Osimertinib- Cardiac Dysfunction with Prolonged QTc Interval: A Case Report
Lalitha C. Medepalli¹*, Pradeep C. Jolly2, Ajay Vallakati3, Anita M. Medepalli4
1Department of Cardiology, Northside Hospital Cardiovascular Institute, Northside Hospital, Atlanta, USA
2Department of Oncology. Georgia cancer specialists, affiliated with Northside Hospital Cancer Institute, Atlanta, USA
3Division of Cardiovascular Diseases, the Ohio State University Wexner Medical Center, Columbus, Ohio, USA
4Department of Medicine, Mercer University School of Medicine, USA
*Corresponding author: Lalitha C. Medepalli, Department of Cardiology, Northside Hospital Cardiovascular Institute, Northside Hospital, Atlanta, USA
Received: 19 May 2022; Accepted: 31 May 2022; Published: 13 June 2022
Article Information
Citation: Lalitha C. Medepalli, Pradeep C. Jolly, Ajay Vallakati, Anita M. Medepalli. Osimertinib- Cardiac Dysfunction with Prolonged QTc Interval: A Case Report. Cardiology and Cardiovascular Medicine 6 (2022): 284-291.
View / Download Pdf Share at FacebookAbstract
Background: Osimertinib has improved outcomes and is indicated by Food and Drug Administration (FDA) for epidermal growth factor receptor (EGFR)-mutated lung cancer. Osimertinib use is associated with cardiac-related adverse events, including cardiac dysfunction and prolonged QTc interval. There is an increasing need for a comprehensive evaluation prior to the initiation of Osimertinib with a detailed assessment of clinical history particularly looking at the cardiovascular risk factors, a protocol-driven close cardio-oncology with periodic electrolyte and ECG monitoring during the therapy, as these high-risk patients are associated with higher rates of cardiotoxicity compared with the low-risk patient population.
Introduction: We report a patient who presented with cardiomyopathy, manifested by prolonged QTc interval, new valvular and left ventricular systolic dysfunction, six months into her treatment with Osimertinib. With timely detection and treatment of her electrolyte abnormality and poor oral intake, we were able to correct the markedly prolonged QTc interval and prevent potential life-threatening arrhythmia.
Conclusions: Patients receiving third-generation tyrosine kinase inhibitor, Osimertinib, should be carefully monitored for the development of cancer treatment-related cardiac dysfunction (CTRCD). A significant number of these patients also have other associated cardiac risk factors and comorbidities including poor nutrition. With poor per oral (PO) intake due to comorbidities, this subgroup of patients is even more susceptible to significant electrolyte abnormalities. Our case highlights the importance of close monitoring of especially these patients with regular electrocardiograms (EKGs), and electrolyte panels, along with the assessment of left ventricular function when needed to detect and interven
Keywords
<p>Osimertinib, prolonged QTc interval; hypokalemia, left ventricular ejection fraction, cancer treatment-related cardiac dysfunction (CTRCD)</p>
Article Details
1. Case Presentation
A 70-year-old female ex-smoker with a past medical history significant for depression initially presented to the emergency room with aphasia. Her workup included computerized tomography (CT) of her head, which revealed a right frontal lobe mass. Her chest X-ray showed a 7-8 centimeter large right upper lobe lung mass and multiple bilateral lung nodules with nodal metastasis. Magnetic resonance imaging (MRI) brain with and without contrast revealed ring-enhancing lesions within the right frontal lobe suggestive of metastatic lesions in the brain. Findings were consistent with metastatic lung adenocarcinoma with brain metastasis. Left axillary node biopsy revealed metastatic adenocarcinoma that was also positive for programmed death-ligand 1 (PD-L1) stain and a 90% epidermal growth factor receptor (EGFR) Exon 21 mutation. Her transthoracic echocardiogram showed a left ventricular ejection fraction greater than 55%, mild mitral, and mild tricuspid regurgitation. The patient initially completed whole-brain radiation therapy for her brain metastasis. With the stabilization of her neurological manifestations, she has been initiated on Osimertinib 80 mg daily.
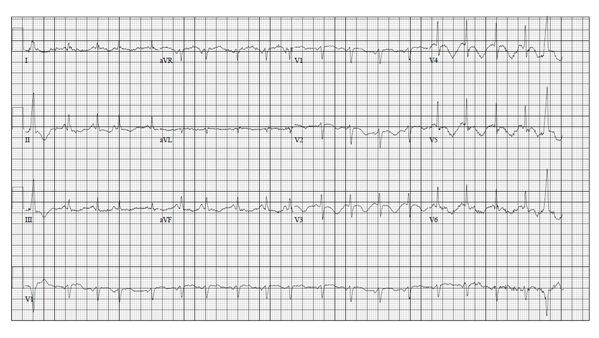
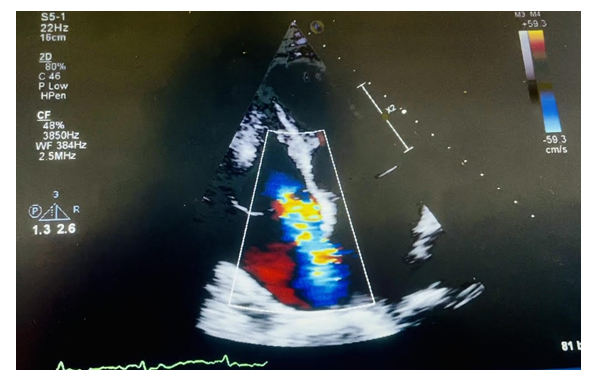
Approximately six months later, the patient was admitted to our hospital with complaints of anorexia, nausea, decreased oral intake, and weight loss. The patient also had a restaging CT scan done as an outpatient, which showed improvement in her metastatic disease; however, a small pulmonary embolus was noted as an incidental finding. The patient was then admitted to the hospital for further management. Her home medication Osimertinib was held. Laboratory tests on admission revealed a brain natriuretic peptide (BNP) level of 680 picograms per milliliter (pg/ml), and a potassium level of 2.4 millimoles per liter (mmol/L), and a creatinine of 0.8 milligrams per deciliter (mg/dl). Her troponin-I level was also elevated at 0.062 nanograms per milliliter (ng/mL). The lower extremity venous duplex showed no sonographic evidence of deep venous thrombosis. Her electrocardiogram (EKG) revealed sinus tachycardia, nonspecific ST and T wave abnormality, and a prolonged QTc interval at 581 milliseconds (msec) (Figure-1). A transthoracic echocardiogram (TTE) showed mild to moderate left ventricular systolic dysfunction with an estimated left ventricular ejection fraction (LVEF) of 40-45%, moderate to severe tricuspid regurgitation, (Figure-2), mild to moderate mitral regurgitation, mild aortic regurgitation, and mild pulmonary hypertension with an estimated pulmonary artery systolic pressure to be 40 mmHg. Compared to her baseline TTE, performed before initiation of her chemotherapy, there are many changes, including new cardiomyopathy, worsening mitral and tricuspid regurgitation, and new pulmonary hypertension.
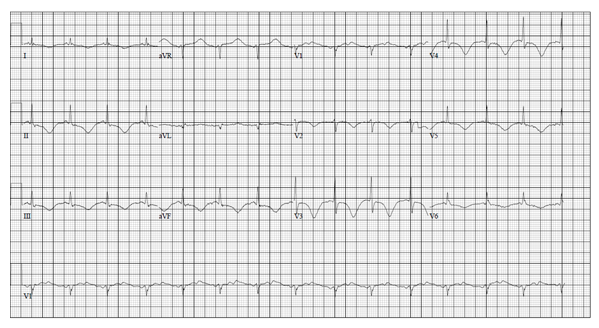
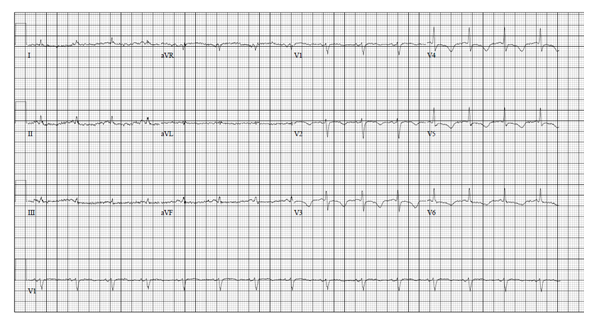
The patient was treated with electrolyte replacement along with parenteral nutrition initially. The potassium level improved to 4.0 mmol/L over the next 24 hours. Repeat EKG (Figure-3) with a potassium level of 3.7 mmol/L still showed a prolonged QTc interval of 552 msec. QTc interval normalized in the next 72 hours to 408 msec (Figure-4). After percutaneous endoscopic gastrostomy (PEG) tube placement, enteral feeding was initiated, and total parenteral nutrition was discontinued. Osimertinib dose was decreased to 40 mg at the time of discharge with a plan to check EKG and potassium level 48 hours later. However, the patient refused further cancer treatment and decided to go on for hospice management with supportive care.
2. Discussion
Osimertinib is a third-generation, orally available EGFR- tyrosine kinase inhibitor (TKI), which selectively inhibits the EGFR TKI sensitizing and EGFR T790M resistance mutations with less activity against wild-type EGFR. Current FDA approved indications for this drug include a) as a first-line treatment for patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R mutations, b) for the treatment of patients with metastatic EGFR T790M mutation-positive NSCLC, whose disease has progressed on or after a different EGFRTKI therapy [1]. Osimertinib has improved outcomes for epidermal growth factor receptor (EGFR)-mutated lung cancer. Osimertinib use is associated with cardiac-related adverse events. A retrospective review of the FDA Adverse Events Reporting System (FAERS) for the incidence of the cardiotoxicity due to Osimertinib compared with other FDA approved EGFR –TKI drugs revealed an increased incidence of cardiac adverse events (AE) accounting for 6% due to Osimertinib alone and 5% due to Osimertinib combined with other TKIs. Cardiac failure was the most common AE, followed by QT prolongation, atrial fibrillation, and pericardial effusion. Of all the AEs reported, those due to cardiac failure and QT prolongation were serious. The median time from initiation of the drug to development of heart failure and QT prolongation was 29 and 23 days respectively [2].
In a phase III trial, 419 patients were randomized to receive Osimertinib or platinum-based therapy with Pemetrexed. QT prolongation was noted in ten patients (4%) in the Osimertinib group and one patient (1%) in the platinum–pemetrexed group [3]. Of the approximately 1100 Osimertinib-treated patients in clinical trials, 0.9% were found to have a QTc >500 msec, and 3.6% of patients had an increase from baseline QTc >60 msec [1]. In a canine animal model study by Lu, Z et al., suppression or downregulation of phosphoinositide 3-kinase signaling by the TKI affected different ion channels, thereby leading to QTc prolongation and subsequently risk for life-threatening arrhythmias [4]. Cardiomyopathy occurred in 2.6% of the approximately 1100 Osimertinib-treated patients and 0.1% of cardiomyopathy cases were fatal. A decline in left ventricular ejection fraction (LVEF) ≥10% from baseline and to <50% LVEF occurred in 3.9% of 908 patients who had baseline and at least one follow-up LVEF assessment [1]. A phase III trial reported a significant change in QT interval in a higher percentage of patients in the Osimertinib treated group (29 patients [10%]) than in the standard EGFR-TKI group (13 patients [5%]). The majority (58%) of the treated patients developed diarrhea [5]. Commonly reported Osimertinib-associated AEs in Phase 1 to 3 clinical trials included diarrhea, rash, nausea, and decreased appetite [6]. The gastrointestinal side effects of nausea, vomiting, diarrhea and other related side effects can potentially lead to electrolyte imbalance, i.e., hypokalemia and hypomagnesemia, which may potentiate the prolonged QTc interval and exacerbate the potential cardiac AE risk associated with this drug.
In a meta-analysis by Kunimasa et al., most cardiac AEs occurred in patients with underlying cardiovascular risk factors or cardiovascular disease. The authors postulated that the Osimertinib might further worsen underlying cardiac pathology [7]. In a recently reported post hoc analysis, Ewer et al., specifically looked at heart dysfunction and heart failureadverse events using data from a pooled population of patients who received Osimertinib 80 mg once daily in FLAURA and the AURA trials. LVEF reduction was observed in patients specifically with cardiovascular risk factors before Osimertinib treatment [8].
According to the recently published Brazilian cardio-oncology guidelines [9], as well as several other guidelines, cardiotoxicity is defined in the presence of QTc prolongation > 500 ms and, or QT variation > 60ms from baseline. The QT interval and the risk factors associated with QT prolongation should be assessed before and after treatment with drugs known to be related to cardiac arrhythmias, such as tyrosine kinase inhibitors (crizotinib, dasatinib, and sunitinib) and arsenic trioxide. Baseline electrolyte and ECG assessments should be performed 1-2 weeks post-initiation of the treatment and any dose adjustments should be performed in the first three months, depending on the therapy frequency. If a prolonged QTc interval is noted by the ECG, other drugs or electrolyte abnormalities that may contribute to the QTc interval prolongation should be addressed. The medications known to prolong the QTc interval included but were not limited to antiemetics, antidepressants, antiarrhythmic, antifungal drugs, and antipsychotics. Electrolyte abnormalities, if present, should be corrected. Osimertinib is potentially another associated drug for which this protocol can be extended.
Our case highlights the need for protocol-driven cardio-oncology evaluation prior to initiation of Osimertinib with a detailed assessment of clinical history specifically looking at the cardiovascular risk factors such as hypertension, hyperlipidemia, diabetes mellitus, history of congestive heart failure, pulmonary embolism, valvular heart disease, prior tobacco use, atrial fibrillation, and other conduction abnormalities [7, 8]. Additionally, a routine baseline assessment of LVEF is recommended. Regular monitoring of LV function by TTE during treatment should be considered in patients with cardiac risk factors, or those who develop relevant cardiac signs or symptoms. Patients with cancer therapy-related AE or comorbidities due to concomitant use of multiple agents should also undergo a periodic assessment of ECG and electrolyte abnormalities. This should especially be performed in patients with poor oral intake or nausea as well as patients on other antiemetic or antidepressant drugs.
3. Conclusion
Our patient demonstrated an increase in her QTc interval approximately six months into her treatment with a third-generation tyrosine kinase inhibitor. This occurred in a setting of significant nausea and decreased oral intake. LVEF and cardiac biomarkers were also abnormal, suggesting potential cancer therapy-related cardiac dysfunction (CTRCD). Monitoring electrolytes, potassium, and magnesium levels, and QTc interval in addition to TTE with strain imaging may help guide the prevention and management of CTRCD with early detection of subclinical cardiomyopathy in patients receiving Osimertinib, particularly in those patients with poor oral intake leading to potential electrolyte abnormalities. This case report emphasizes the importance of increased awareness, vigilant monitoring, and multidisciplinary team involvement for high-risk cardiac patients undergoing Osimertinib-based CT. This can lead to earlier diagnosis of potential Osimertinib-related cardiotoxicity, even in asymptomatic patients, and can also prevent life-threatening arrhythmias due to a potential QTc interval prolongation from an otherwise highly effective life-saving cancer therapy. Such multidisciplinary teams can also provide and optimize medical and nonpharmacological therapy for these patients who are at high risk for developing CTRCD. A coordinated care team approach may include temporarily withholding the drug until the QTc interval normalizes, initiating the drug at a lower dose (40 mg daily), or considering alternative therapy with a plan for a careful follow-up.
Declaration
All authors have no conflict of interest relevant to the topic in discussion
Consent for publication:
Ethical Approval and Consent to participate. Dr. Jolly Pradeep, the Oncologist on the case received consent from the patient`s husband as the patient is now expired. Also, N/A -- Patient`s name or identity was never disclosed.
Availability of data and materials:
Patient care, record review
Competing interests:
None
Funding:
None
Authors' contributions:
Dr. Lalitha C. Medepalli-Lead author, Cardio-Oncologist on the case involved in the patient care, preparing, coordinating, and editing the manuscript
Dr. Pradeep C. Jolly- 2nd author, Oncologist on the case, preparing the manuscript from an Oncology standpoint
Dr. Ajay Vallakati-Cardio-Oncologist- editing and expert opinion
Anita M. Medepalli – Writing and Editing
Acknowledgements: The authors would like to thank, Dr. Ragavendra R. Baliga- Cardio-Oncologist, The Ohio State University Wexner Medical center, for his contribution towards his expert opinion.
References
- US Food and Drug Administration: TAGRISSO (Osimertinib) Highlights of Prescribing Information(2018).
- Anand K, Ensor J, Trachtenberg B & Bernicker EH. Osimertinib-induced cardiotoxicity: a retrospective review of the FDA adverse events reporting system (FAERS).JACC: CardioOncology1 (2019): 172-178.
- Mok TS, Wu YL, Ahn M, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer.New England Journal of Medicine376 (2017): 629-640.
- Lu Z, Wu CY, Jiang YP, et al. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome.Sci Transl Med 131 (2012): 131ra50.
- Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC.New England Journal of Medicine382 (2020): 41-50.
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer.New England journal of medicine378 (2018): 113-125.
- Kunimasa K, Kamada R, Oka T, Oboshi M, Kimura M, et al, Cardiac adverse events in EGFR-mutated non-small cell lung cancer treated with osimertinib.JACC: CardioOncology2 (2020): 1-10.
- Ewer, Michael S, Sri Harsha Tekumalla, Andrew Walding, and Kwame N. Atuah. "Cardiac Safety of Osimertinib: A Review of Data."Journal of Clinical Oncology(2020): JCO-20.
- Hajjar LA, Costa IBSDSD, Lopes MACQ, Hoff PMG, et al,. Brazilian Cardio-oncology Guideline–2020.Arquivos Brasileiros de Cardiologia115 (2020): 1006-1043.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 