Personalized Care with DPP4i/SGLT2i Strategy as Initial-Line in the Management of Type 2 Diabetes Mellitus with Cardiovascular Risk: DELPHI Statement
Rakesh Kumar Sahay1, Manash Baruah2, Prakash Keswani3, Sanjeev Phatak4, Manoj Chawla5, Basavaraj G Sooragonda6, Ashutosh Kakkad7*, Narendra Chouksey7, Krishnaprasad Korukonda7 on behalf of CONTROL panel members
1Department of Endocrinology, Osmania Medical College, Hyderabad, Telangana, India
2Department of Endocrinology, Excel Centre, Guwahati, Assam, India
3Department of Medicine, Sawai Man Singh Medical College & Hospital, Jaipur, Rajasthan, India
4Department of Internal Medicine, Vijayratna Diabetes Diagnosis & Treatment Centre, Ahmedabad, Gujarat, India
5Department of Diabetes, Lina Diabetes Care and Mumbai Diabetes Research Centre, Mumbai, Maharashtra, India
6Department of Endocrinology, Diabetes & Metabolism, Narayana Health, Bengaluru, Karnataka, India
7Medical Services Department, Torrent Pharmaceuticals Limited, Ahmedabad, Gujarat, India
*Corresponding author: Ashutosh Kakkad, Medical Services, Torrent Pharmaceuticals Limited, Ahmedabad, Gujarat, India.
Received: 27 February 2024; Accepted: 07 March 2024; Published: 28 March 2024
Article Information
Citation: Rakesh Kumar Sahay, Manash Baruah, Prakash Keswani, Sanjeev Phatak, Manoj Chawla, Basavaraj G Sooragonda, Ashutosh Kakkad, Narendra Chouksey, Krishnaprasad Korukonda. Personalized Care with DPP4i/SGLT2i Strategy as Initial-Line in the Management of Type 2 Diabetes Mellitus with Cardiovascular Risk: DELPHI Statement. Cardiology and Cardiovascular Medicine. 8 (2024): 111-117.
View / Download Pdf Share at FacebookAbstract
Background: Type 2 diabetes mellitus (T2DM) is a highly prevalent metabolic disorder in Asian-Indians owning to distinct phenotypic traits including impaired Insulin sensitivity and resistance, necessitating personalized management to avoid progression towards cardiometabolicbased chronic disease (CMBCD) or complications.
Objective: To develop Consensus on the Indian perspective for management of T2DM with high cardiovascular (CV) risk by a National Expert panel.
Methods: A steering committee (n=6) developed DELPHI statements using a Knowledge, Attitude, and Perception questionnaire while analyzing the literature review and responses from a National panel (n=152) utilizing AHRQ criteria defined evidence strength and quality. A Likert scale assessed panel responses, with ≥70% of Steering committee agreement documented for clinical recommendations as DELPHI statement.
Results: National panel (n=152) emphasized need for early add-on strategies for T2DM patients, as half of the patients present with macrovascular complications. Panel agreed on personalizing treatment being crucial, especially for uncontrolled patients, geriatrics, young patients, and those with additional risk factors or microvascular complications. Favourable risk-benefit profile and clinical outcomes with DPP4 inhibitors & SGLT2 inhibitors combination makes it relevant for early use in T2DM cases including those with ASCVD, heart failure, older and obese individuals who are not controlled on metformin.
Conclusion: Need for personalized care strategies combining DPP4i and SGLT2i for T2DM patients with risk stratification score for CMBCD development or progression as highlighted by the CGM studies to avoid the risk of Glycemic variability and/or Hypoglycemia.
Keywords
<p>Type 2 diabetes mellitus; KAP; DELPHI; SGLT2 inhibitors; DPP4 inhibitors; Combination therapy; Cardiovascular disease; Personalized care</p>
Article Details
1. Introduction
Diabetes is a serious, chronic, and progressive cardiometabolic disorder. It is characterised by elevated blood glycemia related to the effects of abnormal β-cell biology on insulin action [1]. According to the report of the International Diabetes Federation (IDF) of 2021, diabetes is the fastest growing global health emergency of the 21st century, with estimates of approximately 537 million cases worldwide that will increase to 1.3 billion by 2050, almost double the existing pool of patients, with every country worldwide seeing an increase [2]. Diabetes was the eighth leading cause of death and disability combined in the world as per estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 [3,4].
ICMR-INDIAB-17, a nationally representative epidemiological study, reports that 101 million people suffer from Type 2 Diabetes Mellitus (T2DM) and at least 136 million people (15.3% of the population) have prediabetes in India. Approximately 39.4 million (53.1%) adults in India are underdiagnosed, and 1 in 7 diabetic patients in the world is Indian [5].
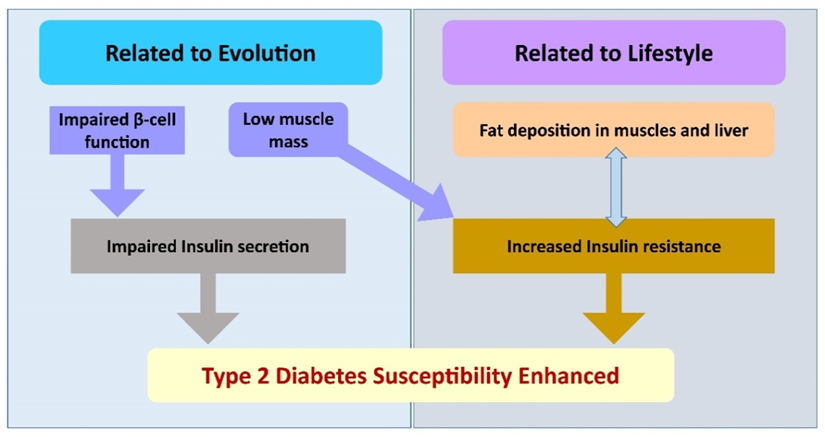
Type 2 diabetes (T2D) in Asian Indians differs from that in white Caucasians in several significant ways and is referred to as the ‘Asian Indian Phenotype’ or ‘thin–fat phenotype’ [6]. The Asian-Indian phenotype is uniquely characterized for cardiometabolic risk with a high predilection for the development of type 2 diabetes. Dual interacting pathologies, i.e., reduced beta cell function and impaired insulin action due to low lean mass, which is further enhanced by ectopic fat deposition in the liver and muscles, appear to be at play, making Asian Indians highly susceptible to type 2 diabetes [7,8]. (Figure 1) The Asian Indian phenotype fits into the model of metabolically obese people of normal weight [8,9].
Figure 1 – South Asians have evolved to have both impaired insulin secretion and increased insulin resistance owning to their low lean muscle mass and low beta cell function. Unhealthy lifestyle choices worsen the effects of insulin by causing ectopic fat accumulation in the muscles and liver.
Cardiovascular disease (CVD) is the most prevalent cause of mortality and morbidity in diabetic populations, as there is a very close link between T2DM & CVD [10]. 4 out of 5 patients with T2DM develop CV complications that account for approximately 65% of deaths in this group [11]. Indian Epidemiology studies highlight the risk of coronary artery disease (CAD) to be almost two to four times higher in diabetic subjects and indicate a premature occurrence of CAD, which is as early as two decades compared to the western diabetes population [12].
In the last few years, the approach to diabetes management has moved away from a glucose-centric, HbA1c-lowering approach to a complication prevention approach, as well as a pathogenesis-centric approach, which looks at the underlying metabolic dysfunction that causes and complicates diabetes management [13]. The primary objective of T2DM treatment should be to reduce CV risk, as CVD is the most prevalent cause of mortality and morbidity in patients with DM. The deep mechanistic link between T2DM and CVD necessitates a focus on treatment options that offer the greatest clinical impact on improving CV outcomes [14].
Choosing therapeutic options such as glucagon-like peptide-1 receptor agonists (GLP1-RAs), sodium-glucose co-transporter-2 inhibitors (SGLT2i) and Dipeptidyl Peptidase IV (DPP IV) Inhibitors, which have proven CV benefits along with glycemic control, is very important for overcoming residual CV risk in T2DM patients. Fortunately, in India, we have the availability of these options as monotherapy and as fixed-dose combination (FDC) to achieve individualization of therapy according to the patient's need [15].
In this line to further assess the scope, potential, and current contemporary practices in relation to the role and clinical positioning of DPP4i/SGLT2i as an initial-line strategy in the management of T2DM patients with CV risk traits in real world settings of India, a Knowledge, Attitude, Perception (KAP) survey was planned to be conducted to develop a consensus on a personalized care approach in the management of T2DM with CV risk.
2. Materials and Methods
2.1 Overview and Delphi process structure
The CONTROL project was a nationwide multicenter two-round Delphi exercise to seek expert opinion on the clinical management of the T2DM patient with high CV risk in India.
The Delphi process is a widely accepted & well-established scientific methodological tool, which is used to assess and integrate the opinions of a group of experts on complex topics [16].
The DELPHI statement was generated by the Steering Committee as in following steps:
- Steering Committee panelists with Scientific, Academic and Clinical expertise or standing
- Literature review
- KAP questionnaire – Development and Validation (Round I)
- Web–based KAP survey rollout to National panel of Clinical experts
- KAP response analyses with DELPHI based Clinical Recommendations generation (Round II)
2.2 Preparation of the questionnaire
First, a steering committee of six eminent practicing endocrinologists and diabetologists with complementary expertise and experience in the management of patients with T2DM was formed. The steering committee carried out a systematic review of the literature on the management of patients with T2DM, focusing on the high CV risk subset. After a careful and critical review of the selected literature and based on their clinical management knowledge, the steering committee generated a set of KAP survey questionnaire relevant to the project objective to be rolled out to the National Clinical expert panel.
2.3 Survey procedures
A total of 152 specialists or clinical experts distributed across India were invited to participate in the project as members of the Delphi expert panel. These panel experts were selected based on their extensive experience and expertise in the management of T2DM patients. Each panel member was empowered with standardized information on the goal of the project and details on the process prior to participating. Upon agreement, the panel members were shared the link to web-based KAP survey and asked to provide their responses within 30 days.
2.4 Survey Response Analysis & Clinical Recommendation Generation
All of the responses generated to the 10 questions were analyzed by descriptive statistics for mean, median and proportion analyses using Microsoft Excel version 2019. The response rate calculated as a percentage was also highlighted using descriptive statistics. The steering committee discussed the analysis of the response to the survey to develop clinical recommendations based on a successful problem-based clinical assessment method that is widely used in primary care.
All clinical recommendations were assigned a level of evidence (LoE) as ‘I, II, III, IV, and V’, according to the Agency of Health Care and Quality Systems (AHRQ) criteria and the general opinion of experts based on the clinical experience and insights shared by the panel. Level of Agreement (LoA) was developed and assigned using LIKERT scale assessment on each clinical recommendation as ‘Strongly Agree (Personal experience with level of evidence); Agree (personal interpretation on the level of evidence suggested); Neutral (neither in agreement or disagreement); Disagree (negligible experience); Strongly Disagree (negligible experience with no level of evidence as Real World Evidence (RWE) or Randomized Clinical Trail (RCT))’. Steering committee’s agreement of ≥70% was a pre-requisite for documenting each clinical recommendations as DELPHI statement.
The finalized statements and associated ratings for level of evidence and agreement provided the basis for the compilation of this consensus document.
2.5 Compliance with Ethics Guidelines
No approval from the Institutional Review Board (IRB) or ethics committee(s) was needed, as this Delphi study does not involve human subject research, and no patient data were collected. It is based entirely on the feedback and opinions provided by experts. All participants freely accepted to contribute to the project and to permit public dissemination of the results, while preserving the anonymity of individual responses.
3. Results
One hundred and fifty-two practicing endocrinologists and diabetologists were approached to provide clinical insights on the current positioning of DPP4i/SGLT2i for early initiation or add-on strategy to metformin in the management of T2DM with CV risk using a structured KAP based survey questionnaire. Within 30 days, responses were received from all 152 HCPs based in different geographical locations across India, which were collated and analyzed by the Steering Committee for the development of DELPHI Statements on the pertinent clinical approach in the management of T2DM with fixed dose combination for diverse clinical profiles. (Response rate – 100%) (Table 1)
Table 1: Structured KAP Survey Questionnaire with Responses
T2DM – Type 2 diabetes mellitus; OAD – oral antidiabetic drugs; CV – Cardiovascular; HbA1c – glycated hemoglobin; SU – Sulfonylureas; DPP4i – Dipeptidyl peptidase 4 inhibitor; SGLT2i – Sodium-glucose cotransporter-2 (SGLT2) inhibitors; CGM – Continuous Glucose Monitoring; TIR - Time in range
4. Discussion
Management of T2DM has been fraught with several challenges for clinical responsiveness to monocomponent use of insulin sensitizers especially in context of delineation of Indian subcontinent specifier Clusters as Severe Insulin Deficient Diabetes (SIDD) and CIRDD (Combined Insulin Resistant and Deficient Diabetes). Role of subtypes identification or "clusters" becomes crucial for understanding the etiology of diabetes and selecting the most suitable antidiabetic therapy. Identified clusters are Severe Insulin Deficient Diabetes (SIDD), Mild Age-Related Diabetes (MARD), IROD (Insulin Resistant Obese Diabetes) and CIRDD (Combined Insulin Resistant and Deficient Diabetes) with later two being unique to Indian population signifying the twin traits of impaired insulin signaling and resistance warranting the use of DPP4i and SGLT2is [17]. DPP4i and SGLT2is complement for glucose stimulated insulin secretion (GSIS) and Sirtuins (SIRT) activity correcting the Beta cell defect, clinical Adiposity and high carb Diet (BAD) lead glucotoxicity characteristics in the Indians. Gan et al. and DIVERSITY CVR subgroup analyses findings corroborates “cluster” approach with the reported response to various classes of antidiabetic medication being different between individuals of different ethnicity [18,19]. The results of the REAL DAPSI study evaluating the real-world effectiveness and safety of dapagliflozin-sitagliptin FDC in the Indian population showed a significant reduction in HbA1c (-1.7%) along with significant reductions in fasting and postprandial blood glucose levels. The study also highlighted the non-glycemic benefits of the dapagliflozin-sitagliptin FDC on weight loss (2-4 kg) and reduction in systolic and diastolic blood pressure without majority of patients experiencing hypoglycemia and genitourinary infections [20].
Unique characteristics of Asian-Indian phenotype demands a personalized approach addressing multiple pathophysiological aspects of T2DM especially in the uncontrolled cases. The combination of DPP4i and SGLT2i may be very appropriate in this regard and this combination will be best suited with metformin to provide good clinically meaningful HbA1c control along with improvement in the overall metabolic profile of the Indian diabetic patient. [15] Mechanistically, combined use of DPP4i and SGLT2i after the initiation of metformin offers a synergistic effect and has the potential to correct seven pathophysiologic defects of Ominous Octet, including β-cell dysfunction, hepatic IR and hyperglucagonemia while pre–empting the risk of diabetes disease induction or progression as highlighted by TODAY and GRADE studies [21-23].
Emerging evidence indicates that incretin-based therapies & SGLT2 inhibitors may provide cardiovascular (CV) benefits beyond glycemic control. This is of vital importance looking at a strong association between glycemia and cardiovascular (CV) morbidity and mortality in diabetic patients while offering similar benefits in cases with hypoglycemic events or risk [24].
The international T2DM management guidelines including ADA/EASD statement (2022) recommend the use of DPP4is and/or SGLT2is with little clarity on the place and positioning of the fixed dose combination in treatment–naïve or uncontrolled cases receiving Sulfonylureas or Biguanides.
The responses to the KAP survey clearly document the current challenges faced by Indian clinicians and their clinical approach towards management of T2DM. Clinicians voiced growing conviction on the need for personalized care in T2DM patients, particularly those with additional risk traits such as CV risk or other microvascular complications to pre–empt further disease progression. The survey responses reflect the adoption of early use of combination therapy with DPP4i/SGLT2i on metformin background that aligns well with the recommendations made by various T2DM management guidelines.
Further clinical recommendations were developed to promote personalized care strategies in the management of T2DM patients with CV risk uncontrolled on initial metformin therapy and who require early treatment intensification.
5. Clinical Recommendations
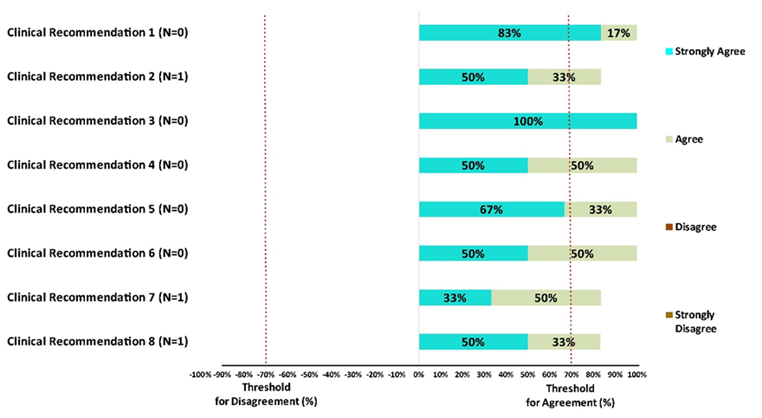
The final clinical recommendations are listed below with level of evidence along a graphical depiction on levels of agreement among experts. (Figure 2)
- Indian phenotype is exemplified by the twin traits of impaired Insulin secretion and resistance requiring combination therapy in treatment naïve or uncontrolled cases of T2DM. [LoE: Expert opinion; LoA: 100%]
- Early fixed dose Combination with DPP4i as one of the component is particularly preferred with HbA1c level 1 to 1.5% above target for T2DM, T2DM with multiple CV risk traits or established CVD. [LoE: Level IIb; LoA: 83%]
- Early fixed dose Combination with SGLT2i like Dapagliflozin as one of the components is particularly preferred in T2DM with multiple Risk traits for its glycemic and extraglycemic effects including blood pressure (¯3 to 5 mmHg) and weight (¯3 to 5%) modulation [LoE: Level IIa; LoA: 100%]
- Combination of DPP4i and SGLT2i offers clinical synergy for modulation of Hyperglucagonemia and hepatic glucose output by the former with modulation of lipolysis and its insulin sensitizing action of the latter. [LoE: Expert opinion; LoA: 100%]
- DPP4i and SGLT2is as combination offer incremental glycemic benefits and excellent safety profile with respect to hypoglycemia and weight modulation. [LoE: Expert opinion; LoA: 100%]
- Combination of Sitagliptin & Dapagliflozin can be useful add-on strategy for those uncontrolled T2DM with HbA1c ≥8% and 10y CV risk score >10% for providing glycemic benefit and cardiometabolic protection [LoE: Level III; LoA: 100%]
- Combination of Sitagliptin and Dapagliflozin can be considered as first line strategy for treatment naive T2DM (≥7.5%) with cardiometabolic risk traits including sarcopenic obesity. [LoE: Expert opinion; LoA: 83%]
- Combination of Sitagliptin and Dapagliflozin can be considered in patients of T2DM with high risk of hypoglycemia and glycemic variability to provide consistent Time In Range [LoE: Expert opinion; LoA: 83%]
Figure 2 – Each clinical recommendation has a corresponding bar graph where the level of agreement/disagreement (%) is depicted. All experts voted to assign ‘Level of Agreement (LoA)’ to each recommendation using LIKERT scale as Strongly Agree (Personal experience with level of evidence); Agree (personal interpretation on the level of evidence suggested); Neutral (neither in agreement or disagreement); Disagree (negligible experience); Strongly Disagree (negligible experience with no level of evidence as RWE or RCT). N – Neutral
6. Limitations
The KAP responses and DELPHI statements reflect the paucity of clinical evidence as controlled trials suggesting the validation of them with the conduct of large sample long term studies on surrogate endpoints on HbA1c, Postprandial Blood Glucose (PPBG) and Glycemic variability or TIR for foreseeable traction or implementation in real life settings of India.
7. Conclusion
The growing burden of patients with uncontrolled Type 2 diabetes with vascular complications emphasizes the need for personalized care. The use of DPP4i/ SGLT2i combination as an early add-on strategy is vital for achieving and maintaining treatment goals in patients with the Asian Indian T2DM phenotype. This combination not only corrects T2DM-related pathophysiological defects such as β-cell dysfunction, hepatic IR, and hyperglucagonemia but also has extraglycemic effects, including positive effects on weight, blood pressure, and cholesterol levels for additional improvement in Indian diabetic patients with CV risk or metabolic traits. Furthermore, this strategy has adequate support from clinical evidence for cardiorenal protection in T2DM patients. The summarized expert views and opinions on the potential therapeutic role of DPP4i/SGLT2i combination as an early add-on strategy can assist clinicians in making informed decisions for T2DM patients especially with CV risk >10%.
Prior publication:
None
Support:
The Authors have received no fees for the development of manuscript & clinical recommendations
Conflicts of interest:
Krishnaprasad K, Narendra Chouksey, and Ashutosh Kakkad declare(s) being paid employees of Torrent Pharmaceuticals Ltd. The authors report that there are no other potential conflicts of interest associated with this work.
Permissions:
Not applicable
References
- Kumar Jha P, Shukla H, Makwana A, et al. Pharmacotherapy of Type 2 Diabetes Mellitus. Type 2 Diabetes - From Diagnosis to Effective Management. IntechOpen (2023).
- Magliano DJ, Boyko EJ. IDF DIABETES ATLAS. 10th edition (2021).
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 (2020): 1204-1222.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375 (2010): 2215-22.
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402 (2023): 203-234.
- Anjana RM, Baskar V, Nair ATN, et al. Novel subgroups of type 2 diabetes and their association with microvascular outcomes in an Asian Indian population: a data-driven cluster analysis: the INSPIRED study. BMJ Open Diabetes Res Care 8 (2020): e001506.
- Kalra S, Mithal A, Zargar AH, et al. Indian Phenotype Characteristics Among Patients with Type 2 Diabetes Mellitus: Insights from a Non-interventional Nationwide Registry in India. touchREV Endocrinol 18 (2022): 63-70.
- Mohan V, Sandeep S, Deepa R, et al. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res 125 (2007): 217-230.
- Ruderman N, Chisholm D, Pi-Sunyer X, et al. The metabolically obese, normal-weight individual revisited. Diabetes 47 (1998): 699-713.
- Matheus AS, Tannus LR, Cobas RA, et al. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens (2013): 653789.
- Kosiborod M, Gomes MB, Nicolucci A, et al. DISCOVER investigators. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc Diabetol 17 (2018): 150.
- Mohan V, Venkatraman JV, Pradeepa R. Epidemiology of cardiovascular disease in type 2 diabetes: the Indian scenario. J Diabetes Sci Technol 4 (2010): 158-170.
- Galindo RJ, Trujillo JM, Low Wang CC, et al. Advances in the management of type 2 diabetes in adults. BMJ Med 2 (2023): e000372.
- Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 6 (2015):1246-158.
- Chadha M, Das AK, Deb P, et al. Expert Opinion: Optimum Clinical Approach to Combination-Use of SGLT2i + DPP4i in the Indian Diabetes Setting. Diabetes Ther 13 (2022): 1097-1114.
- Yeh JS, Van Hoof TJ, Fischer MA. Key Features of Academic Detailing: Development of an Expert Consensus Using the Delphi Method. Am Health Drug Benefits 9 (2016): 42-50.
- Anjana RM, Pradeepa R, Unnikrishnan R, et al. New and Unique Clusters of Type 2 Diabetes Identified in Indians. J Assoc Physicians India 69 (2021): 58-61.
- Gan S, Dawed AY, Donnelly LA, et al. Efficacy of Modern Diabetes Treatments DPP-4i, SGLT-2i, and GLP-1RA in White and Asian Patients With Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 43 (2020): 1948-1957.
- Takuma K, Fuchigami A, Shigiyama F, et al. Comparison of the effects of sitagliptin and dapagliflozin on time in range in Japanese patients with type 2 diabetes stratified by body mass index: A sub-analysis of the DIVERSITY-CVR study. Diabetes Obes Metab 25 (2023): 2131-2141.
- Bhattacharjee R, Rai M, Joshi P, et al. The Real DAPSI: A Real-World Retrospective Study on Assessing the Efficacy and Safety of a Fixed-Dose Combination of Dapagliflozin and Sitagliptin in the Indian Population. Cureus 15 (2023): e46767.
- Singh AK, Singh A, Singh R. Cardiovascular and Renal Outcomes With Sodium-Glucose Cotransporter-2 Inhibitors and Dipeptidyl Peptidase-4 Inhibitors Combination Therapy: A Meta-Analysis of Randomized Cardiovascular Outcome Trials. Endocr Pract 29 (2023): 509-516.
- Bjornstad P, Drews KL, Caprio S, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med 385 (2021): 416-426.
- Nathan DM, Lachin JM, Bebu I, et al. Glycemia Reduction in Type 2 Diabetes - Microvascular and Cardiovascular Outcomes. N Engl J Med 387 (2022): 1075-1088.
- Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364 (2011): 829-841.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 