Parafoveal vessel Density Dropout May Predict Glaucoma Progression in The Long-Term Follow Up
Natalia Ivanovna Kurysheva*, Ekaterina Olegovna Shatalova
The Diagnostic Department of the Ophthalmological Center of the Federal Medical and Biological Agency of the Russian Federation
*Corresponding author: Natalia Ivanovna Kurysheva, The Diagnostic Department of the Ophthalmological Center of the Federal Medical and Biological Agency of the Russian Federation.
Received: 03 November 2022; Accepted: 11 November 2022; Published: 07 December 2022
Article Information
Citation: Natalia Ivanovna Kurysheva, Ekaterina Olegovna Shatalova. Parafoveal vessel density dropout may predict glaucoma progression in the long-term follow up. Journal of Ophthalmology and Research 5 (2022): 150-166.
DOI: 10.26502/fjor.2644-00240073
View / Download Pdf Share at FacebookAbstract
Relevance: Successful glaucoma monitoring strongly depends on its progression prediction with the search for new clinical biomarkers.
Purpose: To study new progression predictors of primary open angle glaucoma (POAG).
Materials and methods: The 2-year clinical data of 85 POAG patients were prospectively analyzed. Optical coherence tomography-angiography was used to assess retinal microcirculation. Retrobulbar blood flow was assessed using color Doppler imaging. Functional progression was determined using Humphrey perimetry trend and event analyses, and structural loss – by means of SD-OCT negative slope of retinal nerve fiber layer and ganglion cells complex (GCC). The area under the receiver operating characteristic curve (AUC), the Wald Chi-Squared test of the generalized logistic mixed models and the multilevel mixed-effects models analysis were applied to differentiate between progression and non-progression eyes. A clinical biomarker was considered to be a predictor if the disease progression was confirmed by both perimetry and SD-OCT.
Results: Vessel density (VD) of the parafoveal superficial plexus (AUC 0.776±0.07; Wald Chi-Squared test 12.5), macular thickness measured from the internal limiting membrane to the inner plexiform layer (0.751±0.06; 12.8), peak follow-up intraocular pressure (0.768±0.07; 9.3), end-diastolic velocity (EDV) of central retinal artery (0.715±0.11; 6.29), mean ocular perfusion pressure (0.682±0.08; 5.6) and focal loss volume of GCC (0.636±0.08; 5.0) were detected as significant predictors of glaucoma progression. According to the multilevel mixed-effects models analysis, only four predictors were determined: VD of the parafoveal superficial plexus (z -4.77), EDV of central retinal artery (z -3.08), focal loss volume of GCC (z 3.53) and peak follow-up intraocular pressure (z 3.20).
Keywords
<p>POAG, glaucoma progression, ocular blood flow, OCT-angiography, matrix metalloproteinases</p>
Article Details
1. Introduction
Successful monitoring of primary open angle glaucoma (POAG) depends greatly on early detection of its progression. In each individual case a treatment option should be based on the knowledge of risk factors and specific clinical markers that allow predicting the terms and the rate of disease progression and avoiding unreasonable prescriptions.
Increased intraocular pressure (IOP) [1-5] and its fluctuations [6] are commonly considered to be the main recognized factors for POAG progression. However, there is an increasing interest in the influence of other factors as it is known that the disease can progress at normal IOP [7-9]. These factors include a thin cornea [3, 10], low corneal hysteresis [11], optic disc hemorrhages [5, 12], peripapillary atrophy of the choroid [1, 11], age of patients [1, 5, 13], female sex [5,14], presence of pseudoexfoliations [2], late detection of glaucoma [3], and arterial hypotension [15, 16] or/and hypertension [14, 17]. Nevertheless, researchers disagree on many issues regarding progression risk factors and recommend to take into account only highly reliable results concerning significant parameters [5, 18].
The mathematical models to predict visual field progression also demonstrate a moderate accuracy with some limitations due to possible measurement errors, biological variations, and non-linear relations between outcome and predictor variables [19]. Ernest and co-authors developed a prediction model for glaucomatous visual field progression using easily accessible baseline clinical data. According to the proposed model, age, baseline intraocular pressure, and baseline visual field status could predict only 10.3% of the observed variation in visual field index (VFI) rates. Moreover, they revealed a relatively high absolute difference between observed and predicted visual field index rates [20].
Therefore, it is very important to use the clinical parameters revealed at the detection stage as markers of the disease progression. In many cases, these parameters are structural variables. Sehi et al. investigated the relation between glaucomatous structural abnormalities at baseline and the visual field progression involving the patients from the Advanced Imaging for Glaucoma Study suggesting that structural abnormalities of the optic nerve head and the presence of retinal nerve fiber layer (RNFL) defects accelerate the progression [21]. This data coincides with the opinion of other researchers [20, 22]. However, one should keep in mind the dependence of RNFL thickness on different ocular and systemic factors like IOP (even in individuals without any history of glaucoma), stroke, hypertension, smoking, dementia, etc [23].
Currently, there is much evidence that ocular blood flow is as significant as IOP in the progression of glaucoma [24-27], and that reduced baseline optic nerve head (ONH) blood flow is associated with visual field progression [28]. Moreover, some studies have recently suggested that reduced ocular blood flow is a primary independent parameter of visual function outside other structural parameters supporting a vascular role in the development of glaucoma [29, 30].
Optical coherence tomography angiography (OCTA), a new non-invasive method for examination of microcirculation of retina, optic nerve and choroid, opens up new perspectives for the study of blood supply to the key structures affected by glaucoma – optic disc, peripapillary retina and inner macular layers [31]. The studies have consistently demonstrated reduced ONH [31,32], peripapillary [33] and macular [33-37] perfusion in glaucoma patients using OCTA.
Inter-visit coefficients of variation (CV) have been reported as 3.2% to 9.0% for the global OCTA parameters of macular and peripapillary region [32], and 5.0% to 6.9% for the peripapillary region [38]. Some authors have assumed that the use of OCTA vessel density measurements may complement existing structural parameters to detect glaucoma and its progression by revealing the changes in microvasculature supplying the ganglion cells and axons prior to the changes in structural thickness measurements [29,30, 35-37, 39, 40].
We have not found any single work devoted to the comparative study of microcirculatory parameters as glaucoma progression predictors and other clinical parameters. The purpose of the present work is to study new predictors of the POAG progression, including retinal microcirculation.
2. Materials and methods
2.1 Experimental design
The present prospective longitudinal study evaluated the patients in accordance with the protocol including regular follow-up visits (over a 4-month interval) with clinical examinations and several imaging and functional tests. The study was approved by the Ethical Committee (Institutional Review Board) of the Institution of Federal Medical and Biological Agency of Russia and was conducted in accordance with Good Clinical Practice within the tenets of the Declaration of Helsinki. Each subject was required to sign an informed consent form before being enrolled in the study and prior to any measurements being taken.
POAG was diagnosed on the basis of an open anterior chamber angle (no less than 30 °, see the Study Examinations below), typical changes in the optic disc detected during ophthalmoscopy (abnormal proportions of neural rim, glaucomatous excavation of optic disc, peripapillary atrophy, retinal nerve fiber layer wedge-shaped defects close to the edge of the optic disc, and hemorrhages at the optic disc edges). The diagnosis of glaucoma according to ophthalmoscopy was confirmed by two independent glaucoma specialists. The results of standard automated perimetry (SAP) performed at Humphrey perimeter (Carl Zeiss Meditec, Dublin, CA) were abnormal (see below). The inclusion criteria were the following: ametropia 0.5 dpt, an open anterior chamber angle (not less than 30 °, See Study Examinations below), and no ocular pathology other than glaucoma.
The exclusion criteria included large refractive errors (outside of ±6.00 dpt sphere or 2.00 dpt cylinder), pupil diameter < 3 mm, systemic administration of beta-blockers and calcium-channel blockers, concomitant ocular disease (except for early cataract), chronic autoimmune diseases, diabetes mellitus, Parkinson disease, Alzheimer disease, or dementia, history of stroke, acute circulatory disorders, including ocular arterial or venous obstruction (branch or central occlusion) in past medical history, and any concomitant disease involving the administration of steroid drugs.
2.2 Study examinations
All participants underwent complete ophthalmologic examinations including best corrected acuity, slit lamp examination, IOP measurement using analyzer of biomechanical properties of the eye (Ocular Response Analyzer, ORA, Reichert Ophthalmic Instruments Inc, Depew, NY, USA), gonioscopy, anterior chamber angle measurement (Visante OCT, Carl Zeiss, Germany), pachymetry (SP-100, Tomey, GmbH, Erlangen, Germany), dilated fundus biomicroscopy using a 78-diopter lens, stereoscopic optic disc photography, and SAP using a Humphrey Field Analyzer (HFA, Carl Zeiss Meditec Inc, Dublin, CA, USA) with the 24–2 SITA. Only reliable SAP results, which were defined as false-negative and false-positive responses <33% and fixation losses <20%, were eligible for the study. Glaucomatous visual field defects were determined as having a cluster of 3 or more non-edge points with p < 0.05 and at least 1 point with p < 0.01 in the pattern deviation probability plot; pattern standard deviation (PSD) of less than 5%; or glaucoma hemifield test results outside normal limits.
Mean ocular perfusion pressure (MOPP) was calculated on the basis of IOP and arterial blood pressure (BP) measurements immediately before the OCT scanning and investigation of retrobulbar blood flow, after a 10-minute resting period in the sitting position. Systemic BP was measured using the Riva Rocci technique. MOPP was calculated using the formula: MOPP = (2/3 diastolic BP + 1/3 systolic BP) × 2/3 –IOP.
The subjects were instructed to avoid caffeine intake, smoking, and exercise for 5 hours prior to the study visit. The subjects, who previously used antiglaucoma drops, were asked to discontinue the drug for a period of 21 days (drug washout period). The medical histories of all subjects were carefully obtained with special attention paid to the signs of primary or secondary cardiovascular dysregulation (migraine, vasospasm, neurocirculatory dystonia). All participants were white Europeans.
IOP was measured between 10.00 a.m. and 15.30 p.m. at every visit using the Ocular Response Analyzer (Reichert Ophthalmic Instruments, Depew, NY). The device is based on a burst of air directed toward the cornea and uses two applanation pressure measurements – one during the depression of the cornea and another during the recovery. The corneal hysteresis (CH) is used for calculation of corneal compensated IOP (IOPcc), which appears to be less affected by the corneal properties than conventional applanation tonometry [11].
2.3 CDI examination
The method used for investigating blood flow velocity in retrobulbar vessels included gray-scale ultrasound, CDI, and pulsed-wave Doppler (PWD). The ultrasound examinations were performed with a VOLUSON 730 Pro ultrasound system (GE Medical Systems Kretztechnik GmbH & Co OHG, Austria) and an SP 10-16 transducer. With the patient in supine position, sterile ophthalmic gel was applied as a coupling to the closed eyelid, and the probe was positioned gently with minimal pressure. The application of gray-scale ultrasound enabled us to obtain images of the globe and the orbit. The CDI method was used to display the fine orbital vessels directly, including the ophthalmic artery (OA) and its branches, the central retinal artery (CRA), the lateral (temporal) and medial (nasal) short posterior ciliary arteries (PCAs), and central retinal vein (CRV). It was done according to the expected anatomical position of the vessels and its color code. The blood flow in the OA was evaluated at a depth of 35 mm. The CRA blood flow velocity was examined in the optic nerve canal at a distance of 5–6 mm from the posterior wall of the globe. The PCAs were identified on either side of the optic nerve, about the same distance from the fundus as the central retinal artery and vein. PWD was used to measure the blood flow spectrum of vessels and its main indices: peak systolic velocity (PSV), end-diastolic velocity (EDV), mean velocity (Vmean), resistive index (RI), and the pulsatility index (PI).
2.4 OCT image acquisition and processing
All subjects also underwent optic disc area measurement on Avanti SD-OCT (Optovue. Inc, Fremont. CA, USA) using the traditional ONH scan. This scan consists of 12 radial scans of 3.4 mm in length and 6 concentric ring scans ranging from 2.5 to 4.0 mm in diameter. All scans are centered on the optic disc. The retinal pigment epithelium (RPE) tips are automatically detected by the software and are joined together to delineate the optic disc margin and to calculate the disc area. All the examinations for a particular subject were performed on the same day. OCT in the macular area was performed as well. The tracking mode was used.
The GCC scan mode measured macular inner retinal layer thickness from the internal limiting membrane (ILM) to the inner plexiform layer – Avg. GCC. The GCC scan was centered on the fovea and covered a square grid on the central macula of 7.0×7.0 mm. The GCC thickness was determined with the GCC scanning protocol, which consists of 15 vertical line scans covering a 7.0×7.0 mm area centered about 1 mm temporal to the fovea. The GCC scanning protocol also included a central horizontal line scan for registration of vertical scans and fovea center searching. The characteristics of GCC (global loss volume – GLV; focal loss volume – FLV) were also measured.
The RNFL measurements in each area were automatically obtained and calculated using the retinal map protocol in the Avanti software. The full RNFL thickness was defined by the algorithm as the distance between the ILM and the middle of the RPE. The inner retinal layer thickness was defined by the algorithm as the distance between the ILM and the outer boundary of the inner plexiform layer (IPL). The average and regional information (superior hemisphere and inferior hemisphere) was obtained directly from this system. The choroid thickness (CT) was examined in the tracking mode (Retina Cross Line protocol) as has been previously described [34].
2.5 OCTA imaging of the optic disc, peripapillary region, and macula
OCTA scans were collected using the spectral-domain system (Avanti. Optovue Inc, Fremont. CA. USA): AngioVue OCTA software revision 2016.1.0.26. The method was described in detail elsewhere. In short, this method may be described as follows. The optic disc scan covers an area of 4.5×4.5 mm. The software automatically fits an ellipse to the optic disc margin (as detected by software) and calculates the average VD within the ONH (referred to as “inside disc” VD). The peripapillary region of 750-μm-wide elliptical annulus extending from the optic disc boundary is divided into 6 sectors, and the peripapillary VDs are calculated in each sector (nasal, inferonasal, inferotemporal, superotemporal, superonasal, and temporal). Whole image vessel density (wiVD Disc), VD inside disc and peripapillary VDs were evaluated. VD is defined as the proportion of the area occupied by vessels (white pixels) out of the whole area of the measurement sector. The peripapillary VD were analyzed in the radial peripapillary capillary (RPC) slab. The RPC slab extends from ILM to the posterior boundary of RNFL.
Macular scans cover a 6.0×6.0 mm area centered on the fovea. Two vascular plexuses were studied in macula: a superficial plexus located in a retinal slab with the upper boundary 3 μm below the surface of ILM and the lower boundary 15 μm below IPL, and a deep plexus located in the retinal slab with boundaries of 15 μm to 70 μm below IPL. The measurements were performed in the fovea (1 mm diameter central zone) and the parafovea (1-3 mm diameter ring). The parafoveal region is divided into 4 sectors of 90 degrees each (nasal, inferior, superior, and temporal sectors). In addition, average VD for wiVD macula was studied. Image quality was assessed for all OCTA scans. The retinal thickness measurements in each area were automatically obtained using the retinal map protocol in the Avanti software. The parafoveal thickness was measured within a circular annulus centered on the fovea using an OCT angiogram. Only the images with optimal image quality (signal strength index >50), no motion artifacts, vitreous floaters or other artifacts were selected.
2.6 Detection of glaucoma progression
The Guided Progression Analysis (GPA) software on the Humphrey Field Analyzer II was used to detect glaucoma progression. The analysis includes a visual field (VF) trend analyses defined with either visual field index (VFI) or mean deviation (MD), and a pointwise event analysis. The event analysis defined progression as a significant change (compared to two significant VFI or MD negative slope was observed. The probability levels considered to be statistically significant where p was less than 0.05 for the slope of the global 24-2 area. Only significant values were selected for the calculation of mean progression rates. SAP was performed every 4 months. From the entire dataset containing VF obtained with the Humphrey Field Analyzer, those patients with ≥ 8 VF examinations were included in accordance with literature recommendation [41]. The VF progression endpoint was detected when either the event analysis or the trend analysis showed significant progression. Only visits with both VF and OCT data were used.
2.7 The detection of structural changes
The peripapillary nerve fiber layer (NFL), and macular GCC were imaged and measured by FD-OCT (RTVue, Optovue. Inc, Fremont. CA, USA). During each visit, participants had three GCC and ONH scans. Only ONH scans with a signal strength index (SSI) above 37 and GCC scans above 42 were selected for analysis. We used two OCT parameters to track glaucoma disease progression: overall GCC thickness and RNFL thickness. The series of OCT thickness parameters from baseline up to the current visit was made at each visit. A progression case was registered if a significant (p < 0.05) negative slope (thinning trend) was observed during the visit. This visit was considered to be the date of progression detection [42]. To eliminate the interference of cataract on the visual field measurements, we also excluded eyes with any significant cataract progression detected during the follow-up. Significant cataract progression was understood as confirmed worsening of visual acuity scores by two or more lines at two or more follow-up visits. All examinations were performed at each visit.
2.8 Statistical processing
We applied Mann-Whitney U test using Rosner-Glynn-Lee method and Pearson chi-squared test to compare two independent groups by one characteristic. 199 clinical parameters of each eye were taken for the study. They included 120 variables assessed by means of SD-OCT (structural parameters - average RNFL thickness, macular, choroidal and GCC thickness, etc) and 38 parameters related to the ocular blood flow including the parameters measured by means of Color Doppler images and OCT-angiography. The biomechanical characteristics of the cornea, biometrical parameters of the eye were also included as well as age of patients. A parameter was considered to be a predictor of glaucoma progression, if it was confirmed by both perimetry and SD-OCT. We used the area under receiver operating characteristic curve (AUC) as a significant measure for distinguishing between the groups.
Cut-off value was determined by means of Youden’s index. The numerical data are represented as the mean value ± SD. Statistical processing of the obtained results was carried out using the standard package of statistical analysis software “SPSS 16.0 for Windows.” Parameters with p < 0.05 were considered statistically significant.
Generalized logistic mixed models (GLMM) were used for evaluation of the most likely predicted value of the outcome variable. GLMMs allow simulating diverse response distributions and multiple sources of random variation called random effects [43]. The analysis used a logistic regression model based on data from both eyes, including a random effect to account for the inter-eye correlation within subjects. The groups were included in the model as a “factor”: 1 = progression. 2 = no progression. Each dependent variable was introduced into the analysis separately. Such indexes of the statistical model as the Wald test, the regression coefficient β and odds ratio were analyzed. The Wald test in the context of logistic regression was used to determine whether a certain predictor variable was significant or not. It rejects the null hypothesis of the corresponding coefficient being zero. The regression coefficient β describes the relation between a predictor variable and its response. If the coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable, the outcome variable will decrease by the beta coefficient value. Odds ratio represents the constant effect of a predictor on the likelihood that studied event will occur. Analysis was carried out using SPSS 16.0. JAMOVI 0.9.2.8 for Windows. Significant progression predictors identified during GLMM were further studied in the multilevel mixed-effects models analysis (Stata, 16.1). All the studied parameters were adjusted for age.
3. Results
The study included 353 patients with initial to advanced stages of POAG. The enrollment of patients was carried out from June 2016 to May 2017. Later, eighty-five patients (152 eyes) were selected from this group in accordance with the accepted inclusion/exclusion criteria. The remaining 268 patients were excluded as follows: 98 patients had fewer reliable VF examinations than OCT examinations, 110 patients did not have 7 OCT examinations with SSI more than 45 for both ONH and GCC, 43 patients underwent the cataract phacoemulsification and 17 patients underwent trabeculectomy. The selected patients were prospectively monitored until June 2019 and were divided into two groups on the basis of presence/absence of progression detected using standard automated perimetry and SD-OCT. The clinical characteristics of all included patients are represented in Table 1.
Table 1: Clinical characteristics of patients
|
Characteristic |
mean±SD |
Min/max |
|
Age, years |
65.06±7.07 |
44.00/75.00 |
|
BCVA |
1 |
1.0/1.0 |
|
Refractive error (spheric equivalent, diopters) |
-0.5 |
#DIV/0! |
|
MD, dB |
-6.04±6.68 |
-8.1 |
|
Corneal hysteresis, mmHg |
9.85±1.43 |
13-May |
|
Baseline IOP cc , mmHg |
20.31±5.19 |
Aug-34 |
|
IOP cc max at the observation period, mmHg |
22.86±3.46 |
15.0/34.0 |
|
IOP cc min at the observation period (peak follow-up IOP), mmHg |
16.32 ±2.33 |
10.10/20.90 |
|
CCT, μm |
549.25±28.55 |
500/600 |
|
MOPP, mmHg |
45 .23±7.31 |
23.29/62.64 |
|
Systolic BP, mmHg |
134.69±17.02 |
100/180 |
|
Diastolic BP, mmHg |
84.18±10.57 |
60/100 |
|
Mean peripapillary RNFL (classic ONH scan), μm |
83.37±15.86 |
47/105 |
|
Mean GCC thickness, (classic GCC scan), μm |
80.92±14.34 |
45.79/100.58 |
|
Mean FLV (classic GCC scan), % |
6.35±11.33 |
0/36 |
|
Mean GLV(classic GCC scan), % |
14.81±13.46 |
0.04/47 |
|
Mean Anterior-posterior axis, mm |
23.28±1.20 |
20.65/25.10 |
|
Mean Anterior chamber depth, mm |
3.00 ±0.39 |
2.22/3.50 |
|
Mean Lens thickness, mm |
4.58 ±0.44 |
3.32/5.18 |
Abbreviations: p – reliability of difference between the groups according to Mann-Whitney U test and Student’s t-test for 2 independent samples, MD – mean deviation, IOP – intraocular pressure, MOPP - mean ocular perfusion pressure, BP – blood pressure, RNFL – retinal nerve fiber layer, GCC – ganglion cell complex, FLV – focal loss volume, GLV – global loss volume.
The progression of glaucoma, as confirmed by both perimetry and SD-OCT, was revealed in 50 eyes (33%). The predictors of glaucoma progression and their prognostic significance and cut-off values detected in this study are presented in Table 2. The results have revealed that the decrease in circulatory parameters including retinal microcirculation and retrobulbar blood flow was associated with glaucoma progression. Table 2 represents the clinical variables with statistically significant AUC for distinguishing between progression and non-progression eyes with glaucoma. Table 3 demonstrates the selected predictors according to the generalized linear mixed models. In the both tables the studied variables were related to predictors if the progression was detected by means of functional or/and structural deterioration. However, the final decision to relate the parameter to a predictor was made if the parameter was significant for distinguishing between progression and non-progression eyes using both AUC and the generalized linear mixed models and if the progression was revealed on the basis of both structural and functional deterioration.
Table 2: Diagnostic ability of studied clinical parameters in differentiating between patients with progressive vs. non-progressive damage, separately for the functional and morphological damage
|
Variables |
Visual field progression |
OCT progression |
||||
|
AUC ± S.E. 95% CI |
p |
Criterion (Cut-off) |
AUC ± S.E. 95% CI |
p |
Criterion (Cut-off) |
|
|
MD |
0.677±0.07 |
0.017 |
<=-2 .73 |
0.666±0 .07 |
0.029 |
<=-2.73 |
|
(0.539 - 0.814) |
(0.524 - 0.808) |
|||||
|
PSD |
0.633±0.06 |
0.049 |
>1 .62 |
0.651±0 .07 |
0.048 |
>2.85 |
|
(0.510 - 0.757) |
(0.510 - 0.757) |
|||||
|
TPCA, RI |
0.801±0.12 |
0.025 |
> 0.6 |
0.517±0.07 |
0.82 |
> 0.6 |
|
(0.557 - 0.946) |
(0.382 - 0.650) |
|||||
|
TPCA, PSV, cm/s |
0.606±0.08 |
0.232 |
<=11 .1 |
0.690±0.07 |
0.0084 |
<=11.4 |
|
(0.454 - 0.744) |
(0.554 - 0.806) |
|||||
|
CRA, PSV, cm/s |
0.552±0.08 |
0.599 |
<=9.7 |
0.520±0.07 |
0.005 |
<=10 |
|
(0.400 - 0.697) |
(0.557 - 0.810) |
|||||
|
CRA, EDV, cm/s |
0.715±0.11 |
0.008 |
< =2.5 |
0.712±0.06 |
0.0018 |
<=3.5 |
|
(0.508 - 0.876) |
(0.578 - 0.823) |
|||||
|
Peak follow-up IOP, mm Hg |
0.768±0.07 |
0.0002 |
> 23.8 |
0.741±0.06 |
||
|
(0.631 - 0.905) |
(0.612 - 0.848) |
0.0002 |
>=22.1 |
|||
|
Initial IOPcc, mm Hg |
0.583±0.08 |
0.328 |
>16 |
0.714±0.06 |
0.001 |
>20 |
|
(0.432 - 0.724) |
(0.582 - 0.823) |
|||||
|
Corneal hysteresis, mm Hg |
0.755±0.07 |
0.0001 |
<=9.6 |
0.623±0.07 |
0.09 |
<=9.7 |
|
(0.606 - 0.870) |
(0.486 - 0.747) |
|||||
|
Peripapillary CT, μm |
0.752±0.09 |
0.01 |
< =235 |
0.597±0.08 |
0.221 |
<=313 |
|
(0.574 - 0.885) |
(0.439 - 0.756) |
|||||
|
Foveal CT, μm |
0.740±0.09 |
0.012 |
< =222 |
0.579±0.08 |
0.338 |
< =256 |
|
(0.587 - 0.893) |
(0.436 - 0.712) |
|||||
|
OCT thickness ILM-IPL inferior- Hemi, μm |
0.754±0.07 (0.607 - 0.901) |
0.004 |
<=105 |
0.748±0.06 (0.611 - 0.856) |
0.0004 |
<=105 |
|
OCT Thickness ILM-IPL (μm) superior- Hemi, μm |
0.684±0.08 (0.532 - 0.812) |
0 .022 |
<=109 |
0.759±0.07 (0.623 - 0.865) |
0.0002 |
<=109 |
|
OCT Thickness ILM-IPL parafovea, μm |
0.745±0.07 (0.595 - 0.890) |
0.007 |
<=111 |
0.751±0.06 (0.615 - 0.859) |
0.0003 |
<=107 |
|
wiVD Disc, % |
0.715±0.07 (0.566 - 0.865) |
0.001 |
<=45.2 |
0.622±0.07 (0.481 - 0.749) |
0.116 |
<=47.15 |
|
Parafovea vessel density superficial |
0.752±0.07 (0.609 - 0.895) |
0.0004 |
<=45 |
0.776±0.07 (0.593 - 0.835) |
0.0008 |
<=44 |
|
Age, years along with |
0.710±0.07 |
0.001 |
>70 |
0.520±0.06 |
0.79 |
>60 |
|
(0.588 - 0.813) |
(0.388 - 0.650) |
|||||
|
Avg, RNFL, μm |
0.654±0.08 |
0.02 |
<=93.28 |
0.613±0.07 |
0.122 |
<=72 |
|
(0.555 - 0.627) |
(0.479 - 0.736) |
|||||
|
Avg infer. RNFL, μm |
0.687±0.08 |
0.018 |
<=82 |
0.647±0.07 |
0.0455 |
<=85 |
|
(0.533 - 0.813) |
(0.511 - 0.767) |
|||||
|
Avg. GCC, μm |
0 .658±0.08 |
0.015 |
<=92.51 |
0.705±0.06 |
0.0024 |
<=82.52 |
|
(0.527 - 0.698) |
(0.573 - 0.815) |
|||||
|
FLV, % |
0.636±0.08 |
0.022 |
>1.769 |
0.626±0.07 |
0.019 |
>0.854 |
|
(0.540 - 0.726) |
(0.491 - 0.747) |
|||||
|
GLV, % |
0.622±0.08 |
0.028 |
>13.22 |
0.665±0.07 |
0.091 |
>13.32 |
|
(0.525 - 0.712) |
(0.532 - 0.782) |
|||||
|
Inf. GCC, μm |
0.674±0.08 |
0.046 |
<=95.2 |
0.667±0.07 |
0 .018 |
<=83.7 |
|
(0.516 - 0.832) |
(0.534 - 0.784) |
|||||
|
CRV, PSV, cm/s |
0.691±0.07 |
0.015 |
<=5.07 |
0.622±0.07 |
0.097 |
<=5.4 |
|
(0.538 - 0.819) |
(0.486 - 0.745) |
|||||
|
MOPP, mm Hg |
0.682±0.08 |
0.03 |
<=40 |
0.674±0.07 |
0.0223 |
<=51.11 |
|
(0.521 - 0.819) |
(0.528 - 0.798) |
|||||
|
Initial IOPcc, mm Hg |
0.583±0.08 |
0.328 |
>16 |
0.714±0.06 |
0.001 |
>20 |
|
(0.432 - 0.724) |
(0.582 - 0.823) |
|||||
Note: the progression detected by the visual field method is based on the visual field event analysis by Guided Progression Analysis (GPA) and the visual field trend analysis. The progression detected by the optical coherence tomography method is based on the analysis of thinning trend in macular ganglion cell complex and peripapillary retinal nerve fiber layer.
The significance of AUC is marked in bold if the parameter was referred to a “predictor” of glaucoma progression, detected only by functional or structural deterioration. If the parameter was associated with progression confirmed by both methods, the significance of its AUC is marked in bold italics.
Abbreviations: MD – mean deviation, PSD – pattern standard deviation, AUC – area under ROC curve; CI – confidence interval, S.E. – standard error; TPCA – temporal posterior ciliary artery, CRA – central retinal artery, EDV – end diastolic velocity, CT – choroid thickness, OCT – optical coherence tomography, ILM – internal limiting membrane, IPL – inner plexiform layer, CRV – central retinal vein, PSV – peak systolic velocity, MOPP – mean ocular perfusion pressure.
Table 3: Predictors of glaucoma progression according to the generalized logistic mixed models
|
Variables |
Visual field progression |
OCT progression |
||||||||
|
Wald Chi-Squared |
B |
95% CI |
OR |
p |
Wald Chi-Squared |
B |
95% CI |
OR |
p |
|
|
Test |
Test |
|||||||||
|
Parafovea vessel density superficial |
12.5 |
-0.6 |
-1.2 |
0.55 |
0.0001 |
7.43 |
-0 .429 |
-0.859 |
0 .65 |
0 .006 |
|
wiVD Disc, % |
8.1 |
-0.11 |
-0.16 |
0.89 |
0.004 |
1.88 |
-0.06 |
-0 .156 0 .02 |
0.94 |
0.17 |
|
CRA, EDV, cm/s |
6 |
-0.25 |
-0.44 |
0.78 |
0.02 |
6.29 |
-0.228 |
-0.456 |
0.8 |
0.012 |
|
TPCA, EDV, cm/s |
7.6 |
-0.26 |
-0.55 |
0.77 |
0.005 |
2.72 |
-0.1 |
-0.452 |
0.9 |
0.385 |
|
TPCA, PSV, cm/s |
1.17 |
-0.314 |
-0.881 - 0 .254 |
0.73 |
0.279 |
5.77 |
-0.163 |
-0.327 |
0.85 |
0.016 |
|
MOPP, mm Hg |
5.6 |
-0.15 |
-0.3 |
0.85 |
0.018 |
5.54 |
-0.109 |
-0.217 |
0.89 |
0.019 |
|
Corneal Hysteresis, mm Hg |
9 |
-0.12 |
-0.24 |
0.88 |
0.003 |
1.09 |
-0.04 |
-0.155 |
0.96 |
0.295 |
|
Peak follow-up IOP, mm Hg |
9.3 |
0.1 |
0.03 - 0.15 |
1.18 |
0.002 |
7.81 |
0.09 |
0.03 - 0.16 |
1.09 |
0.005 |
|
Initial IOP, mm Hg |
2.69 |
0.12 |
-0.29 |
1.12 |
0.1 |
7.38 |
0.18 |
0.05 - 0.31 |
1.18 |
0.007 |
|
RNFL inferior, μm |
6.7 |
-0.16 |
-0.31 |
0.85 |
0.011 |
3.89 |
-0.14 |
-0.29 |
0.86 |
0.049 |
|
Avg RNFL, μm |
5.7 |
-0.11 |
-0.33 |
0.89 |
0.015 |
2.55 |
-0.07 |
-0.18 |
0.93 |
0.111 |
|
Avg GCC, μm |
4.7 |
-0.08 |
-0.43 |
0.92 |
0.03 |
4 .10 |
-0.102 |
-0.203 |
0.9 |
0.045 |
|
FLV, % |
5 |
0.5 |
0.08 - 1.26 |
1.64 |
0.028 |
2.08 |
0.43 |
-1.16 |
1.53 |
0.028 |
|
GLV, % |
4.5 |
0.34 |
0.05 - 1.28 |
1.4 |
0.036 |
5.3 |
0.47 |
0.06 - 1.16 |
1.59 |
0.149 |
|
Peripapillary CT, μm |
5.4 |
-0.21 |
-0.4 |
0.81 |
0.012 |
3.2 |
-0.15 |
-0.323 |
0.86 |
0.72 |
|
OCT Thickness ILM-IPL in inferior hemisphere (μm) |
12 .8 |
-0.13 |
-0.25 |
0.86 |
0.0001 |
8.97 |
-0.109 |
-0.142 |
0.89 |
0.003 |
|
OCT Thickness ILM-IPL parafovea (μm) |
10.8 |
-0.12 |
-0.23 |
0.88 |
0.0001 |
8.5 |
-0.107 |
-0.214 |
0.9 |
0.004 |
|
Inferior GCC |
6.45 |
-0.12 |
-0.23 |
0.88 |
0.011 |
4.71 |
-0.11 |
-0.22 |
0.89 |
0.03 |
|
Superior GCC |
2.13 |
-0.06 |
-0.016 0.02 |
0.94 |
0.144 |
4.37 |
-0.12 |
-0.283 |
0.86 |
0.038 |
|
OCT Thickness ILM-IPL (μm) superior- Hemi |
6.5 |
-0.11 |
-0.21 |
0.89 |
0.011 |
6.44 |
-0.106 |
-0.212 |
0.89 |
0.011 |
Note: the progression detected by the visual field method is based on the visual field event analysis by Guided Progression Analysis (GPA) and the visual field trend analysis. The progression detected by the optical coherence tomography method is based on the analysis of thinning trend in macular ganglion cell complex and peripapillary retinal nerve fiber layer.
The significance of AUC is marked in bold if the parameter was referred to a “predictor” of glaucoma progression, detected only by functional or structural deterioration. If the parameter was associated with progression confirmed by both methods, the significance of its AUC is marked in bold italics. Wald Chi-Squared Test characterizes that the explanatory variables in a model are significant.
Coefficient β – regression coefficient, the negative coefficient means that the increase of the value of the certain variable will decreases glaucoma progression CI - confidence interval of β OR - odds ratio representing the constant effect of a predictor on the likelihood that glaucoma progression will occur. OR in brackets is represented in the percentage. p indicates an evidence against the null hypothesis.
Abbreviations: wiVD Disc – whole en face image vessel density (Disc scan), CRA – central retinal artery, EDV – end diastolic velocity, CRV – central retinal vein, PSV – peak systolic velocity, TPCA – temporal posterior ciliary artery, MOPP – mean ocular perfusion pressure, IOP – intraocular pressure, RNFL – retinal nerve fiber layer, GCC – ganglion cell complex, CT – choroid thickness, FLV – focal loss volume, GLV – global loss volume, OCT – optical coherence tomography, ILM – internal limiting membrane, IPL – inner plexiform layer.
According to this data, vessel density of the parafoveal superficial plexus, macular thickness from the internal limiting membrane to the inner plexiform layer, peak follow-up intraocular pressure, end-diastolic velocity of central retinal artery, focal loss volume of ganglion cells complex and mean ocular perfusion pressure were detected as the significant predictors of glaucoma progression.
According to the multilevel mixed-effects models analysis, only four predictors were determined: VD of the parafoveal superficial plexus (z -4.77), EDV of central retinal artery (z -3.08), focal loss volume of GCC (z 3.53) and peak follow-up intraocular pressure (z 3.20) (Table 4).
Table 4: Predictors of glaucoma progression according to the multilevel mixed-effects models
|
Variables |
Coefficient |
Std.Error |
Z |
P (z) |
95% CI |
|
|
Parafovea vessel density superficial |
-0.38 |
0.08 |
-4.77 |
p≤0.001 |
-0.54 |
-0.26 |
|
CRA, EDV, cm/s |
-2.16 |
0.7 |
-3.08 |
0.002 |
-3.54 |
-0.78 |
|
FLV, % |
2.89 |
0.82 |
3.53 |
p≤0.001 |
1.28 |
4.49 |
|
Peak follow-up IOP, mm Hg |
5.82 |
1.82 |
3.2 |
0.001 |
2.26 |
9.34 |
Abbreviations: CRA – central retinal artery, EDV – end diastolic velocity, FLV – focal loss volume, IOP – intraocular pressure
The results showed that the rate of disease progression expressed as a change of perimetric index MD of visual field (ROP1) or RNFL thinning (ROP2) correlates with the peak follow-up IOP (r = 0.22, p = 0.01) for ROP1 and the end diastolic velocity in PCAs (r = -0.23, p = 0.01) for ROP2 (Table 5).
Table 5: Correlation between the rate of glaucoma progression* and blood flow velocity in retrobulbar vessels and IOP
|
Variables |
TPCA, PSV |
TPCA, EDV |
Peak follow-up IOP |
|
ROP1, dB/year |
r = 0.23 |
||
|
? = 0.01 |
|||
|
ROP2, μm /year |
r = -0.24 |
r = -0.23 |
|
|
p = 0.01 |
p = 0.03 |
ROP1 – rate of progression according to the change of perimetric index MD
ROP 2 – rate of progression according to the thinning of the retinal nerve fiber layer
*glaucoma progression was detected on the base of structural and functional deterioration
Abbreviations: PSV – peak systolic velocity, EDV – end diastolic velocity, CRV – central retinal vein, TPCA – temporal posterior ciliary artery, IOP – intraocular pressure.
A positive correlation was also observed between the thickness of the retinal inner layers in parafovea and the parafovea vessel density in superficial layer (r = 0.4, p = 0.01). MOPP correlated with the average GCC thickness and the GCC thickness in the inferior hemisphere, as well as with FLV and GLV (Table 6). Finally, the obtained results have revealed a correlation between parafovea VD in the superficial plexus with corneal hysteresis (Fig. 1).
Table 6: Correlation between the mean ocular perfusion pressure and the ganglion cells complex characteristics
|
Variables |
Avg.GCC, |
GCC in inferior hemisphere, μm |
FLV, % |
GLV, % |
|
μm |
||||
|
MOPP, mm Hg |
r = 0.36 |
r = 0.55 |
r = -0.4 |
r = -0.39 |
|
p = 0.01 |
p = 0.01 |
p = 0.01 |
p = 0.01 |
Abbreviations: GCC – ganglion cell complex, MOPP – mean ocular perfusion pressure, FLV – focal loss volume, GLV – global loss volume.
4. Discussion
The present study is devoted to the search for new biomarkers-predictors of glaucoma progression in a comparative aspect with well-known predictors. In this regard, we have analyzed 199 clinical parameters, which could be potentially associated with glaucoma progression, in two statistical models (with the calculation of AUC and the LGLM). The results showed that circulatory parameters, such as the blood flow in CRA, as well as the parafoveal vessel density measured by OCTA, have the highest AUC, the LGLM characteristics and were determined as predictors of glaucoma progression in the multilevel mixed-effects models analysis. There is currently strong evidence that the reduced ocular blood flow is an important risk factor for the development and progression of glaucoma [24, 25]. Meng et al. (2013) showed that despite the effective treatment of increased IOP, the differences in the parameters of ocular blood flow still persisted in IOP-controlled patients. This fact proves that lower rates of retrobulbar blood flow in glaucoma patients do not always result from increased IOP [44].
In this regard, the present study also involved the methods for measuring ocular blood flow along with traditional examination methods. As a result, the high prognostic significance of retrobulbar blood flow parameters as predictors of glaucoma progression was confirmed, and it coincides with the literature [45, 46, 47, 48, 49]. In present study the parameters of blood flow not only in CRA, but also in SPCA had a significant value in differentiating the progression and non-progression eyes, and SPCA PSV correlated with the rate of thinning of RNFL (ROP2). According to Martinez A, the disease progression is mainly associated with low end diastolic velocity in SPCA [50], that is consistent with our result on the high RI in SPCA as the predictor of glaucoma progression. High significance of blood flow parameters in CRA that have been revealed in this study is also consistent with the results of other authors. Therefore, according to Januleviciene, the odds of higher nerve fiber indicator (NFI) at the final examination of glaucoma patients was nearly 14 times greater in those, who had had higher than 0.67 baseline CRA RI (p = 0.028) [51].
It is worth noting that color Doppler imaging (CDI) is not a reliable method for measuring ocular blood flow in terms of interpretation of results. In fact, CDI data as the subjective aspects of CDI measurements have been pointed out in literature. Meanwhile the reproducibility is rather good in the CRA and ophthalmic artery, but it has higher variability in the SPCAs [52]. Development of a new diagnostic method – OCTA – has significantly expanded the possibilities for studying retinal microcirculation. Applying this method in the present study, we have found out that such parameters as wiVD Disc and parafovelal VD superficial parameters are among the predictors of glaucoma progression. However, only the reduced parafoveal VD in the superficial layer was associated with glaucoma progression that had been confirmed by both structural and functional deterioration in all applied statistical models. Several existing studies have shown that OCTA allows detecting the disease at pre-perimetric stage, and the parameters of OCTA correlated better with functional parameters including the electrophysiological variables than with structural ones [31, 53, 54].
Different studies show variable diagnostic ability of macular and peripapillary vascular parameters. Nevertheless, the question on the role of OCTA in the diagnosis and monitoring of glaucoma compared to the detection of structural parameters by OCT remains open. The literature on this subject is rather contradictory. Some authors have found inferior diagnostic ability of vascular parameters compared with such structural parameters as RNFL thickness [55, 56], while other studies showed comparable diagnostic accuracy [54, 57]. In general, the literature on retinal microcirculation damage in glaucoma is limited. In 2012, Hwang et al. were the first who showed that the relationship between total retinal microcirculation investigated by Doppler-OCT has a strong correlation with visual field defects which is not dependent on structural defects. The authors suggested that the decreased retinal and optic nerve blood flow might be the main cause of glaucoma progression [29]. G. Hollo et al. were the first who described VD loss in the peripapillary retina in the dynamics of glaucoma process [58]. T. Shojl and coauthors described the rate of change of retinal microcirculation during the follow-up of 13.9 months and noticed that it was significantly faster in glaucomatous eyes compared to healthy eyes. Moreover, they detected, by means of serial OCTA measurements, a glaucomatous change in macular VD without evidence of change in GCC thickness [37]. The authors concluded that change of VD in parafovea may precede the significant structural and functional changes that should be considered in longitudinal studies.
However, the question on the priority of studying microcirculatory parameters over structural ones remains open. For example, Chen et al. [33] studied POAG and normal eyes and demonstrated that peripapillary RNFL thickness and macula GCC thickness had the highest AUROCs for discriminating between glaucomatous and normal eyes (0.95 for both) when compared to wiVD and peripapillary vessel density (0.93 and 0.89, respectively). One more issue needs to be solved is whether OCT or OCTA parameters are more important for predicting the disease progression. For example, it has been noted, that although there is a loss of capillaries in the peripapillary retina in dynamics, it is not a good biomarker of glaucoma progression as RNFL analysis [59]. On the other hand, according to the same author, the superficial macular capillary VD in the central 6-mm area can be more informative than GCC thickness in glaucoma monitoring [60]. There is evidence in literature that OCTA VD decrease may actually precede both structural and functional loss, and therefore might be useful in the diagnosis and monitoring of very early glaucoma [61, 62]. Though it does not solve the question, if the reduced parafovea VD imply causation of structural and functional loss or just only reflect the metabolic changes of “sick” retinal ganglion cells, this reduction detected at the very beginning of glaucoma monitoring may be used as the glaucoma progression predictor.
According to our data, the reduced VD in parafovea may serve as the harbingers of the impending cell death and subsequent tissue thinning with functional loss. In general, the role of OCTA in determining the predictors of glaucoma progression and the dynamic range for vessel density is sparse. We have found only one publication on this issue. Moghimi and coauthors investigated prospectively the relation between macular and peripapillary vessel density and progressive RNFL loss in patients with early to moderate POAG [40]. According to these results, the OCTA parameters and central corneal thickness were the only predictive independently of the rate of RNFL loss. Each 1% lower macular wiVD and ONH whole image vessel density (ONH-wiVD) was associated with a 0.11-mm/year (p < 0.001) and 0.06-mm/year (p = 1⁄4 0.031) faster rate of RNFL decline, respectively [40]. It is worth noting that we did not find any correlation between the OCTA parameters and rate of RNFL thinning in the present study. Moghimi et al. also have underlined that the relation between VD measurements and rate of RNFL loss was weak. However, the authors have concluded that the parameters measured by OCTA may predict the RNFL thinning in long follow-up. They assume that OCTA may provide information about the early dysfunction of RGCs with lower metabolic demands. This is consistent with our previous data [54]. Performing the electrophysiological studies and OCTA in the same patients, we have revealed the strong correspondence between the amplitude of transient pattern electroretinogram (PERG) and VD in the superficial macular plexus. On the basis of this data we have assumed that ganglion cells dysfunction may be related to the reduction of retinal blood supply and is preceding their death that may be reflected in PERG results. It is worth noting that in contrast to Moghimi et al, we used the 6x6 macula scan that covers larger field and therefore is more informative in respect to ganglion cells blood supply.
Our study has revealed that structural and microcirculatory parameters are comparable in accordance with AUC analysis. The same can be noted about the results of regression analysis. According to the OR, the risk of glaucoma progression is equally defined as VD reduction in parafovea and the loss of RGCs. Besides, the results of this study have shown that initial low RNFL thickness was also associated with progression that coincides with the literature [21, 22]. However, according to the multilevel mixed-effects models analysis, only GCC focal loss volume among all other structural parameters was the most significant predictive factor (Table 4). Zhang and co-authors in a study of a large cohort of perimetric glaucoma patients followed for 1.5 to 6.5 years also determined the GCC focal loss volume as the strongest predictor for VF progression [63]. Many authors have reported about the importance of evaluation of RNFL and retinal ganglion cell (RGC) layer with inner plexiform layer (CGIPL) in glaucoma monitoring [19,21], some of them have emphasized the role of RGC assessment that might have a priority over RNFL in glaucoma monitoring, particular, the role of the inferior perifoveal region [64].
In the present study OCT ILM-IPL inferior-Hemi thickness was also determined as an important structural predictor of glaucoma progression, according to the generalized logistic mixed models, but not in the multilevel mixed-effects models analysis. Sung and co-authors, who also studied the OCT ILM-IPL inferior-Hemi thickness, have reported that its five times excess of age-related changes in measuring retinal thickness around fovea with a diameter of 6 mm2 (-0.26 μm/year) allows predicting the progression of glaucoma irrespective of the absence of changes in the perimetric results [65]. Moreover, some authors emphasized that the macular parameters had high discriminating power and high reproducibility for early glaucoma detection in comparison with the peripapillary RNFL parameters [66]. Since the ganglion cells are more concentrated in the macula than in the peripapillary region and have a retinal capillary plexus that supply with blood flow this region, the macular VD may change as part of a glaucoma progression. Furthermore, there is a growing body of evidence that early glaucomatous damage involves the macula, especially its inferior hemisphere [67].
The inferior macula sector as a vulnerable region for glaucoma damage makes sense since most of the nerve fibers of the inferior region of the macula project to the inferior quadrant of the optic disc, a region which is particularly susceptible to glaucomatous damage. Hood et al. described that thinning of RGC and the nerve fiber layer is already present in pre-perimetric glaucoma patients and progresses with increasing loss of mean deviation (MD) [68]. Lommatzsch et al. confirmed first that the VD of the inferior perimacular sector is lower than in all other sectors and that this value is reduced in early forms of glaucoma with progressive losses with deteriorating MD [69]. The recent study has demonstrated that each 1 dB decrease in VF MD was associated with a reduction of 0.43% in macular wiVD and 0.46% in pfVD. The authors also have revealed that the association between visual field MD and macula whole image vessel density (R2 =0.40) was stronger than ONH whole image capillary density (R2=0.31) and GCC and RNFL thicknesses [70]. The reasons for early involvement of macula into the glaucoma process are not studied enough. Probably, it is related to ischemia of the indicated retinal area and with increased metabolic demand of the area with the highest concentration of RGC. According to the results of our study, RGC thickness and its characteristics (GLV and FLV) correlated with mean ocular perfusion pressure. A correlation was also revealed between the thickness of the inner retinal layers in the parafovea and the parafovea vessel density in superficial layer that coincides with our previous results [54] and literature [71].
Another reason to look more closely at the macula VD in glaucoma monitoring is the possibility to determine the disease progression when structural parameters are not acceptable due to the upcoming floor effect. According to Moghimi, even a pronounced loss of visual function (MD reached -19 dB) did not result in the “floor effect” of VD [72]. Similar results were obtained by Rao [73] who demonstrated that the “floor effect” for the specified parameter did not occur at MD -15 dB. Other authors report that the “floor effect” occurs a little earlier for the peripapillary retina VD that is observed at MD below -14.0 dB, but at the same time later than for such morphometric parameters as RNFL and RGC thickness [68, 74]. Thus, according to Hood, this effect for RNFL is already seen at MD -10 dB [68]. Other authors have also noted the advantages of studying GCC thickness compared to RNFL [75]. At the same time, recent studies have shown the importance of assessment of the peripapillary VD at advanced stages of the disease [76]. The results of the present study also demonstrated the high predictor ability of Optic Nerve Head WI VD in patients with initial and moderate glaucoma, but only in the cases, when the progression was detected on the basis of functional deterioration. It has been recognized that the detection of the early glaucoma progression is more reliable using SD-OCT, while functional deterioration is more notable in the moderate and advanced stages. According to our previous data, structural parameters (in particular, retinal GCC) have the priority over functional ones in the patients with initial glaucoma compared to the advanced stages [54]. On the other hand, the peripapillary VD had the highest diagnostic ability for distinguishing between early and moderate to advanced stages, while parafoveal VD in the superficial layer had the highest ability for distinguishing the early glaucoma from healthy eyes. In general, the diagnostic ability of the OCTA parameters in early glaucoma was higher compared to RNFL and GCC. Thus, the results of the current study are consistent with this data.
The most important issue is the relation between the retinal microcirculation measured by OCT as the predictor of functional-structural loss. Some authors have assumed that VD decrease in macula, optic disc and peripapillary retina is associated with a faster rate of RNFL progression in mild to moderate glaucoma suggesting that decreased vessel density may be a risk indicator for progression [72]. Their results have shown that vessel density measures tend to be more strongly associated with severity of visual field damage than thickness measures and may be an additional tool to monitor progression in advanced disease. These data are consistent with our results, according to which OCTA parameters serve as the predictors of glaucoma progression. Besides, this study has revealed that the initially subtle peripapillary choroid is also a predictor of glaucoma progression as it was based on the detection of functional loss. The peripapillary choroidal vasculature is downstream from the posterior ciliary artery, which also perfuses the prelaminar and laminar tissues [77]. According to the results of the present study, reduced blood flow in PCA was one of the main predictors of the disease progression. It also can explain the fact that the peripapillary choroid thickness has been detected as the predictor with reliable diagnostic ability. According to Kim and coauthors, the eyes with capillary loss in the lower temporal quadrant of the peripapillary choroid have more pronounced visual field defects compared to the eyes with the preserved peripapillary choroidal microcirculation. The authors noted an inverse relation between VD in the peripapillary choroid and RNFL thickness [78]. Our data on the predictive role of peripapillary choroid thickness is consistent with these results.
Having followed the patients for two years, Park H. and et al. investigated the peripapillary choroidal microvasculature dropout (MvD) in glaucomatous eyes with or without disc hemorrhage (DH). They revealed that patients with progressive glaucoma exhibited significantly more MvD than the stable patients in both DH and no-DH groups. The authors concluded that presence of MvD associated with progressive RNFL thinning. They assumed that OCTA may provide a new biomarker for glaucoma progression and this biomarker is the peripapillary choroidal microvasculature [39]. The authors explained this phenomenon by the choroidal vascular insufficiency that may play a role in the lack of prelaminar optic nerve nutrition of glaucoma progression. The present study has also revealed that the peak follow-up IOP is among the parameters with very high predictive ability. These data confirm the well-known fact that IOP is the main risk factor for the progression of glaucoma [1, 5, 7, 8, 9,11,14,22,41]. According to the Early Manifest Glaucoma Trial, each increase of IOP mmHg creates a possible 10% growth of progression risk. A 25% IOP reduction allowed a slowdown in the disease progression from 62% to 45% after 6 years of follow-up [2]. The Canadian Glaucoma Study confirmed that the average IOP at follow up (before demonstrating a progression) was directly proportional to the progression itself. Each mmHg increase of IOP caused a risk increase approximately by19% [5].
In the present study, the peak follow-up IOP showed weak but significant correlation with MD worsening (ROP1). Our data is consistent with the literature. Many authors noted that peak IOP values play an important role in the progression of glaucoma [79-81]. After the analysis of 587 patients with progressive glaucoma, De Moraes et al. has discovered that peak IOP is the best predictor of progression than average IOP or its fluctuations (OR 1.13; p <0.01) [7]. According to Actis et al, each average increase of IOP mmHg means an average worsening of the MD variable of -8.82 dB equal to -1.34 dB a year [14].
Despite high predictive ability of IOP, this factor is particularly susceptible to changes during glaucoma treatment because the latter is aimed at reducing IOP. Meanwhile, the effect of changing treatment based on IOP could dilute and even reverse the relation between IOP and visual field progression [82]. The abovementioned limits the predictive ability of IOP. It should be also emphasized that the present study does not consider initial IOP as a predictor of glaucoma progression. These data are consistent with the results obtained by Shiga et al: while the initial IOP did not differ significantly between the groups with and without progression, the initial blood flow parameters in optic disc were significantly lower in the group with progression [28]. It is worth noting that Shiga et al have obtained the patients with normotensive pre-perimetric glaucoma (PPG). The authors suggested that ONH blood flow impairment acts as a primary cause of glaucomatous progression in normotensive PPG eyes and concluded that reduced blood flow in the optic disc, even with normal IOP, makes it reasonable to prescribe hypotensive eye drops. According to our results, corneal hysteresis was also among reliable predictors, though only in the cases with the confirmed functional deterioration. This is consistent with the literature [11]. Moreover, we have found out a positive relation between CH and the vessel density in the superficial plexus of parafovea. It is still difficult to explain this result: corneal hysteresis is an important biomechanical characteristic of the cornea that reflects its viscoelastic properties, while VD characterizes hemoperfusion of the corresponding retinal layers. It can be assumed that the identified relation is not accidental. Indeed, corneal viscoelastic features are determined by the features of the extracellular matrix influenced by matrix metalloproteinases (MMP) [83]. It has been described that MMPs, namely MMP-2, MMP-3 and MMP-9, are involved in the remodeling process of extracellular matrix components such as collagen of the trabecular meshwork and, hence, in the IOP regulation. Interestingly, the same MMPs play a role in the regulation and formation of the endothelium, intima, smooth muscle cells and vascular adventitia [84].
Furthermore, it has been recognized that CH correlates with the depth of optic disc cupping and the degree of change in its shape under the conditions of IOP fluctuations [85] which is explained with the single nature of both phenomena, namely, the changes in ocular biomechanics in glaucoma. According to the literature, circulatory disorders of lamina cribrosa lead to laminar disinsertions occurring twice frequently in eyes with optic disc hemorrhages, and they are considered to be a risk factor of glaucomatous visual field progression [86]. Finally, corneal viscoelastic properties may serve as the buffering mechanism for microvolume ocular changes, which protect an eye from IOP fluctuations and help the cornea withstand deformation [87]. The lower IOP fluctuations are, the more stable ocular perfusion pressure is. It is worth noting that the present study has detected MOPP as a parameter with significant predictive ability. Though, MOPP is a “surrogate” of IOP, our results confirm the literature data on significance of this parameter in glaucoma progression [48]. According to the Los Angeles Latino Eye Study, low MOPP is a risk factor of glaucoma progression [88].
Our study has several limitations that must be acknowledged. First of all, we realize that OCTA does not directly quantify the flow rate within the detected vessels. The reduced vessel density may have been the result of slow blood flow within the vessels or capillary dropout and not necessarily due to glaucoma progression. Second, we did not adjust for the OCTA SSI though SSI may affect the results of VD assessment [37]. However, we excluded all protocols with low SSI. We also did not assess reproducibility and repeatability of the method as it has been previously studied [89]. Third, we did not consider some factors with known predictive activities such as disc hemorrhages, vertical cup-disc ratio (C/D), rim-disc area ratio and rim width, or rim volume. We analyzed only one clinical parameter measured during follow-up: IOP. All these factors have been studied many times before. The purpose of our study was to find out new factors and to compare them with the main structural parameters.
Forth, we did not include other forms of glaucoma (normotensive glaucoma (NTG) and pseudoexfoliative glaucoma (PEG)) in this study as each of them has its own progressive features. For example, PEG is characterized with the highest rate of progression, and NTG – with the slowest one. This issue might be the subject of future studies. According to literature, distinguishing between rapidly progressive and slowly progressive glaucoma patients in the studies of glaucoma progression predictors is crucial [22]. In addition, due to pathogenic features, pseudoexpodiative glaucoma is characterized with particularly significant microcirculation reduction [90] that is also true for NTG [91].
The strengths of the present study include its prospective longitudinal study design and standardized follow-up and examinations minimizing the selection bias.
Another strength is that all our subjects underwent wash-out (discontinue using IOP-lowering medications) before the baseline examination. Indeed, one should keep in mind that the treatment options may significantly affect glaucoma progression. The use of topical antihypertensive treatment can improve ocular hemodynamics [92]. At the same time, little is known on the effect of IOP reduction on retinal microcirculation. The results of experimental studies show that the microcirculation in retina, sclera, lamina cribrosa and choriocapillaries remains unchanged even at significant changes in IOP [93]. On the other hand, according to some clinical studies, OCTA vessel density appears to correlate strongly with IOP [94-96]. We believe that OCTA may significantly improve the early detection of glaucoma progression, as formerly OCT has provided more precise diagnostics in regard to this detection. For instance, recently we have found out that glaucoma progression is detected twice often using the OCT method (36.8%) in comparison with SAP (19.1%, p<0.001 [97] that coincide with other authors [98]. The interpretation of glaucoma progression is rather challenging as many different factors may affect the glaucoma course [2, 14]. According to De Moraes, combination of data may be useful when discussing with individual patients their risks and treatment options, as well as standardizing among clinicians the quantification of progression risk in treated glaucoma patients [11]. From this point of view, the application of a new biomarker as the VD of parafovea superficial layer may improve the prediction of glaucoma progression.
5. Conclusion
In conclusion, the present study has revealed a new predictor of glaucoma progression which allows expanding the boundaries of generally accepted standards for glaucoma monitoring. Besides, the obtained results demonstrate the role of circulatory disorders in the progression of POAG.
Funding statement:
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement:
The data that support the findings of this study are available from the corresponding author N.K, upon reasonable request
References
- AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. Am J Opthtalmol 134 (2002): 499-512.
- Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 121 (2003): 48-56.
- Leske MC, Heijl A, Hyman L, et al. EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology 114 (2007): 1965-1972.
- Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study (EGPS) Group. Ophthalmology 112 (2005): 366-375.
- Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian Glaucoma Study 2. Risk factors for the progression of open-angle glaucoma. Arch Opthalmol 126 (2008): 1030-1036.
- Kim SH, Lee EJ, Han JC, et al. The Effect of Diurnal Fluctuation in Intraocular Pressure on the Evaluation of Risk Factors of Progression in Normal Tension Glaucoma. PloS One 11 (2016): e0164876.
- De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol 129 (2011): 562-568.
- Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 131 (2001): 699-708.
- Musch DC, Gillespie BW, Niziol LM, et al. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 118 (2011): 1766-1773.
- Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol 136 (2003): 805-813.
- De Moraes CV, Hill V, Tello C, et al. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma 21 (2012): 209-213.
- Bengtsson B, Leske MC, Yang Z, et al. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology 115 (2008): 2044-2048.
- Martinez A, Sanchez-Salorio M. Predictors for visual field progression and the effects of treatment with dorzolamide 2% or brinzolamide 1% each added to timolol 0.5% in primary open-angle glaucoma. Acta Ophthalmol 88 (2010): 541-552.
- Actis AG, Versino E, Brogliatti B, et al. Risk Factors for Primary Open Angle Glaucoma (POAG) Progression: A Study Ruled in Torino. Open Ophthalmol J 10 (2016): 129-139.
- Leske MC, Wu SY, Nemesure B, et al. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol 120 (2002): 954-959.
- Gugleta K. Vascular risk factors in glaucoma - diagnostics. Praxis (Bern 1994) 98 (2009): 201-207.
- Memarzadeh F, Ying-Lai M, Chung J, et al. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci 51 (2010): 2872-2877.
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 311 (2014): 1901-1911.
- De Moraes CG, Sehi M, Greenfield DS, et al. A Validated Risk Calculator to Assess Risk and Rate of Visual Field Progression in Treated Glaucoma Patients. Invest Ophthalmol Vis Sci 53 (2012): 2702-2707.
- Ernest PJ, Schouten JS, Beckers HJ, et al. Prediction of Glaucomatous Visual Field Progression Using Baseline Clinical Data. J Glaucoma 25 (2016): 228-235.
- Sehi M, Bhardwaj N, Chung YS, et al. Evaluation of baseline structural factors for predicting glaucomatous visual-field progression using optical coherence tomography, scanning laser polarimetry and confocal scanning laser ophthalmoscopy. Eye (Lond) 26 (2012): 1527-1535.
- Nitta K, Wajima R, Tachibana G, et al. Prediction of Visual Field Progression in Patients with Primary Open-Angle Glaucoma, Mainly Including Normal Tension Glaucoma. Sci Rep 7 (2017): 15048.
- Mauschitz MM, Bonnemaijer PWM, Diers K, et al. Systemic and Ocular Determinants of Peripapillary Retinal Nerve Fiber Layer Thickness Measurements in the European Eye Epidemiology (E3) Population. Ophthalmology 125 (2018): 1526-1536.
- Himori N, Kunikata H, Shiga Y, et al. The association between systemic oxidative stress and ocular blood flow in patients with normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol 254 (2016):333-341.
- Nakazawa T. Ocular Blood Flow and Influencing Factors for Glaucoma. Asia Pac J Ophthalmol (Phila) 5 (2016): 38-44.
- Harris A, Gross J, Moore N, et al. The effects of antioxidants on ocular blood flow in patients with glaucoma. Acta Ophthalmol 96 (2018): 237–41.
- Zhu MM, Lai JSM, Choy BNK, et al. Physical exercise and glaucoma: a review on the roles of physical exercise on intraocular pressure control, ocular blood flow regulation, neuroprotection and glaucoma-related mental health. Acta Ophthalmol 96 (2018): 676-691.
- Shiga Y, Aizawa N, Tsuda S, et al. Preperimetric Glaucoma Prospective Study (PPGPS): predicting visual field progression with basal optic nerve head blood flow in normotensive PPG eyes. Trans Vis Sci Tech 7 (2018): 11.
- Hwang JC, Konduru R, Zhang X, et al. Relationship among visual field, blood flow, and neural structure measurements in glaucoma. Invest Ophthalmol Vis Sci 53 (2012): 3020-3026.
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 123 (2016): 2498-2
- Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 121 (2014): 1322-1332.
- Manalastas PIC, Zangwill LM, Saunders LJ, et al. Reproducibility of optical coherence tomography angiography macular and optic nerve head vascular density in glaucoma and healthy eyes. J Glaucoma 26 (2017): 851-859.
- Chen HS, Liu CH, Wu WC, et al. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci 58 (2017): 3637-3645.
- Kurysheva, N. I. Macula in Glaucoma: Vascularity Evaluated by OCT Angiography. Research Journal of Pharmaceutical, Biological and Chemical Sciences 7 (2016): 651-662.
- Xu H, Yu J, Kong X, et al. Macular microvasculature alterations in subjects with primary open-angle glaucoma. Medicine (Baltimore) 95 (2016): e434.
- Akil H, Chopra V, Al-Sheikh M, et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br J Ophthalmol (2017).
- Shoji T, Zangwill LM, Akagi T, et al. Progressive macula vessel density loss in primary open angle glaucoma: a longitudinal study. Am J Ophthalmol 182 (2017): 107-117.
- Hollo G. Intrasession and between-visit variability of sector peripapillary angioflow vessel density values measured with the angiovue optical coherence tomograph in different retinal layers in ocular hypertension and glaucoma. PLoS One 11 (2016): e0161631.
- Park HL, Kim JW, Park CK. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology 125 (2018): 1003-1013.
- Moghimi S, Zangwill LM, Penteado RC, et al. Macular and optic nerve head vessel density and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology 125 (2018): 1720-1728.
- Chauhan BC, Malik R, Shuba LM, et al. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci 55 (2014): 4135-4143.
- Anton A, Pazos M, Martín B, et al. Glaucoma progression detection: agreement, sensitivity, and specificity of expert visual field evaluation, event analysis, and trend analysis. Eur J Ophthalmol 23 (2013): 187-195.
- Bolker BM, Brooks ME, Clark CJ, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24 (2009): 127-1
- Meng N, Zhang P, Huang H, et al. Color Doppler imaging analysis of retrobulbar blood flow velocities in primary open-angle glaucomatous eyes: a meta-analysis. PLoS One 8 (2013): e62723.
- Ates H, Uretmen O, Killi R, et al. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am J Ophthalmol 132 (2001): 598-599.
- Galassi F, Sodi A, Ucci F, et al. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol 121 (2003): 1711-1715.
- Zeitz O, Galambos P, Wagenfeld L, et al. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br J Ophthalmol 90 (2006): 1245-1248.
- Januleviciene I, Sliesoraityte I, Siesky B, et al. Diagnostic compatibility of structural and haemodynamic parameters in open-angle glaucoma patients. Acta Ophthalmol 86 (2008): 552-557.
- Jimenez-Aragon F, Garcia-Martin E, Larrosa-Lopez R, et al. Role of color Doppler imaging in early diagnosis and prediction of progression in glaucoma. Biomed Res Int (2013): 871689.
- Martínez A, Sánchez M. Predictive value of colour Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol 83 (2005): 716-722.
- Janulevi?iene I, Ehrlich R, Siesky B, et al. Evaluation of hemodynamic parameters as predictors of glaucoma progression. J Ophthalmol (2011): 164320.
- Ciulla TA, Kagemann L, Harris A. Imaging fundamentals. Retina and optic nerve imaging. In: Ciulla TA, Regillo CD, Harris A, eds. Philadelphia: Lippincott Williams and Wilkins (2003): 245-254.
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci 57 (2016): 451-459.
- Kurysheva NI, Maslova EV, Zolnikova IV, et al. A comparative study of structural, functional and circulatory parameters in glaucoma diagnostics. PLoS One 13 (2018): e0201599.
- Triolo G, Rabiolo A, Shemonski ND, et al. Optical coherence tomography angiography macular and peripapillary vessel perfusion density in healthy subjects, glaucoma suspects, and glaucoma patients. Invest Ophthalmol Vis Sci 58 (2017): 5713-5722.
- Rao HL, Kadambi SV, Weinreb RN, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol 101 (2017): 1066-1070.
- Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PloS One 12 (2017): e0173930.
- Hollo G. Progressive decrease of peripapillary angioflow vessel density during structural and visual field progression in early primary open-angle glaucoma. J Glaucoma 26 (2017): 661-664.
- Hollo G. Comparison of Peripapillary OCT Angiography Vessel Density and Retinal Nerve Fiber Layer Thickness Measurements for Their Ability to Detect Progression in Glaucoma. J Glaucoma. 27 (2018): 302-305.
- Hollo G. Comparison of thickness – function and vessel density – function relationship in the superior and inferior macula, and the superotemporal and inferotemporal peripapillary sectors J Glaucoma 29 (2020): 168-174.
- Chen CL, Zhang A, Bojikian KD, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography-based microangiography. Invest Ophthalmol Vis Sci 57 (2016): 475-485.
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology 124 (2017): 709-719.
- Zhang X, Dastiridou D, FrancisB, Tan O, Varma R, Greenfield D, et al. Baseline Fourier-Domain OCT Structural Risk Factors for Visual Field Progression in the Advanced Imaging for Glaucoma Study Am J Ophthalmol 172 (2016): 94–103.
- Dong ZM, Wollstein G, Schuman JS. Clinical utility of optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci 57 (2016): 556-567.
- Sung KR, Sun JH, Na JH, et al. Progression Detection Capability of Macular Thickness in Advanced Glaucomatous Eyes. Ophthalmology 119 (2012): 308-313.
- Nakatani Y, Higashide T, Ohkubo S, et al. Evaluation of macular thickness and peripapillary retinal nerve fiber layer thickness for detection of early glaucoma using spectral domain optical coherence tomography. Glaucoma 20 (2011): 252-259.
- Hood DC, Raza AS, de Moraes CGV, et al. The nature of macular damage in glaucoma as revealed by averaging optical coherence tomography data. Vis. Sci. Tech 1 (2012): 1–15.
- Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res 57 (2017): 46-75.
- Lommatzsch C, Rothaus K, Koch JM, et al. OCTA vessel density changes in the macular zone in glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol 256 (2018): 1499-1508.
- Ghahari E, Bowd C, Zangwill LM, et al. Association of Macular and Circumpapillary Microvasculature with Visual Field Sensitivity in Advanced Glaucoma. Am J Ophthalmol 204 (2019): 51-61.
- Richter GM. The Promise of Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 124 (2017): 1577-1578.
- Moghimi S, Bowd C, Zangwill LM, et al. Measurement floors and dynamic ranges of optical coherence tomography and angiography in glaucoma. Ophthalmology 126 (2019): 980-988.
- Rao HL, Pradhan ZS, Weinreb RN, et al. Relationship of Optic Nerve Structure and Function to Peripapillary Vessel Density Measurements of Optical Coherence Tomography Angiography in Glaucoma. J Glaucoma 26 (2017): 548-554.
- Mwanza JC, Budenz DL, Warren JL, et al. Retinal nerve fiber layer thickness floor and 4 corresponding functional loss in glaucoma. Br J Ophthalmol 99 (2015): 732-737.
- Belghith A, Medeiros FA, Bowd C, et al. Structural Change Can Be Detected in Advanced-Glaucoma Eyes. Invest Ophthalmol Vis Sci 57 (2016): 511-518.
- Hollo G. Peripapillary capillary vessel density progression in advanced glaucoma: a case report. BMC Ophthalmol 19 (2019): 2.
- Onda E, Cioffi GA, Bacon DR, et al. Microvasculature of the human optic nerve. Am J Ophthalmol 120 (1995): 92–102.
- Kim SH, Park KH. The relationship between recurrent optic disc hemorrhage and glaucoma progression. Ophthalmology 113 (2006): 598-602.
- Loukil I, Korchène N, Hachicha F, et al. Ocular risk factors for progression of primary open angle glaucoma in the Tunisian population. J Fr Ophtalmol 36 (2013): 324-330.
- McMonnies CW. Intraocular pressure and glaucoma: Is physical exercise beneficial or a risk? J Optom 9 (2016): 139-147.
- Kyari F, Abdull MM, Wormald R, et al. Risk factors for open-angle glaucoma in Nigeria: results from the Nigeria National Blindness and Visual Impairment Survey. BMC Ophthalmol 16 (2016): 78.
- Aptel F, Bron A, Lachkar Y, et al. Change in Visual Field Progression Following Treatment Escalation in Primary Open-angle Glaucoma. J Glaucoma 26 (2017): 875-880.
- Sharifipour F, Panahi-bazaz M, Bidar R, Idani A, and Cheraghian B. Age-related variations in corneal biomechanical properties. Journal of Current Ophthalmology 28 (2016): 117–122.
- Weinreb R, Robinson M, Dibas M, Stamer W. Matrix Metalloproteinases and Glaucoma Treatment J. of Ocular Pharmacology and Therapeutics 36 (2020): 4.
- Prata TS, Lima VC, Guedes LM, et al. Association between corneal biomechanical properties and optic nerve head morphology in newly diagnosed glaucoma patients. Clin Exp Ophthalmol 40 (2012): 682-688.
- Sharpe GP, Danthurebandara VM, Vianna JR, et al. Optic Disc Hemorrhages and Laminar Disinsertions in Glaucoma. Ophthalmology 123 (2016): 1949-1956.
- Johnson CS, Mian SI, Moroi S, Epstein D, Izatt J, Afshari NA. Role of corneal elasticity in damping of intraocular pressure. Investigative Opthalmology and Visual Science 48 (2007): 2540-2544.
- Memarzadeh F, Ying-Lai M, Azen SP, et al. Associations with intraocular pressure in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol 146 (2008): 69-
- Mansoori T, Sivaswamy J, Gamalapati JS, et al. Measurement of radial peripapillary capillary density in the normal human retina using optical coherence tomography angiography. J Glaucoma 26 (2017): 241-246.
- Park JH, Yoo C, Girard MJA, et al. Peripapillary Vessel Density in Glaucomatous Eyes: Comparison Between Pseudoexfoliation Glaucoma and Primary Open-angle Glaucoma. J Glaucoma 27 (2018): 1009-1016.
- Mastropasqua R, Agnifili L, Borrelli E, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Normal-Tension Glaucoma and Chronic Nonarteritic Anterior Ischemic Optic Neuropathy. Curr Eye Res 43 (2018): 778.
- Vysniauskiene I, Allemann R, Flammer J, et al. Vasoactive responses of U46619, PGF2alpha, latanoprost, and travoprost in isolated porcine ciliary arteries. Invest Ophthalmol Vis Sci 47 (2006): 295-298.
- Xu J, Li Y, Song S, et al. Evaluating changes of blood flow in retina, choroid, and outer choroid in rats in response to elevated intraocular pressure by 1300 nm swept-source OCT. Microvasc Res 121 (2019): 37-45.
- In JH, Lee SY, Cho SH, et al. Peripapillary vessel density reversal after trabeculectomy in glaucoma. J Ophthalmol (2018): 8909714.
- Shin JW, Sung KR, Uhm KB, et al. Peripapillary microvascular improvement and lamina cribrosa depth reduction after trabeculectomy in primary open angle glaucoma. Invest Ophthalmol Vis Sci 58 (2017): 5993-5999.
- Hollo G. Influence of large intraocular pressure reduction on peripapillary OCT vessel density in ocular hypertensive and glaucoma eyes. J Glaucoma 26 (2017): e7–e10.
- Kurysheva NI, Lepeshkina LV. Selective Laser Trabeculoplasty Protects Glaucoma Progression in the Initial Primary Open-Angle Glaucoma and Angle-Closure Glaucoma after Laser Peripheral Iridotomy in the Long Term. Hindawi BioMed Research International (2019).
- Zhang X, Dastiridou A, Francis B. et al.Comparison of glaucoma progression detection by optical coherence tomography and visual field. American Journal of Ophthalmology 184 (2017): 63–74.

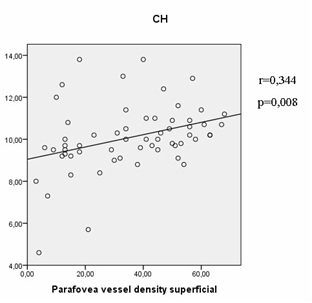

 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
