Impact of Medical Treatments of Colorectal Cancer on Female Fertility and Oncofertility Issues in Young Women with Non-Metastatic Colorectal Cancer: State of the Art
Julie Labrosse1, Laurent Quero2,3, Michael Grynberg1,4,5, Gaetan des Guetz6,7*
1Department of Reproductive Medicine and Fertility Preservation, Hopital Jean Verdier, Bondy, France
2INSERM U1160, Universite de Paris, Paris
3Department of Oncology and Radiotherapy, Hopital Saint Louis, Paris, France
4Department of Reproductive Medicine & Fertility Preservation, Hopital Antoine Beclere, Clamart, France
5University Paris-Sud, Clamart, France
6Oncology Department, Hopital Delafontaine, Saint Denis, France.
7Universite Sorbonne Paris Nord, Laboratoire Éducations et Promotion de la Sante, LEPS, UR 3412, F-93430, Villetaneuse, France
*Corresponding author: Gaetan des Guetz, Universite Sorbonne Paris Nord, Laboratoire Educations et Promotion de la Santé, LEPS, UR 3412, F-93430, Villetaneuse, France.
Received: 16 March 2022; Accepted: 22 March 2022; Published: 09 May 2022
Article Information
Citation: Julie Labrosse, Laurent Quero, Michael Grynberg, Gaetan des Guetz. Impact of Medical Treatments of Colorectal Cancer on Female Fertility and Oncofertility Issues in Young Women with Non-Metastatic Colorectal Cancer: State of the Art. Journal of Women’s Health and Development 5 (2022): 162-174.
DOI: 10.26502/fjwhd.2644-28840086
View / Download Pdf Share at FacebookAbstract
The incidence of Colorectal Cancer (CRC) is increasing among patients of reproductive age. However, very little is known on the impact of medical treatments of CRC on fertility after cancer treatment. We aimed to discuss data existing so far relating to the gonadotoxicity of CRC treatments, fertility issues in CRC female patients of reproductive age and Fertility Preservation (FP) options in this context. We reviewed the literature to identify articles adressing the effect of CRC treatments on female fertility and oncofertility issues using databases EMBASE, the National Library of Medicine (MEDLINE)/PubMed, and the Cochrane Review Library. Studies suggest that although CRC chemotherapy might be midly toxic for ovaries, some cases of persistent amenorrhea have been reported, notably in rectal cancer patients. Pelvic radiotherapy might further impair ovarian reserve, as ovarian tissue is one of the most radiosensitive tissues. Although different FP options exist, it seems that CRC patients are not systematically addressed in FP consultations prior to systemic treatments. In all, data are scarce concerning the impact of medical treatments of CRC on female fertility. To date, in the lack of clear data, CRC patients of reproductive age should be referred to FP units to discuss FP options available.
Keywords
<p>Colorectal cancer; Female fertility; Fertility preservation</p>
Article Details
1. Introduction
Colorectal Cancer (CRC) is the third most common cancer diagnosed in women every year [1]. Due to more widespread screening and surveillance, the incidence of CRC is globally decreasing. However, when looking at the specific population of young adults, the incidence of CRC has been importantly increasing since at least the mid 1990s [2]. According to the American Cancer Society, the incidence of CRC in patients under 50 years old has increased by 2% every year from 2012 to 2016, with mortality rates increasing from 1.3% every year [1,2]. CRC in adolescents and young adults are reported to have more aggressive histological features and more advanced stages at the time of diagnosis [3]. Among CRC in young patients, some correspond to familial forms of the HNPCC spectrum and may be associated to gastric cancer and/or uterine cancers. Due to locally advanced stages and nodal invasion, some of these patients are candidates to systemic treatments [4]. Fortunately, survival rates of CRC are globally improving, with almost 65% of patients surviving at least 5 years from diagnosis [5]. Notably, patients under 50 years old might have a lower risk of death compared with older patients with CRC [6]. Hence, issues relating to life after cancer treatments are an essential part of the management and care of young CRC patients.
Fertility issues after cancer treatments is reported to be one of the major concerns of cancer survivors of reproductive age [7]. It is likely that an increasing number of CRC patients of reproductive age will have a pregnancy desire after CRC treatment [8,9], notably since a global trend towards delaying childbearing has been observed during the past years due to personal, educational or professional reasons [10,11]. Indeed, the proportion of first births to women aged 35 years old or more is eight times higher than 30 years ago [12]. Hence, the desire to start or continue a family project after treatment will become more and more frequent in young CRC patients. In this context, international guidelines recommend an early and prompt discussion to inform on the possible risks and available strategies to preserve fertility [13]. However, it seems that oncofertility care remains to be improved. Indeed, a large proportion of cancer patients of reproductive age report an absence of fertility counseling at diagnosis or unmet fertility needs [14]. The impact of chemotherapy regimens and radiotherapy used in the specific context of CRC on female fertility remains to be established. Moreover, global management of fertility issues in young CRC patients are extremely scarce. Very few data exist on whether fertility preservation (FP) options are adequately discussed to CRC patients of reproductive age, or on their use of FP techniques prior to systemic treatments.
We aim to discuss the questions relating to fertility issues in CRC female patients of reproductive age, the potential impact of CRC medical treatments on female fertility and the different FP options that can be proposed to these patients.
2. Materials and Methods
We performed a review of the literature to identify articles adressing the effect of CRC treatments on female fertility and oncofertility issues using the following databases: EMBASE, the National Library of Medicine (MEDLINE)/PubMed, and the Cochrane Review Library. MeSH terms used, included: colorectal neoplasms, chemotherapy, radiation, radiotherapy, fertility, fertility preservation, infertility, oocyte retrieval, vitrification, cryopreservation, oocyte cryopreservation, embryo preservation, pregnancy and birth outcomes. Randomized-controlled trials, cohort studies, case-control studies, case series, case reports or review articles (systematic reviews, meta-analyses) were included. Articles in English and French language were selected. Articles selected were examined for additional relevant references.
3. Results
3.1. Impact on chemotherapy treatments on female fertility
3.1.1. Adjuvant chemotherapy in locally advanced colorectal cancer
Initially based on fluoropyrimidines, the standard chemotherapy regimen for CRC worlwide is now based on the association of 5-fluorouracil (5-FU) and oxaliplatin (FOLFOX) [15,16]. The addition of oxaliplatin to capecitabine (XELOX) has also shown to improve disease-free survival rates in patients with stage III colon cancer compared to a standard bolus of FU and folinic acid in the adjuvant setting [17]. Irinotecan, an inhibitor of topoisomerase-1, can be also associated in rectal cancers. The combination of FOLFOX with irinotecan is known as FOLFIRINOX. Chemotherapy by FOLFOX is generally administered during 6 months. Secondary effects of these treatments include cumulative neurotoxicity and moderate hematologic toxicities. However, recent international studies aiming to reduce the duration of chemotherapy in order to reduce toxic effects show that the association of capecitabine and oxaliplatin administered during 3 months may be as effective compared to 6 months [18].
3.2. Effects of chemotherapy treatments on fertility
The effect of systemic treatments on fertility in breast cancer patients and time to pregnancy after breast cancer treatments has previously been studied [19]. However, the impact of chemotherapy regimens used in CRC on short-term or long-term fertility remain very ill established. Data are extremely scarce concerning the impact of medical treatments of CRC on fertility and lack high-quality studies and randomized controlled studies [20,21].
Among the scarce data existing so far, potential effects of 5-FU have only been analyzed in animal models [22,23]. The administration of a single dose of 5-FU in adult female mice seems to be mildly toxic for ovaries, as 5-FU did not alter the stock of primordial and primary follicles but significantly increased the atresia of secondary and antral follicles compared to the administration of a single dose of saline [23]. In addition, the impact of oxaliplatin on the reproductive function has been evaluated in 11 women (aged under 43 years old) and 8 men (aged under 45 years old) diagnosed with CRC [24]. Hormone levels and menstrual pattern were assessed at baseline and at 6 months post-treatment. All female patients had continued having menses or had resumed menstruation, and the administration of oxaliplatin did not appear to significantly affect hormone levels. However, these results have to be considered cautiously, as the study suffers from very small effectives and the absence of a control group [24]. In a retrospective series analyzing the risk of chemotherapy-induced amenorrhea after FOLFOX, 16% of women aged under 50 years old had persistent amenorrhea one year after completion of FOLFOX [25]. However, the study did not distinguish patients under 40 compared to those aged from 40 to 50 years old due to small effectives. To date, the largest study focusing on fertility issues after chemotherapy in CRC included 123 premenopausal women aged under 40 years old. Only 4.2% of patients with colon cancer had long-term amenorrhea versus 94.1% of patients with rectal cancer (p < 0.01), highlighting the importance of appropriate fertility counseling for these patients [26]. Concerning irinotecan, present in FOLFIRINOX regimens used in the adjuvant and neoadjuvant settings in rectal cancers, no study so far has analyzed its impact on fertility on patients of reproductive age [27] (Table 1).
Radiotherapy
The indication of treatment by radiotherapy in CRC is exceptional.
Until recently, the treatment of locally advanced rectal cancer (LARC) consisted of preoperative concurrent chemoradiotherapy followed by surgery. Currently, the treatment of locally advanced rectal cancer consists of chemotherapy followed by concurrent chemoradiotherapy and then surgery. Chemoradiotherapy treatment consists of delivering a dose of 44–45 Gy (5x1.8-2 Gy/week) of radiation to the mesorectum, the presacral space and internal iliac nodes +/- a boost to deliver a total dose of 50-54 Gy in combination with chemotherapy. Ovarian tissue is one of the most radiosensitive tissues in the body. Indeed, a dose of 2 Gy at the ovarian level is enough to destroy up to 50% of oocytes [28].
Several parameters may have an impact on procreation/fertility after pelvic radiotherapy. The first one is the radiation dose to which ovaries are exposed. In their mathematical model obtained from data from two cohorts of women with ovarian failure secondary to radiotherapy, Wallace et al. determined the radiation dose delivered to ovaries responsible for ovarian failure [29]. This dose decreased with increasing age at treatment: it was 20.3 Gy at birth; 18.4 Gy at 10 years; 16.5 Gy at 20 years; and 14.3 Gy at 30 years [29]. Some authors reported that a dose of 4 to 5 Gy delivered to both ovaries was sufficient to induce hypofertility [30,31]. The second important factor is the dose of radiation to which the uterus is exposed. Indeed, pelvic radiotherapy may cause damage to the vascularization of the uterus and/or to the endometrium, and may reduce uterine volume and alter uterine distensibility [32]. Loss of elasticity and vascular damage to the uterine body may occur as early as 14-15 Gy. The higher the dose and volume of uterus irradiated, the greater the damage [33]. Chiarelli et al. reported a higher frequency of low-birth-weight newborns (OR= 3.6), premature low-birth-weight newborns (OR= 3.3) and perinatal mortality (OR= 2.4) after abdominopelvic irradiation compared to treatment by surgery alone [34]. Furthermore, to which the vagina is exposed is also important. Indeed, vaginal irradiation can lead to vaginal synechiae and hypofertility through major dyspareunia. This is all the more important when the rectal tumor is located very low.
In order to preserve ovarian function in patients undergoing pelvic irradiation, ovarian transposition can be performed to move the ovaries away from the irradiation volume. Recent radiation techniques such as IMRT, VMAT, IGRT and adaptive radiotherapy can reduce the dose to the uterus and ovaries during pelvic irradiation. By using these techniques in combination with ovarian transposition, it would be possible to preserve a functional uterus and ovaries. In a case report, Mariani et al. reported the case of a 24-year-old nulligravida woman with cT3N1M0 LARC who expressed a desire of childbearing [35]. Before her preoperative chemoradiotherapy treatment, the patient had a left ovarian transposition by laparoscopy and cryopreservation of ovarian tissue. Then, 3 monthly GnRH-agonist injections were given before and during chemoradiotherapy to protect ovarian function. She received VMAT irradiation, delivering a dose of 45 Gy (5x1.8 Gy/week) to the posterior pelvis with a concomitant boost to the tumor delivering 55 Gy (5x2.2 Gy/week), in combination with oral chemotherapy with capecitabine (825 mg/m²x2/day). During radiotherapy planification, particular attention was paid to not exceed the dose of 3 Gy in the transposed ovary. Dosimetric analysis showed that the uterus and vagina (lower third) received a mean dose of 41.8 Gy and 22.1 Gy, respectively. The left ovary received a minimum dose of 0.6 Gy, a maximum dose of 2.1 Gy, and a mean dose of 1.1 Gy. The irradiation was performed with a full bladder. A cone-beam CT (CBCT) scan was performed daily to assess the position of the uterus. Menstrual cycles resumed before surgery. Four months after surgery, follow-up showed no signs of recurrent disease and the patient reported regular menstrual cycles during all the follow-up time after surgery. Furthermore, Kurt et al. reported a spontaneous pregnancy in a 24-year-old woman treated with adjuvant chemoradiotherapy after lateral ovarian transposition for rectal cancer [36]. Menstrual cycles of the patient resumed without performing any medical treatment two months after the completion of chemoradiotherapy. Two years after the end of the treatment, the patient became pregnant spontaneously with no recurrence of rectal cancer. In all, due to the lack of current data, prospective studies with a larger number of patients treated with modern irradiation techniques are needed. The use of modern irradiation techniques such as VMAT with daily cone beam CT to decrease the radiation dose delivered to the ovaries, uterus and lower 1/3 of the vagina, should be priviledged. Given the encouraging survival rates in young patients with locally advanced rectal cancer, questions relating to FP are essential.
3.4. Oncofertility counseling
The greatest reproductive concerns expressed by cancer patients of reproductive age relate to fertility potential and the health of future offspring [14]. However, it seems that an important proportion of cancer patients do not receive adequate and timely information on fertility issues and possibilities of FP [37,38]. Notably, there might be a difference between men and women, as most men report having received information about treatment impact on fertility and FP with more than half of them undergoing sperm cryopreservation prior to systemic treatments, whereas less than half of cancer female patients report receiving information about the impact of treatments on fertility [39]. According to ESHRE guidelines, clinical care of cancer patients of reproductive age should include information on the impact of the disease and treatments on fertility and on the existence of FP techniques [40]. Information on cryopreservation storage after FP, on pregnancy after gonadotoxic treatment and other childbearing and parenting options should also be provided [41,42]. It is recommended that patients be referred to a specific FP consultation and to provide decision aids to patients considering FP [40]. A widespread use of an FP checklist for a better provision of oncofertility issues might also be useful [43]. Furthermore, additional psychological support when dealing with FP decisions might improve the process and quality of life of cancer patients during this crucial point of patient care [7,44]. To predict high and low response to ovarian stimulation, assessment of Antral Follicle Count (AFC) and Anti-Müllerian Hormone (AMH) serum levels is recommended [45,46]. The risk of premature ovarian failure after cancer treatments relies on age, type of gonadotoxic treatment and dose administered, and pre-treatment AMH levels [40]. Hence, assessment of pre-treatment ovarian function, in particular through AMH levels, in premenopausal women is recommended to predict post-treatment recovery of ovarian function [47-49].
3.5. FP techniques
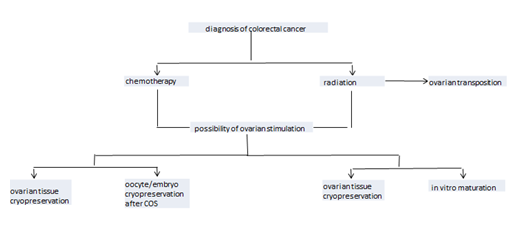
Fertility can be preserved through several procedures, including cryopreservation of oocytes and/or embryos, cryopreservation of ovarian tissue, and medical and surgical methods of ovarian protection (Figure 1).
3.6. Oocyte and/or embryo cryopreservation
Oocyte and/or embryo cryopreservation by vitrification after ovarian stimulation by gonadotropins (when not contraindicated) is the method of choice for women undergoing FP procedures for medical indications [40,50-52]. Ovarian stimulation using an antagonist protocol should be privileged due to its safety (enabes to reduce the risk of ovarian hyperstimulation) and feasibility in urgent conditions [53]. Ovarian stimulation is typically initiated at the onset of menses. However, in urgent FP cycles, starting ovarian stimulation immediately, known as random-start ovarian stimulation, is an option that leads to comparable results in terms of oocyte yield [54,55]. Double stimulation can also be considered for urgent FP cycles [53]. Embryo cryopreservation is also an option in case of the existence of a male partner. However, women should be informed that embryo cryopreservation enables to preserve the fertility of the couple and not of the women by herself. Therefore, use of cryopreserved embryos is not possible in case of separation of the couple or refusal of the male partner. Altogether, women considering oocyte and/or embryo cryopreservation should be fully informed that these techniques do not guarantee a pregnancy after cancer treatments. Success rates, risks, benefits, costs and the possible long-term consequences should be discussed.
3.7. In vitro maturation (IVM)
IVM is still considered as an experimental procedure, but is particularly interesting when ovarian stimulation is contraindicated. IVM consists in retrieving immature cumulus-oocyte complexes at the prophase I stage and maturing them in vitro until the metaphase II stage [56]. Although IVM was first developed for patients with polycystic ovary syndrome (PCOS) since it avoids the risk of ovarian hyperstimulation syndrome [57], indications of IVM have expanded. IVM has become a major option for fertility preservation, notably when ovarian stimulation is unfeasible or contraindicated in an oncologic context [58,59]. One of the great advantages of IVM is that it can be performed at any stage of the menstrual cycle, which is particularly appropriate when urgent fertility preservation is required, for instance prior to oncological treatments [60]. Nevertheless, controlled ovarian stimulation remains the option to be privileged when possible, as significantly higher implantation rates, clinical pregnancy rates and live birth rates have been described in IVF with controlled ovarian stimulation compared to IVM [61].
3.8. Ovarian tissue cryopreservation (OTC)
Ovarian TISSUE CRYOPRESERVATION (OTC) is an important option either through choice, or if there is insufficient time for ovarian stimulation. OTC consists in the laparoscopic removal of a portion, one, or both ovaries, which are then sectioned into strips of tissue less than 2mm thick and cryopreserved [62]. After treatments, the ovarian tissue is transplanted to the patient, either in an orthotopic position (pelvic) or in a heterotopic position (such as the forearm or abdominal wall [63]. Ovarian tissue transplantation requires a multidisciplinary approach. A one-step laparoscopy procedure should be performed as it is considered safe without causing additional surgical risk. The presence of residual neoplastic cells in the ovarian cortex (and in the residual medulla when available) is evaluated before the procedure. Ovarian tissue transplantation is not recommended in cases where the ovary is involved in the malignancy. Overall, it is recommended to offer OTC in patients undergoing moderate/high-risk gonadotoxic treatment where oocyte/embryo cryopreservation is not feasible, or at patient preference [64,65].
3.9. Gonadotropin-Releasing Hormone agonist (GnRHa)
On a physiological rationale, the use of GnRH agonists during chemotherapy may be beneficial by suppressing the follicle-stimulating hormone axis leading to a decreased number of primordial follicles entering development and thus exposed to the potential gonadotoxic effect of treatments. Furthermore, the subsequent hypoestrogenism decreases ovarian perfusion and participates in a reduces exposure of the ovaries to cytotoxic agents [20]. However, limited evidence exists on the real benefit on the use of GnRHa in this context. In malignancies other than breast cancer, GnRH agonists should not be routinely offered as an option for ovarian function protection and FP without discussion of the uncertainty about its benefit [66].
3.10. Ovarian transposition
In case of treatment by pelvic radiotherapy without chemotherapy, ovarian transposition (oophoropexy) can be proposed to prevent the gonadotoxic effects of pelvic radiation [67]. Ovarian transposition consists in surgically mobilizing one or both of the ovaries and fixing them to the abdominal sidewall at the pelvic brim [68]. Because radiotherapy in case of CRC often implies high cumulative doses of radiation, ovarian transposition away from the target area is an interesting and valuable option to reduce ovarian exposure. However, patients should be informed that ovarian transposition do not prevent the risk of ovarian damage [32]. Women with reduced ovarian reserve and women at risk of having ovarian metastases are inappropriate candidates for ovarian transposition. Furthermore, ovarian transposition can be performed in addition to another FP technique such as after oocyte/embryo cryopreservation.
4. Conclusion
The incidence of CRC is increasing in patients of reproductive age, among which patients with familial forms of CRC diagnosed at a young age. Although they are often candidates to medical treatments potentially gonadotoxic such as chemotherapy and radiotherapy, data on the impact of CRC treatments on fertility are extremely scarce. Robust and large-scale studies are required to evaluate the gonadotoxicity of these treatments. In this context, and given the lack of knowledge in this field, it is essential to inform patients on the possibility of FP before treatments and to develop an optimal manadgment of fertility issues in order to improve life after cancer.
Disclosure statement
Authors have no conflict of interest to declare.
References
- Colorectal Cancer Statistics. How Common Is Colorectal Cancer?.
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 70 (2020): 145-64.
- Read B, Sylla P. Aggressive Colorectal Cancer in the Young. Clin Colon Rectal Surg 33 (2020): 298-304.
- Tougeron D, Mouillet G, Trouilloud I, et al. Efficacy of Adjuvant Chemotherapy in Colon Cancer With Microsatellite Instability: A Large Multicenter AGEO Study. J Natl Cancer Inst 108 (2016).
- Colorectal Cancer Survival Rates. Colorectal Cancer Prognosis (2021).
- Cheng E, Blackburn HN, Ng K, et al. Analysis of Survival Among Adults With Early-Onset Colorectal Cancer in the National Cancer Database. JAMA Network Open 4 (2021): 2112539.
- Anazodo A, Laws P, Logan S, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update 25 (2019): 159-79.
- Lambertini M, Peccatori FA, Demeestere I, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Annals of Oncology 31 (2020): 1664-1678.
- Letourneau JM, Ebbel EE, Katz PP, et al. Pre-treatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 118 (2012): 1710-1717.
- Mathews TJ, Hamilton BE. Mean Age of Mothers is on the Rise: United States, 2000-2014. NCHS Data Brief ( 2016): 1-8.
- Mills M, Rindfuss RR, McDonald P, et al. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update 17 (2011): 848-860.
- Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief (2009): 1-8.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 36 (2018): 1994-2001.
- Benedict C, Thom B, N Friedman D, et al. Young adult female cancer survivors’ unmet information needs and reproductive concerns contribute to decisional conflict regarding posttreatment fertility preservation. Cancer 122 (2016): 2101-2109.
- Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 29 (2011): 3768-3774.
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27 (2009): 3109-3116.
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 29 (2011): 1465-1471.
- Grothey A, Sobrero AF, Shields AF, et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. New England Journal of Medicine 378 (2018): 1177-1188.
- Labrosse J, Lecourt A, Hours A, et al. Time to Pregnancy, Obstetrical and Neonatal Outcomes after Breast Cancer: A Study from the Maternity Network for Young Breast Cancer Patients. Cancers (Basel) 13 (2021): 1070.
- Shandley LM, McKenzie LJ. Recent Advances in Fertility Preservation and Counseling for Reproductive-Aged Women with Colorectal Cancer: A Systematic Review. Dis Colon Rectum 62 (2019): 762-771.
- Chae-Kim J, Hayslip CC. Fertility and Endocrine Preservation in the Management of Colorectal Cancer in Women. Dis Colon Rectum 63 (2020): 723-726.
- Hrushesky WJ, Vyzula R, Wood PA. Fertility maintenance and 5-fluorouracil timing within the mammalian fertility cycle. Reprod Toxicol 13 (1999): 413-420.
- Lambouras M, Liew SH, Horvay K, et al. Examination of the ovotoxicity of 5-fluorouracil in mice. J Assist Reprod Genet 35 (2018): 1053-1060.
- Levi M, Shalgi R, Brenner B, et al. The impact of oxaliplatin on the gonads: from bedside to the bench. Mol Hum Reprod 21 (2015): 885-893.
- Cercek A, Siegel CL, Capanu M, et al. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin Colorectal Cancer 12 (2013): 163-167.
- Wan J, Gai Y, Li G, et al. Incidence of chemotherapy- and chemoradiotherapy-induced amenorrhea in premenopausal women with stage II/III colorectal cancer. Clin Colorectal Cancer 14 (2015): 31-34.
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology 22 (2021): 702-715.
- Wallace WHB, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod 18 (2003): 117-121.
- Wallace WHB, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 62 (2005): 738-744.
- Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 27 (2009): 2677-2685.
- Sudour H, Chastagner P, Claude L, et al. Fertility and pregnancy outcome after abdominal irradiation that included or excluded the pelvis in childhood tumor survivors. Int J Radiat Oncol Biol Phys 76 (2010): 867-873.
- Wo JY, Viswanathan AN. The Impact of Radiotherapy on Fertility, Pregnancy, and Neonatal Outcomes of Female Cancer Patients. Int J Radiat Oncol Biol Phys 73 (2009): 1304-1312.
- Critchley HO. Factors of importance for implantation and problems after treatment for childhood cancer. Med Pediatr Oncol 33 (1999): 9-14.
- Chiarelli AM, Marrett LD, Darlington GA. Pregnancy outcomes in females after treatment for childhood cancer. Epidemiology 11 (2000): 161-166.
- Mariani S, Chiloiro G, Villa P, et al. Fertility preservation in chemo-radiotherapy for rectal cancer: A combined approach. Clin Transl Radiat Oncol19 (2019): 77-79.
- Kurt M, Uncu G, Cetintas SK, et al. Successful spontaneous pregnancy in a patient with rectal carcinoma treated with pelvic radiotherapy and concurrent chemotherapy: the unique role of laparoscopic lateral ovary transposition. Eur J Gynaecol Oncol 28 (2007): 408-410.
- Chin HB, Howards PP, Kramer MR, et al. Which female cancer patients fail to receive fertility counseling before treatment in the state of Georgia? Fertil Steril 106 (2016): 1763-1771.
- Niemasik EE, Letourneau J, Dohan D, et al. Patient perceptions of reproductive health counseling at the time of cancer diagnosis: a qualitative study of female California cancer survivors. J Cancer Surviv 6 (2012): 324-332.
- Armuand GM, Rodriguez-Wallberg KA, Wettergren L, et al. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol 30 (2012): 2147-2153.
- ESHRE Guideline Group on Female Fertility Preservation, Anderson RA, Amant F, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open 2020 (2020): hoaa052.
- Goossens J, Delbaere I, Van Lancker A, et al. Cancer patients’ and professional caregivers’ needs, preferences and factors associated with receiving and providing fertility-related information: a mixed-methods systematic review. Int J Nurs Stud 51 (2014): 300-319.
- Silva C, Almeida-Santos AT, Melo C, et al. Antineoplastic Agents and (In)fertility: Informing Patients to Improve Decisions. J Adolesc Young Adult Oncol 7 (2018): 306-314.
- Kemertzis MA, Ranjithakumaran H, Hand M, et al. Fertility Preservation Toolkit: A Clinician Resource to Assist Clinical Discussion and Decision Making in Pediatric and Adolescent Oncology. J Pediatr Hematol Oncol 40 (2018): 133-139.
- Logan S, Perz J, Ussher JM, et al. A systematic review of patient oncofertility support needs in reproductive cancer patients aged 14 to 45 years of age. Psychooncology 27(2018): 401-409.
- Broer SL, Broekmans FJM, Laven JSE, et al. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 20 (2014): 688-701.
- Victoria M, Labrosse J, Krief F, et al. Anti Müllerian Hormone: More than a biomarker of female reproductive function. J Gynecol Obstet Hum Reprod 48 (2019): 19-24.
- Dillon KE, Sammel MD, Prewitt M, et al. Pre-Treatment AMH Determines Rate of Post-Therapy Ovarian Reserve Recovery: Acute Changes in Ovarian Reserve During and After Chemotherapy. Fertil Steril 99 (2013): 477-483.
- Anderson RA, Rosendahl M, Kelsey TW, et al. Pretreatment anti-Müllerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer 49 (2013): 3404-3411.
- Dezellus A, Barriere P, Campone M, et al. Prospective evaluation of serum anti-Müllerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer 79 (2017): 72-80.
- Cobo A, Garcia-Velasco JA, Domingo J, et al. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril 99 (2013): 1485-1495.
- Massarotti C, Scaruffi P, Lambertini M, et al. State of the art on oocyte cryopreservation in female cancer patients: A critical review of the literature. Cancer Treat Rev 57 (2017): 50-57.
- Druckenmiller S, Goldman KN, Labella PA, et al. Successful Oocyte Cryopreservation in Reproductive-Aged Cancer Survivors. Obstet Gynecol 127 (2016): 474-480.
- Ovarian Stimulation TEGGO, Bosch E, Broer S, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open 2020 (2020): hoaa009.
- Marklund A, Eloranta S, Wikander I, et al. Efficacy and safety of controlled ovarian stimulation using GnRH antagonist protocols for emergency fertility preservation in young women with breast cancer—a prospective nationwide Swedish multicenter study. Human Reproduction 35 (2020): 929-938.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol 27 (2015): 215-221.
- In vitro maturation: a committee opinion. Fertility and Sterility 115 (2021): 298-304.
- Siristatidis CS, Maheshwari A, Vaidakis D, et al. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst Rev 11 (2018).
- Donnez J, Dolmans MM. Fertility Preservation in Women. N Engl J Med 378 (2018): 400-401.
- Grynberg M, Sonigo C, Santulli P. Fertility Preservation in Women. N Engl J Med 378 (2018): 400.
- De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet 384 (2014): 1302-1310.
- Gremeau AS, Andreadis N, Fatum M, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case-control study of 194 treatment cycles. Fertil Steril 98 (2012): 355-360.
- Ovarian tissue cryopreservation: a committee opinion. Fertility and Sterility 101 (2014): 1237-1243.
- Oktay K, Buyuk E, Veeck L, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet 363 (2004): 837-840.
- Pacheco F, Oktay K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod Sci 24 (2017): 1111-1120.
- Gellert SE, Pors SE, Kristensen SG, et al. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet 35 (2018): 561-570.
- Senra JC, Roque M, Talim MCT, et al . Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 51 (2018): 77-86.
- Hoekman EJ, Broeders EABJ, Louwe LA, et al. Ovarian function after ovarian transposition and additional pelvic radiotherapy: A systematic review. Eur J Surg Oncol 45 (2019): 1328-1340.
- Arian SE, Goodman L, Flyckt RL, et al. Ovarian transposition: a surgical option for fertility preservation. Fertil Steril 107 (2017): e15.


 Impact Factor: * 3.4
Impact Factor: * 3.4 Acceptance Rate: 78.89%
Acceptance Rate: 78.89%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 