Oral Immunization for Anti-snake Venom Production in Equine Animals
Zameer Ahmed1, Omar Bagasra2*, Sambreen Zameer1, Shahana U. Kazmi3 and Muhammad R. Khanani4, Shaheen Sharafat5
1Department of Laboratory Animal Sciences, Ojha Campus, Dow University of Health Sciences, Karachi, Pakistan
2Department of Biology, South Carolina Center for Biotechnology, Claflin University, Orangeburg, USA
3Reactor and Professor of Microbiology, Dadabhoy Institute of Higher Education, Karachi, Pakistan
4Liaquat College of Medicine and Dentistry & Darul Sehat Hospital, Karachi, Pakistan
5Dow International Medical college, Oja Campus, Karachi, Pakistan
*Corresponding Author: Omar Bagasra, Department of Biology, South Carolina Center for Biotechnology, Claflin University, Orangeburg, USA
Received: 01 July 2019; Accepted: 08 July 2019; Published: 12 July 2019
Article Information
Citation: Zameer Ahmed, Omar Bagasra, Sambreen Zameer, Shahana U. Kazmi and Muhammad R. Khanani. Oral Immunization for Anti-snake Venom Production in Equine Animals. International Journal of Applied Biology and Pharmaceutical Technology 10 (2019): 008-021.
View / Download Pdf Share at FacebookAbstract
The immunization process of current commercial manufacturing of anti-snake venom (ASV), uses injections of bentonite, complete Freund’s adjuvant, or incomplete Freund’s adjuvant, mixed with low doses of the snake venom in horses (but rarely in other large mammals), which frequently cause serious adverse effects in host animals. At the site of injection, horses may develop painful swelling, granuloma, abscess, scar, or systemic neurological and hematological defects, low antibody response, or death due to anaphylactic shock.
We sought to investigate a novel alternate immunization strategy with oral administration of snake venom with adjuvants. We utilized M5904 mineral oil emulsion as an adjuvant that was mixed with sub-lethal doses (LD) of the snake venoms. Our preliminary experiments were initiated in March 2011 and the present data culminated in March 2018. In our initial experiments which were carried out in inbred mice, the LD100 was 10.36 ug/25 grams of mice for Naja. oxins and 10.0 ug/25 gram of Naja. karachians. We extrapolated the sub-LD dose to horses by cutting the LD100 in mice to 20%. This dose did not cause any apparent pathology in horses and therefore, we adopted that dose for the equine.
The antibody titers were measured in vitro by quantitative ELISA and in vivo by neutralization assay in mice. This method resulted in the development of high titer neutralization by the ASV antibodies, without any significant side effects in equine experimental models, against venoms of two cobra species namely Naja oxiana and Naja karachians. The results showed that three oral dosage schedules produced highly significant (P<0.001) neutralizing antibodies with persistent yield of immunoglobulin for 6-months of observation. This novel technique of oral administra
Keywords
<p>Anti-snake venoms; Cobra venoms; Equine model; Novel adjuvant; Oral immunization</p>
Article Details
Introduction
Snake bite incidences and causalities are high in various underdeveloped tropical and sub-tropical countries [1, 2]. A study estimates that 2.5 million people globally are affected with snake bite and over 100,000 fatalities occur annually [3]. The most effective and rational lifesaving treatment of snake bites is the administration of anti-snake venom (ASV). However, due to the shortage and low potency of available ASV, there is an urgent need to enhance the effectiveness and accessibility of anti-venoms [4, 5]. It has been proposed that novel immunization technique to produce potent poly specific antisera is one of the important targets that need to be achieved [2, 6].
The development of a suitable host (generally equine) to prepare ASV faces several challenges. After receiving an intramuscularly injection, generally on their rump, it can result in mild to moderate adverse effects, including muscular swelling, soreness, transient, fever, anorexia and lethargy. In some animals localized edema, abscesses, fistulas, granuloma and fibrosis can develop and may require prolonged treatment and convalescence. Systemic adverse side effects such as urticaria, purpura hemorrhagic colic or anaphylaxis can also occur. Severe anaphylactic shock and death are also reported [6-7].
In our preliminary studies, we applied various methods to optimize production of ASV, including titration of different types of crude venoms, inactivated venoms, fractionated venoms, different adjuvants and immunization strategies. However, we obtained poor antibodies responses, severe side effects, or animal death. Several other investigators have reported similar problems and have stopped projects of ASV production. On the contrary, Sriprapat S. et al. [8] have reported an effective equine immunization protocol using the ‘low dose, low volume, multi-site’ injection protocol, against three Thai elapid venoms, to increase antibodies titer with least adverse reactions [7]. We, at the Dow University of Health Sciences (DUHS) have started experiments to develop a novel strategy for oral venom immunization to produce antibodies against the venom of two common cobra species found in Sindh province of Pakistan [8-10]. We report our preliminary data of experiments on oral immunization protocols and ASV responses.
Material and Methods
Ethical considerations
All horses were kept in a University Approved Facility, supervised and maintained by equine veterinarian team. The immunization protocols were approved by the Ethical Review Board of DUHS, (letter No IRB-685/DUHS
Local venomous snake collections
Ten snakes of two cobra species each namely Naja oxaina, and Naja karachians (Figure 1, 2) were collected and kept in the (DUHS sepentarium. The extraction of cobra venoms was carried out manually, utilizing a sterile cup covered with a sterile latex glove, pooled and centrifuged at 2500 RPM for 30 minutes (at approximately 4x4xG) to clarify the fluid. The venoms were then freeze- dried and stored at -20 0C until used.
Figure 1: Illustrating the morphologic differences between Naja Naja oxiana and N. karachians. Naja Naja oxiana most known characteristic features are the wide black band on the underside of the neck, and the hood marking design which shows half-rings on either side of the hood. It is a smooth-scaled snake with black eyes, a wide neck and head, and a medium-sized body. Its coloring varies from black, to dark brown, to a creamy white. The body is usually covered with a spectacled white or yellow pattern, which sometimes forms ragged bands (http://reptile-database.reptarium.cz/species?genus=Naja&species= oxiana oxianaa).
Experimental animal collections and experimental stations
Inbred NIMRI mice strain, weighing from 20 -25 grams, were obtained from the Department of Laboratory Animal Sciences, DUHS. Local horses weighing 200-300 kg, aged 3 to 8 years were obtained from Karachi Race Course Equine Corporation and the local farmers. After confirmation of their excellent health (based on clinical examination, history of treatment, and health records), were housed in the specially built facility at DUHS. Professional equine caretakers and veterinarians regularly monitored their health.
Snake venom protein estimation
To estimate the amount of proteins in the cobra venoms, the Bradford method was used [11]. Briefly, the Bradford assay relies on the shift of the dye Coomassie Brilliant Blue G-250. This dye exists in three forms: anionic (blue), neutral (green), and cationic (red). Under acidic conditions, the red form of the dye is converted into blue form, In the absence of any protein the solution will remain brown. In the presence of protein the dye forms a strong, noncovalent complex with the protein’s carboxyl group by Van der Waals force and amino group through electrostatic interactions. Subsequently, the binding of the protein stabilizes the blue form of the Coomassie dye and the amount of the complex present in solution represents the protein concentration, which can be estimated by the use of a colorimetric absorbance reading [11]. By utilizing the Bradford assay the results of Cobra N. oxiana protein was 280µg/µl and N. karachians protein 230µg/µl, were used for antigen preparation.
Toxicological studies of cobra’s snake venom for the determination of Naja karachians and Naja oxaina Lethal
doses (LD)
The LD100 was estimated in NIMRI inbred mice by injecting various dilutions of the cobra venoms from the stock venoms. Mice were divided into various groups of five mice per group. We utilized two injections methods: intravenous (IV) and intraperitoneal (IP). For IV we injected 0.2 mL of diluted venom and for IP 0.5 mL of volume. Percentages of dead animals/group with each of the dilutions were recorded and LD100 was estimated after 24-h and 48-h post injections. Full pathologic studies were carried out on the dead animal organs findings were compared including histopathology of organ damage and time of death. The control groups were injecting with normal saline by both routes and were injected with 0.2 ml and 0.5 ml saline IV and IP respectively.
Preparations of adjuvants for oral immunization
The pilot study was carried out in NIMRI mice to determine the optimal concentrations of adjuvants. After the estimation of LD50, serial 1:2 dilution of venoms were mixed in equal volumes (v/v) in Tween 20, Tween 80, Mineral oil, and phosphate buffered saline (PBS). The solutions were mixed by rigorous vortexing, until emulsion appeared creamy white or light brown, depending on the amount of snake venom present in the emulsion [12]. Therefore, generally, low dilutions of emulsions exhibit brownish colorations, whereas the highly diluted emulsion gives a whitish-creamy coloration. Water in-oil and Tween-based emulsions provide an excellent mean to stimulate antibody response and it best suited for the producing the maximum amount of response by oral immunization [12].
Protocols for oral immunization of horses
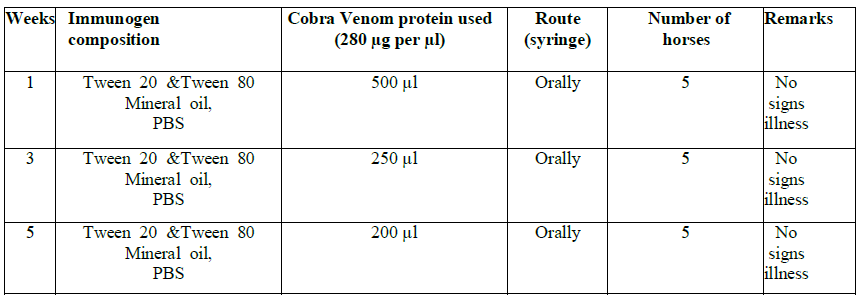
Each individual horse was allotted a unique number and name for identification. The horses were divided in two groups: Group 1 for protocol No. 1-N. oxiana and group 2 for N. karachians. Each group consisted of six horses, five experimental and one negative control. Before immunization of both groups, baseline sera were collected from the jugular veins of each horse and stored as baseline controls (Table 1a, b and 2). The blood collections were carried out in the morning after overnight fast, before animals were fed.
Protocol No. 1 for Naja oxain oral adjuvant formulation group
Protocol No. 2 for Cobra Naja Karachians oral adjuvant formulation group
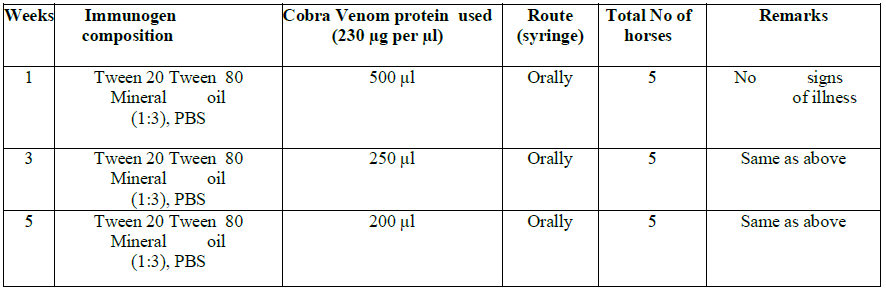
Table 1a: Protocols for Oral Immunizations of Horses
Notes 1: Venom dose was different in 1st, 2nd and 3rd instances.
There was no significant change in week 9 and 11, so the table truncated at week 7.
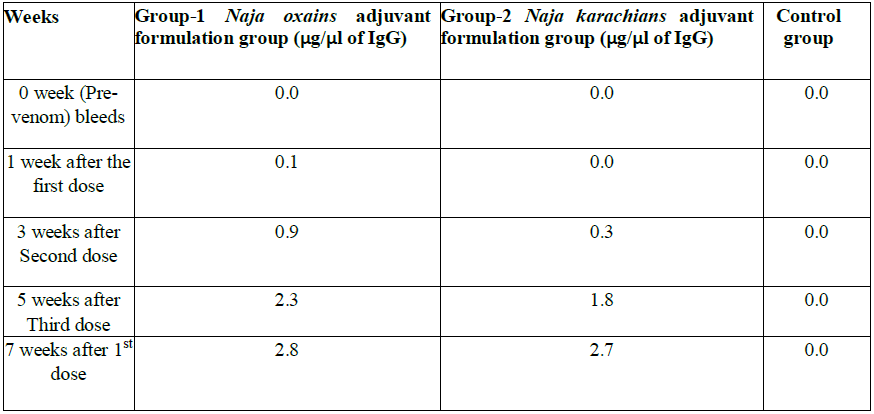
Table 1b: The Immunoglobulin IgG titer at various intervals
Equine Immunoglobulin (IgG) isolation and purification
Equine immunoglobulin (IgG) isolation was carried out by the caprylic acid fractionation method for whole IgG according to the method described by Steinbuch and Audran [13]. All the sera from of unimmunized horses (pre-bleed) and from each week post immunizations from various horses were pooled and mixed well before storage.
Estimation of horse immunoglobulin by enzyme-linked immunosorbent assay ELISA
Fifty microliter L) coating buffer (pH 9.5) was added with each of 10 ng, 100 ng and 1000 ng of Ag (cobra venom) separately into 96-well plates and incubated over night at 37 0C. The plates were washed with PBS + Tween 20 four times, followed by the addition of 250 µl blocking solution 2% containing IgG free bovine albumin, for 1-h at 37 0C. The plates were washed again with PBS+ Tween 20. Serial dilution (1:10) of two groups of sera obtained from each of the horse post-immunizations (ASV) weekly batches were prepared. One hundred microliters of each of the ASV was added in triplicate to cobra venom coated 96-well plates and incubated for 1-h at 37 0C. These plates were then washed with PBS-Tween 20 four times to remove unbound ASV. One hundred µl of secondary anti-horse IgG antibody conjugated with chromogenic (avidin-peroxidase) was added to each well and incubated for 1-h at 37 0C. The unbound secondary antibody was removed by multiple washing with PBS-Tween 20. The color was developed by adding 100 µl of chromogenic TMB (3’, 3′, 5’, 5′-tetramethylbenzidine) to each well and incubating the plates at 37 0C for 15 minutes. Finally, 100 µl of stop solution (0.2% HCl) was added to prevent non-specific color development. The plates were read at absorbance 450 nm on ELISA reader. Results were recorded, printed, and labeled, and plates were photographed as well [14].
Immunodiffusion studies
To detect the presence of specific IgG against snake venoms, agar gel immunodiffusion test was performed using the Ouchterlony double immunodiffusion technique [14]. For this purpose, 0.9% agar was dissolved in PBS, autoclaved for 20 min at 5 Psi and 20 ml dispensed in 100 mm x 15 mm Petri dishes. After solidification of the agar, gel in the plate was cut to form a series of wells. Thirty µl of 1:1,000 diluted cobra venom (antigen) was placed in the center well, and 30 µl of the respective horse serum samples (containing either pre-immune sera or post immunization anti venom antibodies) were placed in the surrounding wells. Pre-immune sera were used as negative controls and the National institute of Health (NIH), Islamabad, Pakistan anti-cobra sera were used as positive control. The plates were incubated at 37 0C for 48 hours in a humidified chamber.
Sero-neutralization assays
Sera were collected from all the horses immunized against N. oxiaina, and karachians snake venom on a weekly bases. These sera were serially diluted in sterile PBS, followed by IP injection in mice 95/group). Pre-immune sera were used a negative controls and the NIHh, Islamabad, Pakistan (NIH) anti sera was used for positive control. Mortality, morbidity and live animal behaviors were observed and recorded as per the World Health Organization (WHO), 2010 document [1].
Statistical analysis
The data for ELISA and neutralization studies were entered into an Excel spreadsheet, and statistical analyses were carried out using Graph Pad Prism (version 3.0; Graph Pad Software Inc. San Diego, CA, USA) with significance of difference between the groups assessed using a Student’s t-test (Origin Laboratory, Northampton, MA, USA).
Results
Immunodiffusion studies demonstrated precipitation lines showing specificity of antibody against the venoms of N.oxiana and N. karachians. ASVs to N. oxiana protected against venoms of both species while anti-snake venoms against N. karachians only partially protected against toxins of naja oxains.
LD100 for cobra snake venoms and the development of optimal anti-snake venom post-immunizations
We observed that the venom tolerability by IP route was better in NIMR mice strain with LD100. The result of LD100 N. oxiana was 10.36+1.244 µg/25 g/mice and N. karachians was 10.0+ 1.272 µg 25 g/mice (Tables 2-5). N. oxiana The Immunoglobulin IgG titer gradually increased after repeated doses and reached a plateau at the 7th week after oral immunization (Tables 2 and 5) and persisted at almost the same levels till end of 6 months.. Neutralization efficacy of serath collected at the 5th and 7 weeks post-immunization showed 100% protection and no mice deaths was recorded (Tables 2 and 3).
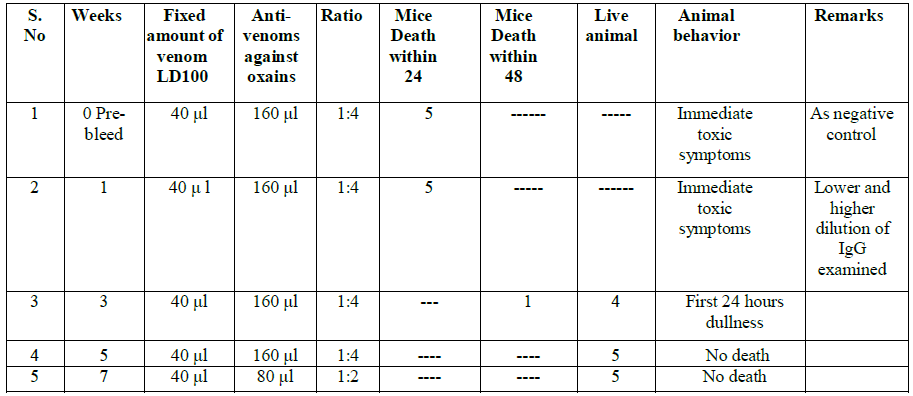
At 5th and 7th weeks there was 100% protective efficacy obtained and no mice death occurred.
Table 2: Neutralization Assay of Cobra karachians.
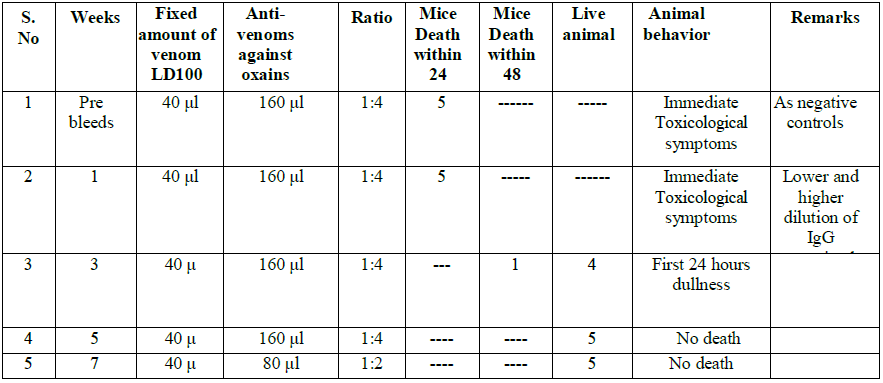
At 5th and 7th weeks there was 100% protective efficacy obtained and no mice death occurred.
Table 3: Neutralization Assays of Cobra oxains

Table 4 (a): Cross protective assessment by animal testing of cobra Oxains venom vs. Karachians
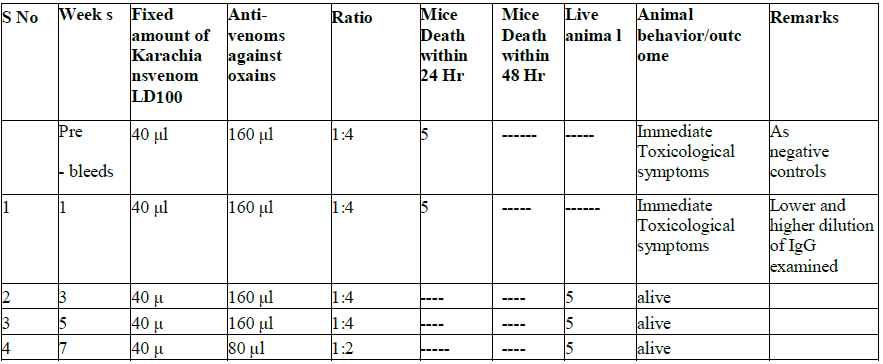
Table 4 (b): Cross protective assessment by animal testing of cobra Karachians venom vs. Oxains antisera.
Table 4: The Immuno-Protective effects of Antivenom in the Experimental Mice Model.
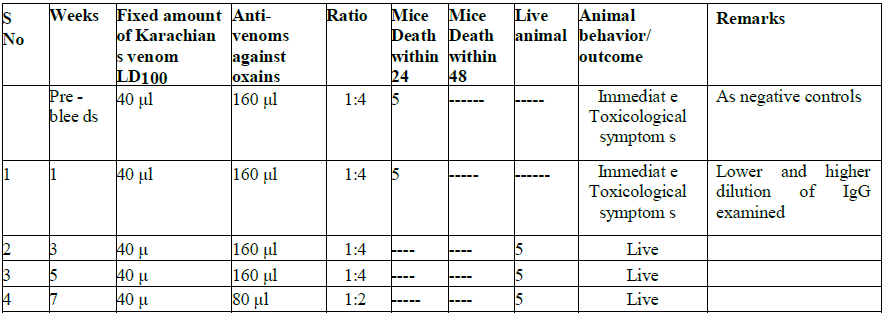
Table 5: Cross protective assessment in animal testing of cobra Karachians venom vs. Oxains antisera
We also carried out cross-protection studies to determine if antisera raised against N. oxiana can protect against N.
karachians venom and vice versa. As shown in Tables 4a and 4b, when animals were injected with 40 l of N. oxiana
venom mixed with 160 ml of ASV from N. karachians, as various weeks of post immunization sera, the cross protection
was limited. However, when mice were tested for cross protection against venom from N. karachians and antisera against
oxains, animals showed a cross-protection, starting from sera obtained at 3 weeks post-immunization. (Table 4a and 4b)
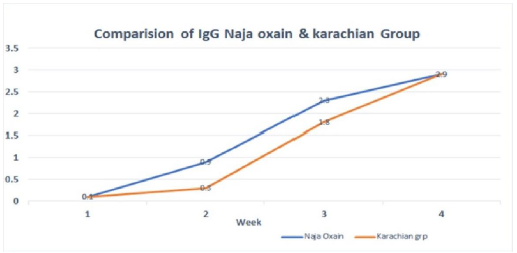
Kinetics of horse antibody response
In N. oxiana oxaina and N. karachians groups, the antibody responses against the orally administered venoms rose rapidly and reached its optimal titer in 7 weeks’ post-immunization in all the immunized horses, irrespective of genetic background, age, weight and gender (Figure 3). The production of ASV persisted at the same level up to 6 months with only three doses of venoms. For our detailed studies, we utilized the sera that were collected on week 10 post- immunization. All the sera from week 10 were pooled to carry the detailed analyses. Until week 10 we carried out separate analyses of each collection.
Discussion
According to the WHO, around 5 million people worldwide are bitten by snakes each year; more than 100,000 of them die and as many as 400,000 endure amputations and permanent disfigurement [15-17]. Most of the snake bites can be treatable if antivenoms are administered on time, before the toxicities of the venom sets in. This is depending on the toxicity of the venom and how much venom is injected into the body and which part of the body the venom entered. A snake bite will cause muscle weakness, nausea, swallowing difficulties, and fatal breathing problems [18-19].
The current protocol for preparing ASV follows a strickt and vigorous steps, staring from collecting the venomous species of snakes indigenous to the area of the country where the ASV is being manufactured. The snakes are bred in clean and healthy sepentarium. These snakes are milked by expert snake milkers. The next step is to collect and cool the venom and stored below -20 0C temperature. Once enough venom are collected, the venoms from different snakes from the same species are mixed and diluted in a well-defined manner so they would not be fatal to the host animals. Traditionally, horses are used to manufacture ASV. Other animals, such as goat, sheep, donkey, rabbits, cats and camels, other animals are also used [16]. In the next step, the correct amount of venom is mixed with an adjuvant(s) and injected into the animal of choice. In horses, the ASV peak between 8 and 10 weeks and in case of horses, 3-6 liters of blood is collected from the jugular vein. Subsequently, ASV is purified for IgG by various immunoprecipitation methods.
As one can appreciate the whole process is very complicated and expansive. Typically an ASV via costs $1,500-$2,200 and it may require 20-25 ASV vials to neutralize a snake bite. In the US treating a snake bit may cost up to $30,000 just in ASV costs alone. Since the costs and efforts required to produce ASV are so large, producers don’t make enough to enter this area of business since it’s financially not feasible, despite high demand for the product. As such, even if these individuals make it to a hospital for treatment, antivenom is in little or no supply [15].
One can easily understand that purchasing such ASV from Europe or the USA to treat snake bites would be cost prohibitive. Furthermore, it is unlikely that these Western nations will produce ASV against local venomous snakes (i.e. N. oxiana or N. karachians) were there is no profitable market. Many Asian nations as well as Latin American countries are producing ASV in their respective government-funded laboratories and distribute the ASV for free [16-18].
We believe that the new oral immunization protocol will significantly reduce the cost of preparing ASV, since this protocol reduces the duration of immunization from 180-45 days and the quantity of the snake venom is reduced from 6 to 8 injection doses to three oral doses. All of these markedly reduce animal care, maintenance and housing cost. Pakistan has various types of venomous snakes, including numerous morphological types of cobras having a variety of habitat [19-20].
Efforts are being made in applied veterinary immunology, biotechnology and other related fields of research to produce commercially potent specific ASVs against a wide range of venomous snake species, particularly the Naja species and variants that appear to differ in their venom composition [19]. In the present study, we developed a novel method to
immunize horses with oral immunization. Here we present two innovations i.e., oral immunization and use of novel oral adjuvant formulation in horses for production of ASV against two Pakistani cobras. Our results are the first of their kind and open up a new avenue of research and development for manufacturing ASV against numerous venoms. In this study, combination of Tween-20, Tween 80 and Mineral oil was used as an adjuvant mixed with pooled snake venom. Mineral oil emulsifying the venom antigen and tween work as surfactant. In summary, our results have shown that oral formulation resulted in production of specific neutralization antibodies (IgG) which are comparable with injectable formulations. This experiment opens a new era of ASV and toxicology research to establish oral adjuvant based vaccines for commercial purposes. We believe that this innovation will bring the technology to developing nations, where appropriate ASV can be produced locally with low budgets.
Conclusion and Recommendation
Oral administration of snake venoms with novel and modified adjuvants are useful for ASV production of potent antibodies at a significantly reduced cost without any significant adverse effects on equine health. Only three oral dosages produced neutralizing antibodies with persistent yield of IgG. Large scale studies for commercial production of ASVs in countries with high prevalence of snakebite cases will go a long way in saving thousands of lives.
Disclosure statement
The authors report no conflicts of interest.
Author’s contribution
The oral immunization idea was conceived by OB in 2011 while setting up the ASV laboratory at DUHS. ZA carried out the research under supervisions MRK and SUK. AZ provided technical support.
Acknowledgment
We highly appreciate the support of Karachi Race Course Equine Corporation, workers of animal care facility, support staff of laboratories and snake care facility of DUHS as well as professional snake handlers for their dedications and support.
References
- World Health Organization. First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases. In First WHO report on neglected tropical diseases: Working to overcome the global impact of neglected tropical diseases 2010 Oct 12. http://www.who.int/neglected_diseases/2010report/en/ (retrived 3/11/2011)
- Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: a review. PLoS Negl Trop Dis 4 (2010): e603.
- Halilu S, Iliyasu G, Hamza M, Chippaux JP, Kuznik A, Habib AG. Snakebite burden in Sub-Saharan Africa: estimates from 41 countries. Toxicon 159 (2019): 1-4.
- Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis 3 (2009): e569
- Williams DJ, Gutiérrez JM, Calvete JJ, Wüster W, Ratanabanangkoon K, Paiva O, et al . Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J Proteomics 74 (2011): 1735-1767.
- Angulo Y, Estrada R, Gutiérrez JM. Clinical and laboratory alterations in horses during immunization with snake venoms for the production of polyvalent (Crotalinae) antivenom. Toxicon 35 (1997): 81-90.
- Gilliam LL, Carmichael RC, Holbrook TC, et al Antibody Responses to Natural Rattlesnake Envenomation and a Rattlesnake Toxoid Vaccine in Horses. Clin Vaccine Immunol 20 (2013): 732–737.
- Sriprapat S, Aeksowan S, Sapsutthipas S, Chotwiwatthanakun C, Suttijitpaisal P, Pratanaphon R, et al. The impact of a low dose, low volume, multi-site immunization on the production of therapeutic antivenoms in Thailand. Toxicon 41 (2003): 57-64.
- Chotwiwatthanakun C, Pratanaphon R, Akesowan S, Sriprapat S, Ratanabanangkoon K. Production of potent polyvalent antivenom against three elapid venoms using a low dose, low volume, multi-site immunization protocol. Toxicon 39 (2001): 1487-1494.
- Cook DA, Owen T, Wagstaff SC, Kinne J, Wernery U, Harrison RA. Analysis of camelid IgG for antivenom development: serological responses of venom-immunised camels to prepare either monospecific or polyspecific antivenoms for West Africa. Toxicon 56 (2010):3 63-372.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry 72 (1976): 248-254..
- Spickler AR, Roth JA. Adjuvants in veterinary vaccines: modes of action and adverse effects. J Vet Intern Med 17 (2003): 273-281.
- Steinbuch M, Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Int Biochem Biophys 134 (1969): 279-284.
- Bereiter M, Young TF, Joo HS, Ross RF. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae in swine serum. Vet Microbiol 25 (1990): 177-192.
- Harrison RA, Cook DA, Renjifo C, Casewell NR, Currier RB, Wagstaff SC. Research strategies to improve snakebite treatment: challenges and progress. J Proteomics 74 (2011): 1768-1780.
- Arnold, C. Vipers, mambas and taipans: the escalating health crisis over snakebites. Nature 537 (2016): 26–28.
- Theakston RD. New techniques in antivenom production and active immunization against snake venoms. Trans R Soc Trop Med Hyg 83 (1989): 433-435.
- Chippaux JP, Massougbodji A, Diouf A, Baldé CM, Boyer LV. Snake bites and antivenom shortage in Africa. Lancet 386 (2015): 2252-2253.
- Waheed H, Moin SF, Choudhary MI. Snake Venom: From Deadly Toxins to Life-saving Therapeutics.Waheed H et al. Curr Med Chem 24 (2017): 1874-1891.
- Wong KY, Tan CH, Tan NH. Venom and Purified Toxins of the Spectacled Cobra (Naja naja) from Pakistan: Insights into Toxicity and Antivenom Neutralization. Am J Trop Med Hyg 94 (2016): 1392-1339.
Citation: Zameer Ahmed, Omar Bagasra, Sambreen Zameer, Shahana U. Kazmi and Muhammad R. Khanani. Oral Immunization for Anti-snake Venom Production in Equine Animals. International Journal of Applied Biology and Pharmaceutical Technology 10 (2019): 008-021.




 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
