Evaluation of D-Dimer Levels in Various Subgroups of Atrial Fibrillation: Role in Risk Stratification
Avinash Mani1, Vineeta Ojha2, Pradip Kumar Sinha1, Jayanta Saha3
1Department of Medicine, Medical College, Kolkata, India
2Department of Radiology, Medical College, Kolkata, India
3Department of Cardiology, Medical College, Kolkata, India
*Corresponding author: Avinash Mani, Department of Medicine, Medical College, Kolkata, India.
Received: 03 November 2022; Accepted: 25 November 2022; Published: 13 December 2022
Article Information
Citation: Avinash Mani, Vineeta Ojha, Pradip Kumar Sinha, Jayanta Saha. Evaluation of D-Dimer Levels in Various Subgroups of Atrial Fibrillation: Role in Risk Stratification. Cardiology and Cardiovascular Medicine 6 (2022): 565-570.
View / Download Pdf Share at FacebookAbstract
Background: D-dimer levels have been shown to be elevated in atrial fibrillation(AF) patients. However, different subgroups of AF have not been evaluated. We aimed to evaluate D-dimer levels in different subgroups of AF and determine association between D-dimer levels and adverse outcomes like heart failure and thromboembolism.
Methods: This was a single center, cross sectional, observational study which included patients with AF treated at a tertiary care hospital, from January 2017 to December 2017. Patients were subdivided into different subgroups based on etiology and D-dimer levels were measured. Reference value for the normal population was taken as < 0.5 μg/ml.
Results: 70 patients with AF were studied over a one-year period. Mean age of study population was 53 years. Valvular AF was the most common etiology(30%) noted followed by non-ischemic cardiomyopathy (NICM) (14.2%). About half of study population had history of heart failure whereas thromboembolism(TE) was noted in 15.7%. 72.8% patients had elevated D-dimer levels in the cohort. D-dimer levels were significantly higher in valvular AF(1.2 μg/ml) and NICM patients(1.4 μg/ml) (p=0.005). Higher D-dimer levels were noted in those with heart failure (HF) events (p=0.016). D-dimer levels were shown to accurately detect prior HF/ TE events with levels of 1.1 μg/ml and higher having a sensitivity and specificity of 59.1% and 81%, respectively (AUC 0.727).
Conclusion: D-dimer levels are significantly higher in valvular AF and NICM patients. D-dimer levels have a strong association with HF/TE events and elevated levels can be used to detect at risk patients.
Keywords
<p>Atrial Fibrillation; D-dimer; Heart Failure; Rheumatic Heart Disease; Thromboembolism</p>
Article Details
1. Background
Atrial fibrillation (AF) is the most common type of supraventricular arrhythmia encountered in clinical practice. The incidence of atrial fibrillation varies amongst the male and female population with males having higher rates as compared to female population (60.7 vs 43.8 per 100,000 person years, respectively) [1]. The average age of patients at risk for developing atrial fibrillation is 60-65 years in the Western population [2]. In the Indian population, atrial fibrillation occurs a decade earlier with epidemiological studies showing average age at onset around 50-55 years [3]. Atrial fibrillation is known to be a pro-inflammatory state, with cytokines like TNF-α, IL-2, IL-6, IL-8, IL-10, MCP-1 and CD-40 implicated in the development of the inflammatory milieu [4]. The persistent state of inflammation causes endothelial dysfunction, coagulation activation, platelet activation, and increased fibrinolytic activity, which may confer a prothrombotic environment in atrial fibrillation [5]. Clinical risk scores like CHADS, CHADS-VASc and ATRIA score have been used for prediction of thromboembolic risk in patients with atrial fibrillation [6]. Biomarkers like CRP and D-dimer have been used to predict the cardiovascular and thromboembolic risk in few primary prevention studies [7,8]. D-dimer is used widely as a prothrombotic marker and has been shown to be elevated in patients with atrial fibrillation [9]. However, there is paucity of data on the association of D-dimer levels with cardiovascular adverse events like heart failure and thromboembolism. Patients with different etiologies of atrial fibrillation may have varied cardiovascular outcomes and D-dimer levels may vary amongst different subgroups. None of the studies have compared D-dimer levels amongst different etiological subgroups of atrial fibrillation. Consequently, D-Dimer levels may be used for predicting outcomes in different subgroup of patients with atrial fibrillation. In our study, we aimed to compare d-dimer levels amongst various groups of patients with atrial fibrillation. We also aimed to evaluate association between d-dimer levels in different subgroups with cardiovascular outcomes like heart failure and thromboembolic events.
2. Materials and Methods
This was a single center, cross sectional study performed at a tertiary care hospital, over a period of 6 months from January 2017 to June 2017. All patients with persistent atrial fibrillation, who attended out-patient department or had hospital admission, were included in the study. Patients with multisystem disorders, sepsis, malignancy and chronic kidney disease on hemodialysis were excluded. Baseline demographics and co-morbidities like hypertension, diabetes, dyslipidemia, thyroid disorders, obstructive airway diseases, obstructive sleep apnea were recorded. Drug history including history of oral anticoagulant intake was also recorded. ECG was done in all patients to document presence of atrial fibrillation. Doppler echocardiography was used for evaluation of valvular heart disease and ventricular function. The study patients were divided into multiple subgroups depending upon etiology of atrial fibrillation. The main subgroups were valvular AF, non-valvular AF, ischemic heart disease, non-ischemic cardiomyopathy and other miscellaneous causes. Doppler echocardiography was performed to determine the etiology of atrial fibrillation. Valvular AF was defined as presence of mitral stenosis (most commonly rheumatic in etiology) or prosthetic heart valves. Non-valvular AF included patients with atrial fibrillation in presence of valvular lesions other than mitral stenosis(mitral regurgitation, aortic stenosis, aortic regurgitation). Ischemic heart disease was defined as documented evidence of coronary artery disease or past history of acute coronary syndrome. Patients with documented evidence of heart failure, ventricular dysfunction on echocardiography with normal epicardial coronaries were considered to have non-ischemic cardiomyopathy (NICM). Other causes of atrial fibrillation like thyroid disorders, lung diseases, obstructive sleep apnea were also noted. Heart failure events were defined as documented episodes of dyspnea and pedal swelling, which may or may not have required hospitalization. Thromboembolic events were defined as documented episodes of stroke, systemic embolism or peripheral vascular thrombosis. Quantitative D-dimer level estimation was done using immunoturbidimetric assay. Normal D-dimer levels at our institution was considered <0.5 mg/ml, which is similar to cut-offs followed worldwide. Other biochemical investigations including complete hemogram, renal function test, liver function test and thyroid profile were also performed. This study was approved by the Institutional ethics committee, Medical College, Kolkata and informed written consent was taken from all patients. No independent grants or funds were received for the purpose of this study.
2.1 Data Analysis
Data was collected using a structure questionnaire and recorded in a tabular format. Categorical variables were presented as proportions whereas continuous variables were presented as mean and standard deviation. Categorical variables were studied using binomial test and Chi-square test. Unpaired t test and ANOVA test were used to compare means of different continuous variables. ROC curve was plotted to determine the specificity and sensitivity of different study variables for prediction of clinical events. A p value of <0.05 was considered to be significant. The data analysis was done using SPSS version 20 (IBM, Armonk, NY).
3. Results
70 patients were included in our study over the period of 1 year. The baseline characteristics of the study population is outlined in Table 1. The mean age of the study population was 53 ± 14 years. Majority of the patients (56%) belonged to the age group 30-59 years. Females comprised 47% of the study population. Around 18% of study population had diabetes and hypertension whereas 3% had thyroid abnormalities. Valvular AF was the most common subgroup noted in our study cohort (30%) followed by non-ischemic cardiomyopathy (NICM) (14%), non-valvular AF(11.4%) and ischemic heart disease (IHD) (11.4%). Other etiologies of AF noted in our cohort were reactive airway diseases (7%), thyroid disorders (3%), diabetes (4%), hypertension (6%), chronic kidney disease (7%), repaired congenital heart disease and respiratory infections (5.7%). Around half of the patients had prior history of heart failure events, with valvular AF subgroup having the highest events (47.2%) amongst all. Past thromboembolic events were noted in 16% of patients, with valvular AF patients having numerically higher events than other groups. However, the difference was not statistically significant (p=0.803). About 40% patients had clinical evidence of heart failure on presentation as evidenced by elevated JVP and/or basal crepitations. Majority of our study patients were on beta blockers(85%) and calcium channel blockers(35%) for rate control. Digoxin use was noted in a small number of patients (14.2%). Only one-fifth of the study population were on oral anticoagulants (Vitamin K antagonists).
D-dimer levels were estimated for all our study patients. Mean D-dimer levels in our study cohort was 0.99 ± 0.77 mg/ml (Table 2). 51 patients (72.8%) had elevated D-dimer levels in the study cohort. There was significant difference in D-dimer levels amongst various subgroups in the study cohort (p=0.005), with NICM subgroup having highest levels followed by valvular AF. Patients with prior heart failure events had significantly higher D-Dimer levels as compared to those without heart failure events (1.21 vs 0.77 mg/ml, respectively; p = 0.002). D-dimer levels were significantly lower in patients on oral anticoagulants (0.71 vs 1.05 mg/ml, respectively; p = 0.001). However, no significant difference in D-dimer levels was noted amongst patients with thromboembolic events as compared to those without any events (1.09 vs 0.96 mg/ml, respectively; p=0.278). D-dimer levels were also significantly higher in patients who had heart failure on presentation (1.22 vs 0.85 mg/ml, respectively; p=0.014). All patients were noted to have enlarged left atrium (LA), with valvular AF patients having highest LA dimensions (56.7 ± 10.4mm). D-dimer levels were shown to have a positive correlation with LA size in our study (r = 0.282, p = 0.018).
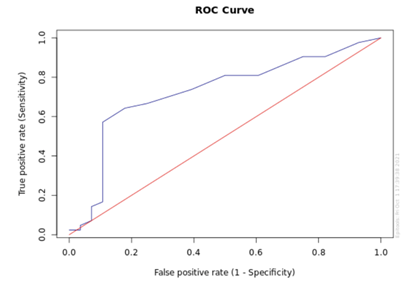
Baseline characteristics of study population were evaluated to determine predictors of adverse outcomes like heart failure and thromboembolism. Univariate analysis revealed that age, diabetes, LA size, valvular AF and D-dimer levels were predictors of heart failure or thromboembolic events. However, none of the above variables independently predicted heart failure or thromboembolic events on multivariate analysis (Table 3). ROC analysis was performed to evaluate accuracy of D-Dimer levels to identify patients at risk of heart failure or thromboembolic events. D-dimer levels were shown to accurately identify patients with heart failure or thromboembolic events (AUC = 0.727, 95% CI 0.60-0.85) (Figure 1). D-dimer level of 1.1 mg/ml and higher can detect past heart failure and thromboembolic events with a sensitivity of 59.1% and specificity of 89.3%.
|
Baseline characteristics |
N = 70 |
|
Age, in years |
53.1 (14) |
|
Females |
33 (47) |
|
Diabetes |
13 (18.5) |
|
Hypertension |
12 (17.1) |
|
Thyroid disorders |
2 (2.8) |
|
Etiology of Atrial Fibrillation |
|
|
Valvular AF |
21(30) |
|
Non valvular AF |
8(11.4) |
|
NICM |
10 (14.2) |
|
IHD |
8 (11.4) |
|
Miscellaneous |
23 (32.8) |
|
Heart Failure events |
34 (49) |
|
Valvular AF |
16 (47.2) |
|
Non valvular AF |
6 (17.6) |
|
NICM |
3 (8.8) |
|
IHD |
3 (8.8) |
|
Miscellaneous |
6 (17.6) |
|
Thromboembolic events |
11 (15.7) |
|
Valvular AF |
6 (54.5) |
|
Non valvular AF |
0 |
|
NICM |
2 (18.2) |
|
IHD |
1 (9.1) |
|
Miscellaneous |
2 (18.2) |
|
Medications |
|
|
Beta blockers (BB) |
60 (85.7) |
|
Calcium channel blockers (CCB) |
25 (35.7) |
|
Digoxin |
10 (14.2) |
|
Oral anticoagulants |
14 (20) |
|
Clinical evidence of heart failure |
26 (37) |
|
Mean LA size, in mm |
44.4 (10.4) |
|
Valvular AF |
56.7 (10.4) |
|
Non valvular AF |
48.3 (10.2) |
|
NICM |
40.5(7.7) |
|
IHD |
39.5(7.3) |
|
Miscellaneous |
35.4 (8.4) |
Table 1: Baseline characteristics of study population. Values are mentioned as mean(SD) or n(%). NICM = Non ischemic cardiomyopathy, IHD = Ischemic heart disease, OAC = oral anticoagulant, 3 Miscellaneous = chronic kidney disease, diabetes, hypertension, thyroid disorders, repaired congenital heart disease (ASD) and respiratory infections.
|
D-dimer level (mg/ml) |
|
|
Study population |
0.99 (0.77) |
|
Subgroups |
|
|
Valvular AF |
1.2 (0.6) |
|
Non-Valvular AF |
1.01 (0.67) |
|
NICM |
1.40 (0.9) |
|
IHD |
0.63 (0.5) |
|
Miscellaneous |
0.72 (0.6) |
|
Patients with heart failure events |
|
|
Yes |
1.21 (0.6) |
|
No |
0.77 (0.8) |
|
Patients with thromboembolic events |
|
|
Yes |
1.09 (1.0) |
|
No |
0.96 (0.7) |
|
Patients on oral anticoagulants |
|
|
Yes |
0.71 (0.5) |
|
No |
1.05 (0.8) |
|
Patients with heart failure on presentation |
|
|
Yes |
1.22 (0.65) |
|
No |
0.85 (0.59) |
Table 2: D-dimer levels in overall population and different subgroups, mean(SD).
NICM- Non-Ischemic Cardiomyopathy; IHD- Ischemic Heart Disease; Miscellaneous- Chronic Kidney Disease; diabetes, hypertension, thyroid disorders, repaired congenital heart disease (ASD) and respiratory infections.
|
Odds ratio |
95% C.I. |
p-value |
|
|
Age |
1.04 |
0.64-1.68 |
0.857 |
|
Diabetes |
0.18 |
0.03-1.04 |
0.055 |
|
Valvular AF |
3.01 |
0.33-27.1 |
0.326 |
|
LA size |
1.28 |
0.83-1.98 |
0.254 |
|
D-dimer levels |
1.56 |
0.96-2.56 |
0.077 |
Table 3: Multivariable analysis for prediction of heart failure or thromboembolic events.
4. Discussion
The current study sought to evaluate D-dimer levels in different subgroups of AF and determine association between D-dimer levels and heart failure or thromboembolic events. Majority of patients in the study had valvular AF followed by NICM. D-dimer levels were significantly different amongst the subgroups, with NICM and valvular AF patients having higher levels. Patients with past history of heart failure had higher D-dimer levels whereas no difference was noted amongst those with or without thromboembolic events. LA size was shown to have a positive correlation with D-dimer levels. ROC analysis should that D-dimer could effectively discriminate patients with history of heart failure or thromboembolic events.
Mean age of our study population was 53 years. Earlier age of onset in our study group is concordant with previous studies which show that mean age of patients with atrial fibrillation in India is around 54 years [10]. Similar findings were noted in a rural Indian population where mean age of atrial fibrillation was 51 years [3]. Globally, patients with atrial fibrillation are a decade older as shown in the REALISE-AF registry, where mean age was 66.2 years [11].
Valvular AF was the most common etiology of atrial fibrillation in the current study group followed by NICM and IHD. Population based registries in Indian population have shown that prevalence of rheumatic heart disease(RHD) in atrial fibrillation patients is around 47% [12]. Ischemic heart disease and cardiomyopathy was noted in about 32% of atrial fibrillation patients in the REALIZE-AF study [11]. 72.8% patients had elevated D-dimer levels in our study. Valvular AF and NICM patients had significantly higher D-Dimer levels as compared to other patients with atrial fibrillation. Similar results were seen in the study by Sadanaga et al. in atrial fibrillation patients, where 23% of the study population had elevated D-Dimer levels [8]. Another study by Cevik et al in patients with rheumatic mitral valve disease showed that D-dimer levels were highest in atrial fibrillation patients with mitral stenosis [13]. D-dimer levels were also significantly higher in patients with past history of heart failure events.
Elevated D-dimer levels in atrial fibrillation are a marker of the existent pro-inflammatory state, which predisposes to heart failure in these patients. Other heart failure biomarkers like BNP and Pro-BNP are available for heart failure detection. However, they are mainly elevated as a result of the increased hemodynamic stress on the myocardium. On the other hand, D-dimer levels signify a generalized pro-inflammatory condition which predisposes to future adverse outcomes like heart failure and thromboembolism. Cytokines like IL-6, IL-1, IL-1ß are elevated in pro-inflammatory states and they have shown to cause myocardial damage leading to heart failure [14]. Previous studies have evaluated role of elevated D-dimer levels in predicting prognosis of patients with atrial fibrillation. Atrial fibrillation patients with elevated D-dimer levels experienced higher rates of thromboembolic and cardiovascular events in the study by Sadanaga et al [8]. Elevated D-Dimer levels are significantly associated with increased risk of stroke, cardiovascular death, and major bleeding outcomes in atrial fibrillation patients [15]. The presence of higher D-dimer levels in certain AF subgroups places them at risk of having further adverse events in future. Identification of at-risk groups amongst AF patients will improve monitoring and management in such cases.
D-dimer levels decrease with oral anticoagulation therapy, thus likely reducing the risk of future adverse events. Surprisingly, no association was noted between thromboembolic events and D-dimer levels in our current study. The plausible explanation was that patients with prior thromboembolic events were already on oral anticoagulants which had reduced their D-dimer levels. Even though D-dimer levels decrease with anticoagulation therapy in AF patients, the levels still remain higher than the normal [16].
In the current study, D-dimer levels has been shown to detect patients with heart failure or thromboembolic events (AUC 0.727). Using a d-dimer level threshold of 60 ng/ml in AF patients, high negative predictive value of 98% was noted for detection of left atrial thrombus [17]. Other smaller studies have also demonstrated that higher D-dimer values may be associated with the presence of atrial thrombus and may predict adverse outcomes and death [18]. Our study showed that D-dimer levels of 1.1 mg/ml and higher could detect patients with history of adverse events. Another study by Kim et al. in patients with non- valvular AF and ischemic stroke showed that D-dimer levels were significantly higher in those having left atrial enlargement [19]. The current study also found a positive correlation between LA size and D-dimer levels. However, the strength of association was not very strong. Patients with increased LA size are more likely to have thromboembolic events in past and would be receiving oral anticoagulants which could have altered D-dimer levels. Larger studies are required to evaluate this association in anticoagulant naïve patients.
Identifying patients with high D-dimer levels and risk stratifying them will help to improve monitoring and management, while preventing future adverse events. D-dimer levels may also be used to modulate the intensity of anticoagulation therapy in high-risk patients. Patient awareness regarding adherence to therapy can also be benefitted by risk stratification using a simple biomarker. All these strategies will help us to reduce the major adverse event rates in patients with atrial fibrillation.
Strengths of the current study include evaluation of D-dimer levels in different subgroups of AF patients, determining association between D-dimer levels and adverse events and finding a cut-off level for risk stratifying high risk patients. There were also inherent limitations in the study. First, it was a single center study with small sample size which makes it difficult to generalize the results obtained on the entire AF population. Second, this was a cross sectional study and patients were not followed up serially due to logistic reasons. Long term follow up studies are needed to prove the hypothesis being postulated in our study. Third, both patients on and off anticoagulation were included in the study which affected the D-dimer levels and may have affected the final result. Fourth, D-dimer levels were compared against standard reference lab values. Using a matched control group from the normal population would have improved the yield of the study. However, the same could not be done due to technical constraints. Fifth, the time difference between adverse events and D-dimer estimation was variable amongst patients, it being a cross-sectional study. This could have influenced the D-dimer levels.
5. Conclusion
D-dimer levels are high in atrial fibrillation patients with rheumatic heart disease and non-ischemic cardiomyopathy. Higher D-dimer levels in this subset of patients place them at high risk of developing cardiovascular adverse events in the long term.
Author Contributions
AM and PKS had access to all data regarding the study. AM, PKS and JS were involved in study design and patient selection process. AM and VO were involved in manuscript preparation, statistical analysis and figure designs. All the authors reviewed the final manuscript and approved it.
Funding
Nil
Conflict of Interest
None of the authors have any conflict of interest to declare.
References
- Chugh Sumeet S, Rasmus H, Kumar N, et al. Worldwide Epidemiology of Atrial Fibrillation. Circulation 129 (2014): 837-847.
- Schnabel RB, Yin X, PhilimonGona, et al. Fifty-Year Trends in Atrial Fibrillation Prevalence, Incidence, Risk Factors, and Mortality in the Community. Lancet Lond Engl 386 (2015): 154-162.
- Bhardwaj R. Atrial fibrillation in a tertiary care institute – A prospective study. Indian Heart J 64 (2012): 476-478.
- Hadi HA, Alsheikh-Ali AA, Mahmeed WA, et al. Inflammatory cytokines and atrial fibrillation: current and prospective views. J Inflamm Res 3 (2010): 75-97.
- Choudhury A, Freestone B, Patel J, et al. Relationship of soluble CD40 ligand to vascular endothelial growth factor, angiopoietins, and tissue factor in atrial fibrillation: a link among platelet activation, angiogenesis, and thrombosis? Chest 132 (2007):1913-1919.
- Aspberg S, Chang Y, Atterman A, et al. Comparison of the ATRIA, CHADS2, and CHA2DS2-VASc stroke risk scores in predicting ischaemic stroke in a large Swedish cohort of patients with atrial fibrillation. Eur Heart J 37 (2016): 3203-3210.
- Devaraj S, Singh U, Jialal I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem 55 (2009): 229-238.
- Sadanaga T, Sadanaga M, Ogawa S. Evidence that D-dimer levels predict subsequent thromboembolic and cardiovascular events in patients with atrial fibrillation during oral anticoagulant therapy. J Am Coll Cardiol 55 (2010): 2225-2231.
- Cohen A, Ederhy S, Meuleman C, et al. d-dimers in atrial fibrillation: a further step in risk stratification of thrombo-embolism? Eur Heart J 28 (2007): 2179-2180.
- Vora A, Kapoor A, Nair M, et al. Clinical presentation, management, and outcomes in the Indian Heart Rhythm Society-Atrial Fibrillation (IHRS-AF) registry. Indian Heart J 69 (2017): 43-47.
- Alam M, Bandeali SJ, Shahzad SA, et al. Real-life global survey evaluating patients with atrial fibrillation (REALISE-AF): results of an international observational registry. Expert Rev Cardiovasc Ther 10 (2012): 283-291.
- J O, Js H, M E, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry [Internet]. Circulation 129 (2014).
- Cevik C, Otahbachi M, Nugent K, et al. Mitral regurgitation reduces systemic coagulation activity in patients with rheumatic heart disease. J Heart Valve Dis 18 (2009): 278-283.
- Hedayat M, Mahmoudi MJ, Rose NR, et al. Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev 15 (2010): 543-562.
- John E, Ziad H, Jonas O, et al. Abstract 18321: D-dimer is Prognostic for Stroke, Major Bleeding and Death During Anticoagulation of Atrial Fibrillation - a RELY Substudy. Circulation 122 (2010): A18321-A18321.
- Nozawa T, Inoue H, Hirai T, et al. D-dimer level influences thromboembolic events in patients with atrial fibrillation. Int J Cardiol 109 (2006): 59-65.
- Somlói M, Tomcsányi J, Nagy E, et al. D-dimer determination as a screening tool to exclude atrial thrombi in atrial fibrillation. Am J Cardiol 92 (2003): 85-87.
- Danese E, Montagnana M, Cervellin G, et al. Hypercoagulability, D-dimer and atrial fibrillation: an overview of biological and clinical evidence. Ann Med 46 (2014): 364-371.
- Kim TW, Song IU, Chung SW, et al. Serum D-dimer Levels Are Proportionally Associated with Left Atrial Enlargement in Patients with an Acute Ischemic Stroke due to Non-valvular Atrial Fibrillation. Intern Med Tokyo Jpn 55 (2016): 1447-1452.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks