Macular and Optic Disc Head Morphology on OCT and Visual Prognosis in Non-Arteritic Anterior Ischemic Optic Neuropathy
Sema Kaya1, Ibrahim Handoko1, Tanja Guthoff1, Marius Ringelstein2,3, Orhan Aktas2, Gerd Geerling1, Rainer Guthoff1
1Department of Ophthalmology, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany
2 Department of Neurology, Medical Faculty, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany
3Department of Neurology, Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany
*Corresponding author: Sema Kaya. Department of Ophthalmology, Medical Faculty, university hospital of Düsseldorf, Heinrich-Heine-University Düsseldorf, Düsseldorf, 40225, Germany. E-mail: sema.kaya2@med.uni-duesseldorf.de.
Received: 8 December 2022; Accepted: 15 December 2022; Published: xxxx
Article Information
Citation:
Sema Kaya, Ibrahim Handokoa, Tanja Guthoffa, Marius Ringelsteinb,c, Orhan Aktasb, Gerd Geerlinga, Rainer Guthoffa. Macular and Optic Disc Head Morphology on OCT and Visual Prognosis In Non-Arteritic Anterior Ischemic Optic Neuropathy. Journal of Ophthalmology and Research 6 (2023): 01-07.
DOI: 10.26502/fjor.2644-00240075
View / Download Pdf Share at FacebookAbstract
Introduction: Non-arteritic anterior ischemic optic neuropathy (NAION) shows an acute painless unilateral visual loss due to a reduced perfusion of the optic nerve head (ONH). Aim of this study was to analyse ONH respective macula morphology after NAION by optical coherence tomography and the correlation to visual acuity.
Methods: In this observational study, 11 patients with NAION were assessed. Patients were examined in the acute (symptoms < 2 weeks) and chronic phase (interval of 6 weeks). The fellow eye served as control. Results: The peripapillary RNFL of the superior sectors showed the strongest thickness alterations in the acute phase (swelling) as well as in the chronic phase (decrease). Early alterations in the macula are the thickening of the RNFL, ganglion cell layer (GCL) and inner plexiform layer (IPL) of outer nasal and superior sectors. The mean thickness increase of the RNFL over all peripapillary regions correlated with initial visual acuity (Pearson correlation coefficient -0.797 at a P-value of 0.003).
Conclusion: The RNFL of the superior optic disc sectors presented the most severe edema in the acute phase and the most thickness loss after 6 weeks, suggesting the strongest affection by hypoxia in NAION. The peripapillary RNFL had the strongest correlation with initial visual acuity.
Keywords
NAION, SD-OCT, visual prognosis, retinal nerve fibre layer, optic nerve head.
NAION articles; SD-OCT articles; visual prognosis articles; retinal nerve fibre layer articles; optic nerve head articles.
Article Details
1. Introuduction
Non-arteritic anterior ischemic optic neuropathy (NAION) is characterized by acute painless unilateral visual loss due to a reduced perfusion of the optic nerve head [1]. The exact pathogenesis of the ischemia is still uncertain, the causes discussed include arteriosclerosis, nocturnal hypotension (as patients often describe a sudden visual loss in the morning hours), impaired vascular autoregulation, embolization, vasculopathic occlusions and venous insufficiency [2]. NAION patients typically exhibit a relative afferent pupillary defect (RAPD, 58.8%), colour vision impairment (73.3%), a swollen optic nerve head (75.6%) and an altitudinal visual field defect (53.3%) [3].Several therapeutic strategies such as antiplatelet therapy with aspirin or vasodilatation with nicotinic acid or acetylcholine have been applied but none is established [4]. The use of high dose systemic steroids for NAION patients to reduce the optic nerve head (ONH) swelling is discussed controversially as recent studies do not show any benefit but rather suggest harm[5,6].
However, it is important for the patients to predict the prognosis regarding visual acuity. In the natural course of NAION, 21% of the patients seen within 2 weeks after onset of symptoms with a visual acuity < 20/70 show an improved visual acuity by at least 3 lines in the Snellen Chart after 6 to 11 weeks [7].
High-resolution power and fast acquisition time of spectral domain-optical coherence tomography (SD-OCT) helps to assess the morphological changes of the optic nerve head and the retina during the course of NAION. Most OCT analyses in NAION focus on the retinal nerve fibre layer (RNFL) and the ganglion cell layer (GCL) [8]. Our objective was to evaluate both the inner and outer retinal layers in acute and atrophic NAION to address the question whether OCT findings correlate with visual prognosis.
2. Materials and Methods:
2.1 Study design and cohort
Patients with unilateral NAION were selected for our longitudinal analysis. The diagnosis was based on the patient´s history and clinical findings of NAION. The patients should have shown the following clinical criteria: sudden painless visual acuity deterioration, altitudinal field defect, relative afferent pupillary defect and unilateral disc swelling. Exclusion criteria were: arteritic anterior ischemic optic neuropathy, other retinal or optic nerve diseases (e.g., diabetic retinopathy, glaucoma, age-related macular degeneration, or macular pucker), high refraction anomalies (myopia of -6 or more diopters (D), or hyperopia of +4 or more D) as well as pathologies of the partner eye. The healthy fellow eye was used as control. Examinations were performed at two time points: within the acute phase defined as up to 2 weeks after onset of symptoms and after an interval of at least 6 weeks after onset of symptoms after regression of optic nerve head swelling (atrophic phase) [9]. Examination included slit lamp examination, funduscopy, evaluation of best corrected visual acuity and static perimetry. This study was approved by the local ethics committee of Heinrich-Heine-University Düsseldorf [Ethikkomission der Medizinischen Fakultät der Heinrich-Heine-Universität Düsseldorf] (4580R).
2.2 Spectral domain OCT
For OCT analysis, peripapillary retinal layers were assessed by 12° peripapillary ring scans (Spectralis®, Heidelberg Engineering Inc., Germany). The Bruch’s membrane opening (BMO) is an area of the border membrane between the choroid and the retinal pigment epithelium through which the axons of the retinal ganglion cells come together and leave the eyeball. The BMO and the volume of the ONH were assessed using 30° horizontally and vertically scans. The scan with the maximal length of 73 ONH scans was chosen to determine the Bruch’s membrane opening (shown in Figure 1C). The ONH volume was measured using the 1-2.22-3.45 mm-grid (shown in Figure 1D). Macular layers were assessed by the standard macula scan protocol (20° horizontally × 20° vertically) derived from 25 horizontal B-scans performed at 9 ART in high-speed mode. All scans were performed using the TruTrack® image alignment system. Retinal thickness was calculated using Heidelberg Eye Explorer software version 1.9.7.0.
2.3 Evaluation of the peripapillary retinal layer thickness
Peripapillary retinal layer thickness was measured automatically (Global, RNFL) or, if necessary, semi-automatically (ganglion cell layer + inner plexiform layer [GCL/IPL], inner nuclear layer + outer plexiform layer [INL/OPL], outer nuclear layer + inner segments of the photoreceptors [ONL/ISP], outer segments of photoreceptors to Bruch’s membrane [OSPBM]). For the evaluation of the peripapillary retinal layer thickness (shown in Figure. 1A) we analysed six sectors (nasal superior [NS], temporal superior [TS], temporal [T], temporal inferior [TI], nasal inferior [NI], nasal [N]) and their means (M).
2.4 Evaluation of the macular retinal layer thickness
The macular retinal layer thickness (shown in Figure. 1B) was analysed at the following 9 regions of the 1, 3, 6-mm Early Treatment of Diabetic Retinopathy Study (ETDRS) grid: outer superior region (S1), inner superior region (S2), outer nasal region (N1), inner nasal region (N2), outer inferior region (I1), inner inferior region (I2), outer temporal region (T1), inner temporal region (T2), and central region (C). The total retinal thickness (Global) of the macula was automatically segmented into the following layers: retinal nerve fibre layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), retinal pigment epithelium (RPE) and a composite layer consisting of the external limiting membrane to Bruch’s membrane (ELMBM).
2.5 Statistical analysis
For the comparison of the retinal layer thickness at different regions and time points, we utilised a multi-way analysis of variance (ANOVA) with repeated measurements. A paired t-test was used to compare visual acuity change of two groups of patients - one with an initial visual acuity greater or equal to 0.4 [LogMAR] (below reading vision), the other with an initial visual acuity under 0.4 [LogMAR]. The correlation of retinal layers in the acute phase, which alters significantly from the normal group and the initial visual acuity, was tested by Pearson’s r. The adjustment for multiple comparison was carried out with a post-hoc test according to Sidak. Two sample t-tests were performed to investigate whether the two groups of patients mentioned above differ in their BMO and ONH volume at the acute phase. Test results with a P-value less than 0.05 were considered, to be statistically significant.
3. Results:
11 patients (6 male, mean age at first presentation 67 ± 10 years [average ± standard deviation]) met the inclusion criteria and were included into this study. Patients were initially examined 8 ± 3 days (acute phase) respectively 79 ± 26 days (atrophic phase) after the onset of symptoms.
3.1 Visual acuity outcome
Initial (acute phase) visual acuity of 0.4 ± 0.6 (LogMAR) increased by one line to 0.3 ± 0.3 (LogMAR) at final follow-up visit (atrophic phase). Patients with poor initial visual acuity of 0.4 or more (Logmar) improved from 0.9 ± 0.6 (Logmar, acute phase) to 0.5 ± 0.4 ([Logmar], t(4)=3.373, p = 0.028) in the atrophic stage. Patients with moderately decreased initial visual acuity of less than 0.4 (Logmar) did not reveal changes in the atrophic phase (from 0.1 ± 0.1 [Logmar] to 0.1 ± 0.1 [Logmar], t(5)=-1.168, p = 0.296).
3.2 Alterations of the peripapillary retinal layer thicknesses
During the acute phase, all RNFL sectors were thickened (shown in Figure 2A and Table 1). The swelling occurred predominantly in the upper sectors of the ONH. Out of the outer retinal layers (such as ONL/ISP, INL/OPL), only some sectors were thickened, also predominantly in the upper sectors of the ONH.
In the atrophic phase, we found a thinning of inner retinal layers (such as RNFL, GCL/IPL) compared to outer layers which were thickened (shown in Figure 2B).
There was no difference of the ONH volume (t(7.706)=-0.395, p=0.705) or the BMO (t(6.929)=-1.54, p=0.168) between acute and atrophic NAION phase.
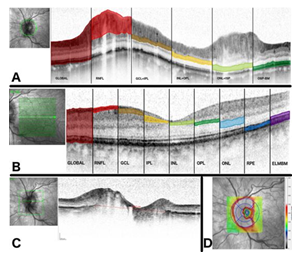
Figure 1: Methodology (A) Segmentation of the peripapillary scan.
While the lines limiting the area global respective RNFL are set automatically by the software algorithm, the remaining lines are set manually but calculated automatically by the software. (B) Segmentation of the macula scan. The coloured areas demonstrate the macular layers which are segmented automatically by the software (C) Measurement of BMO. The line at the longest distance of BMO was chosen for the evaluation of BMO. (D) Measurement of ONH volume. The ONH volume is measured using the 1-2.22-3.45 mm-grid centered at the ONH.
Table 1: Retinal layer thicknesses of the control group compared to the acute phase and the atrophic phase, respectively
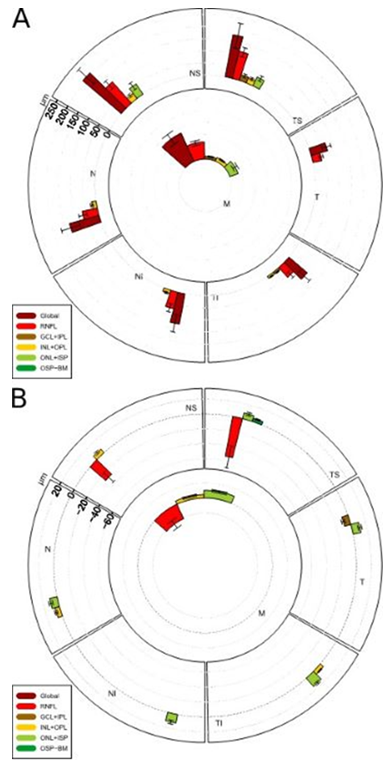
Figure 2: Alterations of the peripapillary retinal layer thicknesses during acute phase (A) and atrophic phase (B).
In the acute stage, global and RNFL thickness has increased in all sectors as well as the ONL-ISP in the upper sectors. In the atrophic phase, RNFL thickness decreased in the upper sectors.
3.3 Alterations of the macular retinal layer thicknesses
In the acute phase, the macula analysis showed a thickened sector (N1) close to the ONH and superiorly (S1) (shown in Figure 3A and Table 1). Moreover, the INL and IPL thickness of the N1 region were slightly increased.
In the atrophic phase, a thinning of the following layers, primarily in the superior and temporal regions was observed: Global, RNFL, GCL, IPL. In contrast, the ONL was almost generally thickened (shown in Figure 3B and Table 1).
3.4 Correlations with initial visual acuity
Of the 26 peripapillary layer region combinations, which were noticeably thickening during the acute phase, 5 correlated to the initial visual acuity and were mainly located in the superior region.(shown in Table 2) The thickness increase (defined as the acute phase value minus the control value) of the mean RNFL over all regions (RNFL-M) offered the highest correlation of -0.797 (Pearson correlation coefficient) at a p-value of 0.003 (shown in Table 2). There was no correlation of the initial visual acuity with any of the 8 macular layer region combinations which have been prominent during the acute phase.
|
Layer and region |
Pearson correlation coefficient |
P-value |
|
Global - NS |
-0.773 |
0.005 |
|
Global - M |
-0.73 |
0.011 |
|
RNFL - NS |
-0.716 |
0.013 |
|
RNFL - M |
-0.797 |
0.003 |
|
ONL+ISP - TS |
-0.698 |
0.017 |
Table 2: correlation with initial visual acuity
Correlation between the peripapillary retinal layer thickness and the initial visual acuity
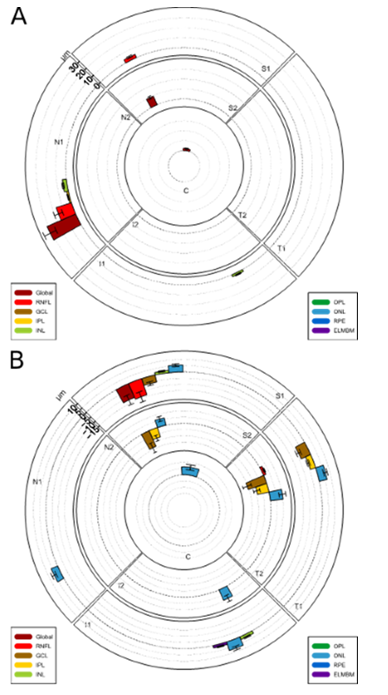
Figure 3: Alterations of the macular retinal layer thicknesses. (A) During acute phase. (B)
During atrophic phase. In the acute phase, sector N1 close to the ONH shows a thickening. In the atrophic phase a thinning of layers primarily in the superior and temporal regions is seen.
4. Discussion/Conclsion:
In clinical practice, prediction of individual disease courses are of utmost importance, even if therapeutic options are limited, as it is the case in NAION. In our study, we aimed to evaluate the prognostic value of SD-OCT analysis of optic nerve head and macular layers regarding visual acuity. In most cases of patients with untreated NAION, visual acuity remains unchanged in comparison to the initial visual acuity [7]. According to other studies like the ischemic optic decompression trial, up to 42,7% of patients in the control group (who does not receive the optic nerve sheath decompression surgery) experience an improvement of visual acuity of 3 or more lines spontaneously [10]. In our study, patients with low initial visual acuity (>0.4) spontaneously improved by 4 lines, while patients with good initial visual acuity (of ≤0.4 LogMar) remained stable. As post-ischemic swelling occurs mostly in the peripapillary retinal layers (up to 116.2%, shown in Table 1), we postulate that the difference in initial visual acuity probably depends on the initial thickness increase. Indeed, our data reveal a strong negative correlation between RNFL-M and initial visual acuity in acute NAION (shown in Table 2): if the retinal layers are thickened, the initial visual acuity in the unit LogMAR will be low, in other words, if the retinal layer gets thicker, the initial visual acuity will improve. A possible hypothesis for this phenomenon could be, that in patients with poor initial visual acuity due to severe ischemia more ganglion cells died immediately with less ability for papillary swelling than in patients with good initial visual acuity. This hypothesis is supported by the fact that Sun et al. determined threshold values for RNFL atrophy in the phase for which the final visual acuity improves: According to them, atrophy not progressing below 65 µm is associated with a good visual outcome [11]. Keller et al. found a similar correlation: Atrophy of the ganglion cell layer and the inner plexiform layer in the macular region is associated with poor final visual acuity [12]. To check whether visual acuity outcome depends on structural crowding due to the cup size of the ONH, we also looked at BMO and global ONH volume. But similar to others, we found no correlation between these ONH parameters and visual function [13]. Another factor may be the degree of stiffness of the lamina cribrosa [14]. Histopathologically, the infarction of ischemic optic neuropathy in a large series of 193 eyes was mainly found in the retrolaminar portion of the optic nerve head, extending prelamilarily only in a few eyes. In NAION cases with a stiff lamina cribrosa, compression of the retrolaminar optic nerve head may present an increased tissue pressure which intensifies ischemia associated with low visual function although the papillary swelling is only moderate. The thickening of the peripapillary retinal layers showed similar patterns with a more prominent superior part of the ONH as also observed in the cross sectional study by Ackermann et al [15].
Han et al. found a thickening of the mean peripapillary RNFL of about 50% within 1 week [16], while we saw a swelling of 70% within 2 weeks (shown in Table 1). In an experimental NAION mouse model by Ho et al. the thickening of the retina during the acute phase was probably caused by an ischemia-induced congestion of axoplasmic flow leading to an elevated water content in the axons, disturbance of microtubules and post-ischemic inflammation [17].
Keller et al. also evaluated macular layers in NAION patients and came to similar results as our study [12]. At the first visit (5.1 ± 5.7 days after onset of symptoms), their patients presented a thickening of RNFL, mostly in the nasal quadrant of the ETDRS grid. They assumed that this occurred due to the topographic proximity of this nasal macular region to the strong peripapillary oedema.
Four to six weeks after onset of NAION, the optic disc is atrophic, and vision gets stabilized [14]. After 6 weeks or more, we saw a thinning of the inner retinal layers reflecting atrophy. This is consistent with previous studies [12-18]. Atrophy may be explained by the initial swelling, which compresses nerve fibres [19]. This may reduce axoplasmic flow, resulting in a decreased amount of neurotrophins, which could cause the retinal ganglion cells to die [20].
During the atrophic phase, we observed a thickening of outer retinal layers which were not altered in the acute phase. Especially, the ONL was predominantly thickened. This was also observed by Keller et al. who therefore conclude that a damage of photoreceptors and a healing process via Müller glial cell proliferation may play a role in the pathomechanism of AION [12]. This repair mechanism, where microglia cells proliferated after photoreceptor death, was observed in an experimental animal model for retinopathia pigmentosa with rd1-mice [21].
Our study has some limitations. The sample size of 11 patients is small, and a bigger sample size might show more clear results. NAION is a relatively rare disease with an estimated incidence of 2.3 per 100,000 [22] so that inclusion of more patients turned out to be rather difficult. For evaluating the peripapillary retinal layers, the BMO and the ONH volume semi-automatic segmentation assessments have been needed.
To sum up, mainly inner retinal layers show a distinct swelling in the acute phase and atrophy in the post-acute phase of NAION. Outer retinal layers, which were inconspicuous in the acute phase, show an increase in thickness in the atrophic phase. The peripapillary RNFL showed the strongest correlation to initial visual acuity, while ONH morphology measured by OCT does not seem to be of prognostic value for initial visual acuity. Further studies combining structural read-outs like OCT with long-term functional parameters are needed to confirm these findings and to further specificy the possible value of this approach for prediction of the individual disease course.
Statement of Ethics
An ethics approval was received by the Ethics Commission of the Medical Faculty of Heinrich Heine University Düsseldorf (register-ID: 2014011666). Written informed consent of participants was obtained.
Conflict of Interest Statement
All authors declare no competing interests related to the presented work.
Funding Sources
The authors did not receive funding for this work.
Author Contributions
Sema Kaya and Ibrahim Handoko contributed to the study design, data acquisition and analysis, drafting and revision of the manuscript. Tanja Guthoff contributed to data analysis, revision of the manuscript for important intellectual content. Marius Ringelstein, Orhan Aktas and Gerd Geerling contributed to the data analysis and revision of the manuscript. Rainer Guthoff contributed to the study design, data acquisition, data analysis, drafting of the manuscript, revision of the manuscript.
References
- Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res 28 (2009): 34-62.
- Kerr NM, Chew SSSL, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci Off J Neurosurg Soc Australas 16 (2009): 994-1000.
- Karti O, Karti DT, Kilic IH, et al. Baseline demographics, clinical features, and treatment protocols of 240 patients with optic neuropathy: experiences from a neuro-ophthalmological clinic in the Aegean region of Turkey. Int Ophthalmol Published Online First: 39 (2019): 155-166.
- Atkins EJ, Bruce BB, Newman NJ, et al. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol 55 (2010): 47-63.
- Kinori M, Ben-Bassat I, Wasserzug Y, et al. Visual outcome of mega-dose intravenous corticosteroid treatment in non-arteritic anterior ischemic optic neuropathy - retrospective analysis. BMC Ophthalmol 14(1) (2014): 62.
- Rebolleda G, Pérez-López M, Casas-LLera P, et al. Visual and anatomical outcomes of non-arteritic anterior ischemic optic neuropathy with high-dose systemic corticosteroids. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 251 (2013): 255-260
- Sawle GV, James CB, Russell RW. The natural history of non-arteritic anterior ischaemic optic neuropathy. J Neurol Neurosurg Psychiatry 53(1990): 830-833.
- Lee TH, Heo H, Park SW. Clinical Usefulness of Spectral-Domain Optical Coherence Tomography in Glaucoma and NAION. Chonnam Med 52 (2016): 194-200.
- Arnold AC. Chapter 7 - Ischemic Optic Neuropathy. In: Walsh and Hoyt’s Clinical Neuro-Ophthalmology (6th edition). Lippincott Williams & Wilkins 10(2004).
- Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. The Ischemic Optic Neuropathy Decompression Trial Research Group. JAMA 273 (1995): 625-632.
- Sun M-H, Liao YJ. Structure-Function Analysis of Nonarteritic Anterior Ischemic Optic Neuropathy and Age-Related Differences in Outcome. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 37 (2017): 258-264.
- Keller J, Oakley JD, Russakoff DB, et al. Changes in macular layers in the early course of non-arteritic ischaemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 254 (2016): 561-567.
- Rebolleda G, García-Montesinos J, De Dompablo E, et al. Bruch’s membrane opening changes and lamina cribrosa displacement in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 101 (2017): 143-149.
- Walsh & Hoyt’s Clinical Neuro-Ophthalmology. 4th ed. Baltimore: Williams & Wilkins 61(2) (1995): 236-237.
- Ackermann P, Brachert M, Albrecht P, et al. Alterations of the outer retina in non-arteritic anterior ischaemic optic neuropathy detected using spectral-domain optical coherence tomography. Clin Experiment Ophthalmol 45 (2017): 496-508.
- Han M, Zhao C, Han Q-H, et al. Change of Retinal Nerve Layer Thickness in Non-Arteritic Anterior Ischemic Optic Neuropathy Revealed by Fourier Domain Optical Coherence Tomography. Curr Eye Res 41 (2016): 1076-1081.
- Ho JK, Stanford MP, Shariati MA, et al. Optical coherence tomography study of experimental anterior ischemic optic neuropathy and histologic confirmation. Invest Ophthalmol Vis Sci 54 (2013):5981-5988.
- Kernstock C, Beisse F, Wiethoff S, et al. Assessment of functional and morphometric endpoints in patients with non-arteritic anterior ischemic optic neuropathy (NAION). Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 252 (2014): 515-521.
- Burde RM. Optic disk risk factors for nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 116 (1993): 759-764.
- Mansour-Robaey S, Clarke DB, Wang YC, et al. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA 91 (1994): 1632-1636.
- Zeiss CJ, Johnson EA. Proliferation of Microglia, but not Photoreceptors, in the Outer Nuclear Layer of the rd-1 Mouse. Invest Ophthalmol Vis Sci 45 (2004): 971-976.
- Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, California. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 14 (1994): 38-44.


 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
