Use of Magnetic Tracer and Magnetic Resonance Imaging for Sentinel Lymph Node Detection after Breast Cancer Recurrence and Previous Axillary Surgery
Eva Vikhe Patil1*, Amalía Segerbard Planoudis2, Kian Chin2, Carlos Dussan1, Henrik Leonhardt3, Pontus Zaar3, Pantelis Gialis4, Fredrik Wärnberg2
1Department of surgery, BKV University Hospital Linkoping, Sweden
2Department of Surgery, Sahlgrenska Academy at Gothenburg University, Sweden
3Department of Radiology, Sahlgrenska Academy at Gothenburg University, Sweden
4Department of Radiology, University Hospital Linkoping, Sweden
Corresponding Author: Eva Vikhe Patil, Department of surgery, BKV University Hospital Linkoping, Sweden.
Received: 19 December 2024; Accepted: 23 December 2024; Published: 28 January 2025
Article Information
Citation: Eva Vikhe Patil, Amalía Segerbard Planoudis, Kian Chin, Carlos Dussan, Henrik Leonhardt, Pontus Zaar, Pantelis Gialis, Fredrik Wärnberg. Use of Magnetic Tracer and Magnetic Resonance Imaging for Sentinel Lymph Node Detection after Breast Cancer Recurrence and Previous Axillary Surgery. Journal of Women’s Health and Development. 8 (2025): 01-06.
DOI: 10.26502/fjwhd.2644-288400132
View / Download Pdf Share at FacebookAbstract
Introduction: Preoperative mapping increases the chance of finding the sentinel lymph node (SLN) in breast cancer (BC) patients with earlier axillary surgery. Today preoperative mapping is usually done using Technetium-99m (Tc99), and lymphoscintigraphy. An alternative method has been proposed using superparamagnetic iron oxide nanoparticles (SPIO) and magnetic resonance imaging (MRI). The aim of this study was to evaluate the feasibility of the magnetic technique for SLN mapping and perioperative localization in patients with breast cancer recurrence and previous axillary surgery. Method: Consecutive patients with breast cancer recurrence, earlier axillary surgery, and planned for SLN-biopsy were included. SPIO was injected and axillary MRI was conducted up to four weeks before surgery. Tc99 and lymphoscintigraphy was performed in all according to clinical routine. SLN detection per method was recorded. Results: In total 22 patients received both SPIO and Tc99. SLN mapping was successful by using SPIO/MRI in 73% (16/22) compared to 50% (11/22) by using Tc99/lymphoscintigraphy (p=0.12). Perioperative SLN detection was 17/22 (77%) using SPIO/magnetic probe and 10/22 (45%) using Tc99/gamma-probe (p=0.03). The magnetic technique detected 26 of totally 27 (96%) removed lymph nodes while Tc99/gamma-probe detected a signal in 12 (44%) (p<0.00003). Conclusion: It is feasible, and perhaps preferable, to perform a repeat SLN-biopsy using the magnetic technique in patients with recurrent breast cancer. Advantage with SPIO are the longer time frame that it remains in the lymph nodes which facilitate logistic planning for surgery as well as the less complicated process in handling the material compared to using Tc99.
Keywords
<p>Breast cancer; Recurrence; SLN; SPIO; MRI</p>
Article Details
Introduction
For primary breast cancer patients, axillary lymph node status is the most significant individual prognostic factor and helps in treatment decisions [1]. In the event of cancer recurrence in the breast, sentinel lymph node biopsy (SLNB) is usually recommended if the patient is clinically node negative [2]. If a SLNB or an axillary lymph node dissection (ALND) has previously been performed, a new SLN can be found in only 52% to 81% of these patients [3]. A new SLN can sometimes be located aberrantly due to changed lymphatic drainage after earlier breast and axillary surgery, e.g., in the contralateral axilla [3, 4]. The success rate of finding a new SLN is related to several factors such as number of lymph nodes extracted at previous SLNB or ALND, type of surgery (breast conserving surgery or mastectomy), and whether the axilla had been previously treated with radiotherapy [2, 5, 6]. Preoperative mapping of the SLN with lymphoscintigraphy has been shown to increase the chance of finding a new SLN, especially if it is aberrantly located [2, 7]. However, there are shortcomings associated with lymphoscintigraphy such as the need of a nuclear medicine department, issues with availability of the SLN tracer Technetium-99m (Tc99), and radiation exposure to healthcare professionals and patients [8]. Also, as the half-life of Tc99 is only 6.1 hours [9], the surgery has to be performed close to the time of injection of Tc99. Thus, a new technique of SLN localization has been proposed, using superparamagnetic iron oxide nanoparticles (SPIO, Magtrace®, Endomag., Cambridge, UK) as SLN tracer and magnetic resonance imaging (MRI) of the axillae after the local SPIO injection for SLN mapping [10-13]. A handheld probe containing a sensitive magnetometer (Sentimag®, Endomag., Cambridge, UK) is used perioperatively to detect SLNs.
The primary aim of this study was to evaluate the feasibility of using SPIO in combination with MRI of the axillae for preoperative lymph node mapping and the magnetic probe to localize the SLNs perioperatively in patients with a breast cancer recurrence and having had previous axillary surgery. The secondary aim was to investigate if same lymph nodes were detected with the magnetic method compared to the standard method of using Tc99 in combination with lymphoscintigraphy and a gamma-probe.
Materials and Methods
In this prospective feasibility study, consecutive patients with a breast cancer recurrence with previous axillary surgery who were planned for a SLNB were included from two Swedish hospitals; Sahlgrenska University Hospital, Gothenburg and Linköping University Hospital, during April 1st, 2022 to October 31st, 2023. The inclusion criteria were age 18 years or older, recurrent DCIS or invasive breast cancer with earlier axillary surgery and planned for a new SLNB. Pregnant women, patients with regional and distant metastases, and patients with contraindications to MRI or iron overload disease were excluded. All patients received an injection of 1.0 ml SPIO at the outpatient clinic up to 30 days before surgery. The SPIO was injected peritumourally in the breast or thoracic wall close to the recurrence. The patients were planned for an MRI of both axillae, preoperatively to identify a SLN and it´s localisation, any time before surgery. The MRI of the axillae was performed according to a specific MRI protocol, 3T system (Achieva dStream; Philips, Best, the Netherlands) (Philips, Amsterdam, The Netherlands). Patient were examined in the supine position with arms up and outwards and the examination took about 30 minutes (Appendix 1). In addition to SPIO, an injection of Tc99 was made preoperatively close to the in-breast recurrence or local thoracic recurrence followed by a lymphoscintigraphy on the day of surgery or the day before. The number and localisation of the SLNs were preoperatively described in the report from the MRI by one of two dedicated radiologists (HL or PZ) in Gothenburg and one dedicated radiologist in Linköping (PG), and from the lymphoscintigraphy by the ordinary physician at the department of nuclear medicine at each hospital. The surgeon had access of images and reports from the MRI and the lymphoscintigraphy at the time of surgery. During surgery, all SLN signal measurements were always firstly done with the magnetic probe and thereafter with the gamma-probe (Gamma Finder®, World of Medicine, Germany). Just before surgery, the transcutaneous signal in the axillae was measured, thereafter separate measurements were made for each retrieved SLN. A SLN was defined as a node identified by any of the probes. Any node with a count exceeding 10% of the SLN with the highest count per tracer method was denoted an additional SLN. All lymph nodes were sent for histopathological analysis separately.
|
Sequence |
Orientation |
FOV (mm2) |
Slice thickness |
Coverage |
|
|
(mm) |
|||||
|
1 |
T2W TSE |
coronal |
For planning |
||
|
2 |
T2W TSE |
trans axial |
150 |
3 |
Thorax aperture -axillae |
|
3 |
3D T1 Dixon all |
trans axial |
150 |
1,5 |
Thorax aperture -axillae |
|
4 |
2D T2*W |
trans axial |
150 |
3 |
Thorax aperture -axillae |
|
Abbreviations: FOV = field of view, T2W = T2-weighted, TSE = turbo spin echo |
|||||
APPENDIX 1, MRI protocol for the multicentre study comparing SPIO (Magtrace) MRI axillae and standard Technetium99 lymphoscintigraphy for sentinel lymph node localization before surgery of breast cancer recurrence.
System: 3 T. Phase-array surface coil.
Patient preparation: After the surgeon has injected SPIO (Magtrace) interstitially close to the tumor.
Note 1: Arms above head if the patient can manage.
Note 2: The breasts are not of main interest in this examination and can if possible be avoided Note 3: Both axillae shall always be examined!
Statistical Analysis
This was a feasibility study, and no power calculation was done. Descriptive data are presented as numbers with percentages and medians with interquartile ranges. Fisher exact test comparing proportions was used with a significance level of 0.05.
The study primary endpoint was SLN detection per patient. Secondary endpoints were concordance and reversed concordance per lymph node. Concordance was defined as the number of lymph nodes detected with both SPIO and Tc99 divided by the total number of nodes detected by Tc99 (Tc99 and SPIO / Tc99 + Tc99 and SPIO), i.e., how many of the SLNs detected by Tc99 were also detected by SPIO. Reversed concordance was defined as the number of lymph nodes detected with both SPIO and Tc99 divided by the total number of nodes detected by SPIO (SPIO and Tc99 / SPIO + SPIO and Tc99), i.e., how many of the SLNs detected by SPIO were also detected by Tc99.
Results
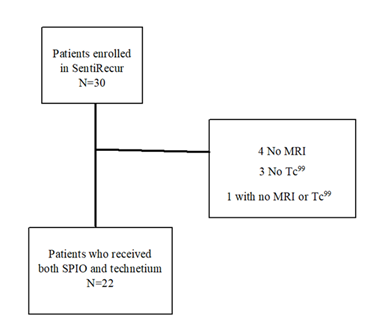
A total of 30 patients were recruited 28 in Gothenburg and two in Linköping. Four patients did not receive Tc99 due to shortages in supply for a couple of weeks in 2022. Five patients did not undergo an MRI, all at Sahlgrenska, one due to claustrophobia and four did not receive MRI due to lack of access, of whom one did not receive Tc99. In total, 22 patients who received both SPIO and Tc99 and performed both MRI and lymphoscintigraphy were eligible for analyses (Figure 1, Table 1).
|
Age at recurrence, median (range) years |
63 (40-82) |
|
Surgery for primary cancer |
|
|
Mastectomy |
5 |
|
Breast Conserving |
17 |
|
Prior axillary staging |
|
|
ALND |
7 |
|
SLN |
15 |
|
Time to recurrence median (range) years |
10.5 (2-31) |
|
Type of recurrent cancer |
|
|
Invasive |
20 |
|
DCIS |
2 |
|
Abbreviations: MRI = magnetic resonance imaging, ALND = axillary lymph node dissection, SLN = sentinel lymph node, DCIS =ductal carcinoma in situ. |
|
Table 1: Demographic of the study cohort, 22 patients with recurrence and undergoing a sentinel node biopsy with magnetic tracer and MRI for visualisation.
Overall, there was no statistically significant difference in SLN localisation per patient using SPIO and MRI compared with Tc99 and lymphoscintigraphy, 73% (16 of 22) vs. 50% (11 of 22), p=0.2. Nevertheless, a clinically significant difference of 23% detection rate in favour of SPIO and MRI was observed. Perioperative SLN detections per patient using SPIO and magnetic probe compared with Tc99 and gamma probe were 77% (17 of 22) vs. 45% (10/22), respectively (p=0.06) (Table 2).
|
|
SPIO/MRI/Sentimag |
Tc99/Scint/Gamma-probe |
|
Preoperative mapping |
16/22 (73%) |
11/22 (50%) |
|
Per-operative signaling |
17/22 (77%) |
11/22 (50%) |
|
Abbreviations: MRI = Magnetic resonance imaging, SPIO = Superparamagnetic iron oxide nanoparticles, Scint = Lymphoscintigraphy, Tc99 = Technetium-99m |
||
Table 2: Result of imaging and per-operative signaling, detection rate per patient with recurrence, receiving both SPIO and Tc99.
In one patient, an area was found to have both magnetic and radioactive signals perioperatively despite negative localisation in the preoperative MRI and lymphoscintigraphy. This area was excised but the final histopathological report did not show any lymph node and therefore the SLNB was regarded as a non-successful attempt. In three patients, SLN nodes were localised preoperatively in the contralateral axilla, two of those patients showed only SPIO signals and the third patient showed both SPIO and Tc99 signals. Five patients had neither magnetic nor radioactive signals perioperatively and in these patients, no nodes were found during surgery in the axillae. However, one of these patients showed signals of both SPIO and Tc99 parasternally, but a perioperative decision was made not to proceed with excision of this node. In total, 27 lymph nodes classified as SLNs were removed and another three lymph nodes were reported by the pathologists. The detection rates for SPIO compared with Tc-99 were 96% (26 of 27) vs. 44% (12 of 27), respectively (p=0).
The median number of nodes removed using SPIO was one (IQR =1), with an average of 27/22 (1.22) and for Tc99 the median was one (IQR =1), with an average of 12/22 (0.55). All lymph nodes that were detected with the gamma-probe except one were also detected with the magnetic probe. Concordance per node was 11/12 (92%), meaning that of the 12 nodes detected by Tc99 and gamma-probe eleven were also detected by SPIO using a magnetic probe. The reversed concordance was 11/26 (42%) as only eleven of the 26 nodes detected by SPIO and the magnetic probe were also detected by Tc99 and gamma-probe. There was only one SLN with a metastasis and this SLN was exclusively identified with the magnetic technique, both preoperatively with SPIO and MRI, and perioperatively with SPIO and magnetic probe.
Discussion
This study showed that the magnetic technique with SPIO and MRI for SLN mapping and SPIO and Sentimag for SLN detection was feasible in recurrent breast cancer patients with a history of earlier axillary surgery. The magnetic technique had higher SLN detection rate than Tc99in combination with lymphoscintigraphy and gamma-probe used in parallel in the same patients. More SLNs per patient, and the only detected SLN metastasis, were detected with the magnetic technique.The magnetic technique enables logistic advantages compared to the radioisotope as the magnetic tracer can be used during a wider time frame [8]. The SPIO stays in the SLN for weeks and thus, an MRI can be performed days to weeks before surgery [10, 14, 15]. Furthermore, the injection of SPIO well before surgery might be related to the better SLN detection than that of radioisotope and lymphoscintigraphy noted in this study and in earlier studies [15]. In this feasibility study the number of included patients was small and comparing SLN detection between the magnetic and radioactive methods was not the primary purpose. Furthermore, SLN detection in the recurrent setting is less successful than in primary surgery as earlier surgery and radiation affects the lymphatic drainage patterns [16]. Nevertheless, the results indicated in this study highlighted that SLNB can be performed using the magnetic technique. A further point of note was that this study had the advantage of using the two different techniques in parallel within the same patients making comparisons more valid. There is an argument against repeating SLNB as the disrupted lymphatic drainage due to fibrosis from previous surgery and radiation, have resulted in low success rates in finding a new SLN leading to an ALND instead [17, 18]. Also, a successful repeat SNLB depends on the extent of previous axillary surgery [19]. We do not know if an ALND would have been beneficial in staging those five patients in this study where no new SLN was found. An axillary sampling was done in two patients and no further surgery was performed in the other three patients. However, mapping with MRI or lymphoscintigraphy can be used to avoid ALND in many cases and especially an unnecessary ALND if the new SLN is located aberrantly [3, 17]. The Dutch study group, Sentinel Node and Recurrent Breast Cancer, (SNARB), has earlier shown that it is feasible to find a new SLN and of course, a SLNB is associated with less co-morbidity than ALND [20]. Furthermore, in a study from Scotland, they showed as high as 73% re-SLN detection success, showing there is high precision in adopting a repeat SLNB approach instead of ALND [21].
In the recurrent setting there is a risk of aberrant lymph drainage. However, by using preoperative mapping, aberrantly located SLNs can be pre-identified thereby avoiding removal of nodes that may not be in the primary drainage pathway [21]. In another study by Schulze, et al. [19], they showed that a repeat-SLNB after earlier mastectomy is feasible. This was also shown in a Danish study where the aberrant lymphatic pathways were clearly seen at the time of re-SLN procedure [2]. It has also been shown that repeat-SLNB is more accurate than ALND due to the aberrant lymph drainage [4, 20]. However, the importance of regional lymph node staging in the recurrent cancer setting is controversial [22]. The prognosis following a recurrence in breast cancer depends on the disease-free interval, extent and location of the recurrent tumour, and prognostic characteristics of the primary breast tumour biology [23]. A study by Vugt et al., showed that among patients with breast cancer recurrences with metastasis in a SLN from a repeat SLNB, the decision regarding adjuvant treatment was made based on the SLN pathology report alone, only in 63% of the patients. To be noticed, 80% of the patients in this study had a non-metastatic SLN [4]. Moreover, the reported risk of lymph node metastases in patients in the recurrent setting was 26% if an earlier SLNB at the time of primary surgery showed a negative SLN [24].
Conclusion
This study show that it is feasible and perhaps preferable to perform a repeat SLNB using the magnetic technique amongst patients with recurrent breast cancer. The advantages with SPIO are the longer time frame that it remains in the lymph nodes which facilitate logistic planning for surgery as well as the less complicated process in handling the material compared to using Tc99.
Data availability
For accesss to study data contact the corresponding author Eva VikhePatil.
Conflict of interest.
There is no conflict of interest.
Funding
There are no disclosure and this study was funded by the Swedish Breast Cancer Society (Bröstcancerförbundet).
Acknowledgments
To all the patients who participated and health personal at the clinic.
Preliminary data was presented as a poster at the San Antonio Breast cancer symposium 2023 and published in Cancer Research online.[25]
References
- Houssami N, J Cuzick, JM Dixon. The prevention, detection, and management of breast cancer. Med J Aust 184 (2006): 230-234.
- Uth CC, et al. Sentinel Lymph Node Dissection in Locally Recurrent Breast Cancer. Annals of Surgical Oncology 22 (2015): 2526-2531.
- Maaskant-Braat AJG, et al. Repeat sentinel node biopsy in patients with locally recurrent breast cancer: a systematic review and meta-analysis of the literature. Breast Cancer Research and Treatment 138 (2013): 13-20.
- Vugts G, et al. Repeat sentinel node biopsy should be considered in patients with locally recurrent breast cancer. Breast Cancer Res Treat 153 (2015): 549-556.
- Sávolt Á, et al. Sentinel lymph node biopsy following previous axillary surgery in recurrent breast cancer. Eur J Surg Oncol 45 (2019): 1835-1838.
- Yoon CI, et al. Repeat Sentinel Lymph Node Biopsy for Ipsilateral Breast Tumor Recurrence After Breast Conserving Surgery With Sentinel Lymph Node Biopsy: Pooled Analysis Using Data From a Systematic Review and Two Institutions. Front Oncol 10 (2020): 518568.
- Ikeda T. Re-sentinel node biopsy after previous breast and axillary surgery. Surg Today 44 (2014): 2015-2021.
- Karakatsanis A, et al. The Nordic SentiMag trial: a comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res Treat 157 (2016): 281-294.
- Shi K, et al. Determination of technetium-99 in environmental samples: a review. Anal Chim Acta 709 (2012): 1-20.
- Motomura K, et al. Superparamagnetic iron oxide-enhanced MRI at 3 T for accurate axillary staging in breast cancer. Br J Surg 103 (2016): 60-69.
- Houpeau JL, et al. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: The French Sentimag feasibility trial. J Surg Oncol 113 (2016): 501-507.
- Hersi AF, et al. Optimizing Dose and Timing in Magnetic Tracer Techniques for Sentinel Lymph Node Detection in Early Breast Cancers: The Prospective Multicenter SentiDose Trial. Cancers (Basel) 13 (2021).
- Douek M, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol 21 (2014): 1237-1245.
- Karakatsanis A, et al. Effect of preoperative injection of superparamagnetic iron oxide particles on rates of sentinel lymph node dissection in women undergoing surgery for ductal carcinoma in situ (SentiNot study). British Journal of Surgery 106 (2019): 720-728.
- Pantiora E, et al. Magnetically guided surgery after primary systemic therapy for breast cancer: implications for enhanced axillary mapping. British Journal of Surgery 111 (2024).
- Ahmed M, R Baker, IT Rubio. Meta-analysis of aberrant lymphatic drainage in recurrent breast cancer. British Journal of Surgery 103 (2016): 1579-1588.
- Maaskant-Braat AJ, et al. Lymphatic mapping after previous breast surgery. Breast 21 (2012): 444-448.
- Intra M, et al. Second Axillary Sentinel Lymph Node Biopsy for Breast Tumor Recurrence: Experience of the European Institute of Oncology. Annals of Surgical Oncology 22 (2015): 2372-2377.
- Schulze AK, et al. Repeat sentinel lymph node surgery for locally recurrent breast cancer after prior mastectomy. J Surg Oncol 129 (2024): 461-467.
- Poodt IGM, et al. Risk of Regional Recurrence After Negative Repeat Sentinel Lymph Node Biopsy in Patients with Ipsilateral Breast Tumor Recurrence. Ann Surg Oncol 25 (2018): 1312-1321.
- Macnab MRF, et al. The Role of redo-Sentinel Lymph Node Biopsy in Patients With Prior Ipsilateral Breast Cancer Surgery. Clinical Breast Cancer 22 (2022): e674-e679.
- Ugras S, et al. Reoperative Sentinel Lymph Node Biopsy is Feasible for Locally Recurrent Breast Cancer, But is it Worthwhile? Ann Surg Oncol 23 (2016): 744-748.
- Voogd AC, et al. Long-term prognosis of patients with local recurrence after conservative surgery and radiotherapy for early breast cancer. Eur J Cancer 41 (2005): 2637-2644.
- Derkx F, et al. Staging and management of axillary lymph nodes in patients with local recurrence in the breast or chest wall after a previous negative sentinel node procedure. Eur J Surg Oncol 36 (2010): 646-651.
- VikhePatil E. Use of magnetic tracer and magnetic resonance imaging for sentinel lymph node detection after breast cancer recurrence and previous axillary surgery: The SentiRecur feasibility study. in SABCS 2023 (2023). Texas US.


 Impact Factor: * 3.4
Impact Factor: * 3.4 Acceptance Rate: 78.89%
Acceptance Rate: 78.89%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 