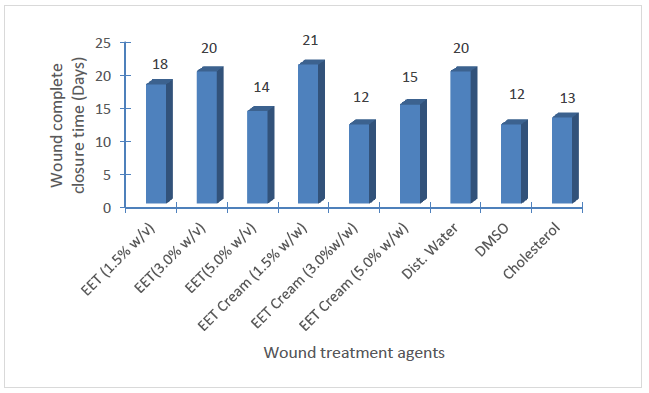
Evaluation of the wound-healing effects of the wound treatment agents
Figure 5 is a graphical representation of the mean wound closure patterns following the days of treatment while Figure 6 depicts the day of 100% wound contractions for the respective wound treatment interventions employed.
The results of tissue HXP assay revealed that a statistically similar highest mean HXP level was obtained in the groups treated with 1.5% w/v EET (crude) and the topical aqueous cream containing 1.5% w/w EET respectively (p>0.05). This value of 1.741±0.01 µg/g was significantly different from every other result obtained from wound treatment agents (Table 2). The statistical equality of the mean tissue HXP levels is an indication that in as much as 1.5% of EET (crude) serves as the mean optimal effective concentration for wound healing in male Wistar rats, obtaining a statistically similar result with its cream shows that its efficacy is retainable in aqueous topical cream, and also proves the consistency and stability of the aqueous creams, especially the batch containing 1.5% w/w EET. The consistency and stability of the creams could be attributed to their content of DMSO and cholesterol which are considered as amphipathic surfactants, providing both hydrophobic and hydrophilic ends to stabilize the bioactive ingredient (EET), hence, its activities was retained in the cream. DMSO is also considered as a penetration enhancer [44,45]. In as much as the groups treated with the controls (distilled water, DMSO and cholesterol) with proven abilities to cause the production of collagen as exhibited by the mean tissue HXP (Table 2), the data displayed in Table 3 indicates that the influence of wound healing brought about by 1.5% EET (crude) and its content in a topical cream was independent of the effects of these controls with statistical differences in the mean tissue HXP of 63.782, 31.495 and 35.592% (p < 0.05) for distilled water, DMSO cholesterol respectively.
The results recorded in this study support the literature survey that turmeric has been useful in the reinstatement of injured bodies, safeguard against contamination (having it as a common practice in Bangladesh to apply it the postpartum excised umbilical cord [27], in the healing of peptic ulcers [28], diabetic wounds [29] with the ability for granulation tissue development, collagen deposition, tissue remodeling and wound contraction [32].
The mechanism of wound healing of the EET may be attributed to its various phytoconstituents [52,53] based on their anti-oxidant, anti-inflammatory and its proven antimicrobial properties, especially imparted by its rich curcumin [26].
Conclusion
Ethanolic extract of turmeric (EET) has been confirmed to possess wound healing properties. Its optimal effective concentration for wound healing in male Wistar rats has been determined as 1.5% w/v. This concentration has also been found to retain effective wound healing properties in its aqueous cream, hence, it could be useful as a bioactive constituent in the development of an aqueous topical cream beneficial in the treatment of body injuries.
Table 2: Mean tissue HXP levels obtained with various wound treatment agents
|
Wound Treatment Agent |
Mean HXP (µg/g) |
|
EET(1.5% w/v) |
1.741±0.01 |
|
EET (3.0% w/v) |
1.469±0.05 |
|
EET(5.0% w/v) |
1.377±0.10 |
|
EET-Cream (1.5% w/w) |
1.741±0.01 |
|
EET-Cream (3.0% w/w) |
1.587±0.10 |
|
EET-Cream (5.0% w/w) |
1.598±0.03 |
|
Dist. water |
1.063±0.05 |
|
DMSO (5.0% v/v) |
1.324±0.49 |
|
Cholesterol (10.0% w/v) |
1.284±0.03 |
Table 3: Difference in the mean tissue HXP levels of treated groups compared to the control groups (distilled water, DMSO and cholesterol)
|
Wound treatment agent |
Mean tissue HXP (µg/g) |
Difference of HXP levels of treated groups to controls groups (%) |
|||
|
Dist. Water |
DMSO |
Cholesterol |
Remark |
||
|
EET(1.5% w/v) |
1.741±0.01 |
63.782 |
31.495 |
35.592 |
P<0.05 |
|
EET (3.0% w/v) |
1.469±0.05 |
38.194 |
10.951 |
14.408 |
P<0.05 |
|
EET(5.0% w/v) |
1.377±0.10 |
29.539 |
4.000 |
7.243 |
P<0.05 |
|
EET-Cream (1.5% w/w) |
1.741±0.01 |
63.782 |
31.495 |
35.592 |
P<0.05 |
|
EET-Cream (3.0% w/w) |
1.587±0.10 |
49.294 |
20.695 |
23.598 |
P<0.05 |
|
EET-Cream (5.0% w/w) |
1.598±0.03 |
50.329 |
19.864 |
24.455 |
P<0.05 |
|
Dist. water |
1.063±0.05 |
- |
- 19.713 |
- 17.212 |
- |
|
DMSO (5.0% v/v) |
1.324±0.49 |
24.553 |
- |
3.115 |
- |
|
Cholesterol (10.0% w/v) |
1.284±0.03 |
20.790 |
-3.021 |
- |
- |
References
- Wounds and injuries. Available from: https://medlineplus.gov/woundsandinjuries.html [accessed 20.01.21].
- Thakur R, Jain N, Pathak R, et al. Practices in Wound Healing Studies of Plants. Evidence-Based Complementary and Alternative Medicine 2011 (2011): 438056.
- Bickers DR, Lim HW, Margolis Dl. The Burden of Skin Diseases: 2004 A Joint Project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 55 (2016): 490-500.
- Shuid AN, Anwar MS, Yusof AA. The Effects of Carica papaya Latex On The Healing of Burn Wounds In Rats,” Malaysian Journal of Medicine and Health Sciences 3 (2005): 39-47.
- Jalalpure SS, Agrawal N, Patil MB, et al. Antimicrobial and Wound Healing Activities of Leaves of Alternanthera sessilisInternational Journal of Green Pharmacy 2 (2008): 141-144.
- Fletcher J. Differences Between Acute And Chronic Wounds and the Role of Wound Bed Preparation. Nurs Stand 22 (2008): 62-68.
- Reddy M. Skin and Wound Care: Important Considerations in the Older Adult. Adv Skin Wound Care 21 (2008): 424-436.
- John Hopkins Medicine. Burns and wounds. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/burns [accessed 21.01.21].
- Martin P. Wound Healing-Aiming For Perfect Skin Regeneration. Science 276 (1997): 75-81.
- Sorg H, Tilkorn DJ, Hager S, et al. Skin wound healing: an update on the current knowledge and concepts. European Surgical Research 58 (2017): 81-94.
- Gilmore MA. Phases of Wounds Healing. Dimens Oncol Nurs 5 (1991): 32-34.
- Maxson S, Lopez EA, Yoo D, et al. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem cells Transl Med 1 (2012): 142-149.
- Guo S, Dipietro LA. Factors Affecting Wound Healing. J Dent Res 89 (2010): 219-229.
- Singer AJ, Clark RA. Cutaneous Wound Healing. N Engl J Med 341 (1999): 738-746.
- Boateng JS, Matthews KH, Stevens HN, et al. Wound Healing Dressings And Drug Delivery Systems: A Review. J Pharm Sci 97 (2008): 2892-2923.
- Hanson D, Langemo D, Thompson P, et al. Understanding Wound Fluid and the Phases of Healing. Adv Skin Wound Care 18 (2005): 360-362.
- Agyare C, Boakye YD, Bekoe EO, et al. Review: African medicinal plants with wound healing properties. J Ethnopharmacol 177 (2016): 85-100.
- Nayak BS, Pereira LM. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complementary and Alternative medicine 6 (2006): 41.
- Maver T, Kurecic M, Smrke DM, et al. Plant Derived Medicines with Potential Use in Wound Treatment (2018a) In: Herbal Medicine (Philip Builders (editor), London, IntechOpen (2019).
- Hwang JK, Kong TW, Baek NI, et al. α-Glycosidase Inhibitory Activity Of hexagalloylglucose From The Galls Of Quercus infectoria, Planta Medica 66 (2000): 273-274.
- Akinmoladun AC, Ibukun EO, Afor E, et al. Chemical Constituents and Antioxidant Activity of Alstonia boonei. African Journal of Biotechnology 6 (2007): 1197-1201.
- Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical Constituents of Some Nigerian Medicinal Plants. African Journal of Biotechnology 4 (2005): 685-688.
- Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Medica 57 (1991): 1-7.
- Eigner D, Scholz D. Ferula asafoetida and Curcuma longa In Traditional Medical Treatment and Diet in Nepal. Journal of Ethnopharmacology 67 (1999): 1-6.
- Prasad S, Aggarwal BB. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; (2011) Chapter 13.
- Karlowicz-Bodalska KA, Han ST, Freier J, et al. Curcuma longa As Medicinal Herb In The Treatment Of Diabetic Complications. Acta Pol Pharm 74 (2017): 605-610.
- Alam MA, Ali NA, Sultana N, et al. Newborn umbilical cord and skin care in Sylhet District, Bangladesh: implications for the promotion of umbilical cord cleansing with topical chlorhexidine. Journal of Perinatology 28 (2008): S61-S68.
- Prucksunand C, Indrasukhsri B, Leethochawalit MH, et al. Phase II Clinical Trial on Effect of the Long Turmeric (Curcuma longa) On Healing Of Peptic Ulcer. Southeast Asian J Trop Med Public Health 32 (2001): 208-215.
- Ammon H, Anazodo M, Safayhi H, et al. Curcumin: A Potent Inhibitor of Leukotriene B4 Formation in Rat Peritoneal Polymorphonuclear Neutrophils (PMNL). Planta Medica 58 (1992): 226.
- Araújo C, Leon L. Biological activities of Curcuma longaMemórias do Instituto Oswaldo Cruz 96 (2001): 723-728.
- Moghaddam NS, Oskouie MN, Butler AE, et al. Hormetic Effects of Curcumin: What is the Evidence?. J Cell Physiol 234 (2019): 10060-10071.
- Akbik D, Ghadiri M, Chrzanowski W, et al. Curcumin as a Wound Healing Agent. Life Sci 116 (2004): 1-7.
- Madhyastha R, Madhyastha H, Nakajima Y, et al. Curcumin Facilitates Fibrinolysis And Cellular Migration During Wound Healing By Modulating Urokinase Plasminogen Activator Expression. Pathophysiol Haemost Thromb 37 (2010): 59-66.
- Farshadi M. Turmeric and the Wound Healing – Beyond Tradition. Journal of Medical Oncology and Therapeutics 2 (2017): 65.
- National Center for Biotechnology information (2021). Pubchem Compound Summary for CID 5810, Hydroxyproline. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/hydroxyproline [accessed 21.01.21].
- Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research. Washington DC, USA: American Psychological Association, 2012. Available from https://www.apa.org/science/leadership/care/guidelines [accessed01.21].
- Suguna L, Singh S, Sivakumar P, et al. Influence of Terminalia chebula On Dermal Wound Healing in Rats. Phytother Res 16 (2006): 223-227.
- Kirker KR, Luo Y, Nielson JH, et al. Glycosaminoglycan Hydrogel films As Bio-Interactive Dressings for Wound Healing. Biomaterials 23 (2002): 3661-3671.
- Bergman I, Loxley R. Two Improved and Simplified Methods for the Spectrophotometric Determination of Hydroxyproline. R Anal Chem 35 (1963): 1961-1965.
- Akhtar N, Khan BA, Haji M, et al. Evaluation of various functional skin parameters using a topical cream of Calendula officinalis extract. African Journal of Pharmacy and Pharmacology 5 (2011): 199-206.
- Surber C, Abels C, Maibach H. pH of the Skin: Issues and Challenges. Curr Probl Dermatol. Basel, Karger 54 (2018): 143-151.
- De Vringer T, de Ronde HA. Preparation and Structure of a Water-in-Oil Cream Containing Lipid Nanoparticles. J Pharm Sci 84 (1995): 466-472.
- Pugh WJ, Degim IT, Hadgraft J. Epidermal permeability–penetrant structure relationships: 4, QSAR of permeant diffusion across human stratum corneum in terms of molecular weight, H-bonding and electronic charge. International Journal of Pharmaceutics 197 (2000): 203-211.
- Junyaprasert VB, Singhsa P, Jintapattanakit A. Influence of Chemical Penetration Enhancers on Skin Permeability of Ellagic Acid-Loaded Niosomes. Asian Journal of Pharmaceutical Sciences 8 (2013): 110-117.
- Moghbel A, Faghiri A. Influence of Dimethyl Sulfoxide as a Penetration Enhancer of Piroxicam Gel through Biological Skin. Iranian Journal of Pharmaceutical Sciences 2 (2006): 177-184.
- Hultén L. Dressings for Surgical Wounds. Am J Surg 167 (1994): S42-S45.
- Metzger S. Clinical and Financial Advantages of Moist Wound Management. Home Healthc Nurse 22 (2004): 586-590.
- Atiyeh BS, Amm CA, El Musa KA. Improved Scar Quality Following Primary and Secondary Healing of Cutaneous Wounds. Aesthetic Plast Surg 27 (2003): 411–417.
- Kikwai L, Babu RJ, Prado R, et al. In vitroand in vivo Evaluation of Topical Formulations of Spantide II. AAPS PharmSciTech 6 (2005): E565–E572.
- Lambers H, Piessens S, Bloem A, et al. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci 28 (2006): 359-370.
- Kristeen C. About Skin pH and Why It Matters. Healthline, March 19, 2019. Available from: https://www.healthline.com/health/whats-so-important-about-skin-ph [accessed 26.01.21].
- Ugoeze KC, Aja PC, Nwachukwu N, et al. Assessment of the Phytoconstituents and Optimal Applicable Concentration of Aqueous Extract of Azadirachta indica Leaves for Wound Healing in Male Wistar Rats. Thai Journal of Pharmaceutical Sciences 45 (2021): 8-15.
- Ugoeze KC, Oluigbo KE, Chinko BC. Phytomedicinal and Nutraceutical Benefits of the GC-FID Quantified Phytocomponents of the Aqueous Extract of Azadirachta indica leaves. Journal of Pharmacy and Pharmacology Research 4 (2020): 149-163.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
