Pharmacological Efficacy of Ethanol Leaf Extract of Justicia secunda in Swiss Albino Mice Experimentally Infected with Plasmodium berghei
Nasiru Ademola ADEYEMI1, Sonnie Joshua ONIYE2, Peter Ibrahim REKWOT4, Abdullateef YUSUF3, Yunusa Adamu WADA1, Muhammad HUSSAINI3
1Department of Zoology, Faculty of Life Sciences, Ahmadu Bello University, Zaria, Nigeria.
2Department of Biology, National Open University, Abuja, Nigeria.
3Department of Biology, Faculty of Life Sciences Ahmadu Bello University, Zaria, Nigeria.
4National Animal Production Research Institute, Ahmadu Bello University, Zaria, Nigeria.
*Corresponding author: Nasiru Ademola ADEYEMI, Department of Zoology, Faculty of Life Sciences, Ahmadu Bello University, Zaria, Nigeria.
Received: 02 August 2024; Accepted: 08 August 2023; Published: 27 August 2024
Article Information
Citation: Nasiru Ademola ADEYEMI, Sonnie Joshua ONIYE, Peter Ibrahim REKWOT, Abdullateef YUSUF, Yunusa Adamu WADA, Muhammad HUSSAINI. Pharmacological Efficacy of Ethanol Leaf Extract of Justicia secunda in Swiss Albino Mice Experimentally Infected with Plasmodium berghei. International Journal of Applied Biology and Pharmaceutical Technology. 15 (2024): 05-16.
View / Download Pdf Share at FacebookAbstract
Plasmodium parasite that causes malaria has developed resistance to most antimalarial medications and this has prompted research on natural products. The plant, Justicia secunda, is domesticated in the tropical region of Africa, and is used for the treatment of anaemia and other debilities. We evaluated the antiplasmodial and immunomodulatory activity of ethanol leaf extract of J. secunda in mice experimentally infected with Plasmodium berghei. Ethanol leaf extract of Justicia secunda contains steroids, tannins, flavonoids, terpenoids, phenols, glycosides and carbohydrate as well as thirty (30) compounds with numerous pharmacological activities. The LD50 was above 5,000mg/kg with no mortality. A dose-dependent percentage suppression of P. berghei was observed in the curative test for the three doses respectively (53.13%, 60.71%, 71.21%). The suppressive test for ethanol leaf extract of J. secunda also showed a similar trend (52.2%, 62.16%, 75.18%). The ethanol leaf extract of J. secunda effectively prevented anaemia, reduced the level of ALT enzyme and also increased the level of TNF-α and IL-10 in the plasma of P. berghei-infected mice. It also significantly reduced the oxidative stress and vascular congestion in the liver of mice infected with P. berghei. This study revealed that ethanol leaf extract of J. secunda could be utilized to treat infection caused by Plasmodium parasite and also ameliorate the pathogenesis of the disease.
Keywords
<p>Antiplasmodial, Phytochemicals, Immunomodulatory, Histopathology</p>
Article Details
1. Introduction
Malaria is more prevalent in African nations than it is worldwide. According to the World Malaria Report 2020; Nigeria (31.3%), the Democratic Republic of Congo (12.6%), the United Republic of Tanzania (4.1%) and Niger (3.9%) account for more than half of all malaria death [1]. Although, sociopolitical issues are connected to the causes and consequences of malaria disease [2]; explicitly, the major contributing factor to the prevalence of malaria is the lack of mosquito protection and appropriate malaria treatment [3].
Malaria is an infectious disease affecting humans and animals [4]. The etiologic agent of the disease is the parasitic protozoans of the genus Plasmodium, and the sole biological vector is the female Anopheles mosquitoes [5]. The asymptomatic human is an unwitting parasite reservoir, allowing continuous transmission [6]. The initial clinical symptom of this illness leads to headaches, fatigue, soreness in the joints and muscles, vomiting, and anorexia. Acidemia, hypoglycemia, anaemia, pulmonary oedema, coma and renal failure are common signs of acute malaria [7].
Antimalarial drug resistance occurs in the malaria parasite due to ineffective chemoprophylaxis [8], fake and irregular pharmaceutical administration leading to genetic mutation [9]. Therefore, searching for a new anti-malarial compound is a continuous process, and numerous studies on African medicinal plants with anti-malarial potentials have been conducted over time. However, there is a dearth of practical information regarding the pharmacological efficacy of J. secunda as an antimalarial agent.
Justicia secunda is taxonomically classified in the family Acanthaceae and is notably utilized for treating anaemia in tropical regions of Africa [10]. It is commonly known as 'Christ blood' and 'Blood herb' in Nigeria. The herbaceous plant has green leaves and pink inflorescence (Figure 1) [11]. According to Irinmwinuwa and Afonne [12], the ethanol leaf extract of J. secunda contains saponin (9.2%), tannins (9.0), flavonoids (7.0%) and alkaloid (2.4%). It has also been established that J. secunda has hematinic effect in rats with anaemia induced by phenylhydrazine [13]. Likewise, it exhibited anti-inflammatory and antioxidant activity in an in vitro study using heat-induced bovine serum albumin (BSA) denaturation and erythrocyte membrane stabilization assay [14].
This study evaluates the phytochemical constituent and lethal dose of the ethanol leaf extract of J. secunda, as well as the effect of the extract on the parasitaemia level, hematological profile, pro-inflammatory (TNF-α) and anti-inflammatory (IL-10) cytokines, liver biochemicals, and histopathology of the liver in Plasmodium berghei NK65-infected mice.
2. Material and Methods
Collection of Plant Sample
Matured leaves were harvested from stands of J. secunda plants from three different sites in Lusada, Ado Odo Ota Local Government Area (LGA) Ogun State, Nigeria.
Preparation of Plant Extract
Cold maceration technique was adopted for the extraction by simply soaking five hundred grams (500g) of the powdered leaves in five (5) litres of eighty percent (80%) ethanol for three days, and the bottle containing the mixtures was agitated at intervals. The liquid extract was filtered into a clean container using a piece of chiffon material after 72 hours. The filtrate was poured into an evaporating dish and placed on a water bath to obtain a solid and more concentrated form of the extract [15].
Evaluation of Phytochemical Constituent of Plant Extract
A standard method [16] was adopted in the phytochemical screening of the extract. The Gas Chromatography-Mass Spectrometry (GC-MS) analysis was conducted using a 7890B GC System (Agilent Technologies, USA) coupled with a 5977A Mass Selective Detector (Agilent Technologies, USA). The leaf extract of J. secunda was diluted in methanol, and 2μL of the mixture was injected into the GC-MS machine using a micro-syringe. The experimental parameters for the GC-MS system were: initial oven temperature: 70oC, Equilibration Time: 1 min, Max Temperature: 325 °C, Slow Fan: Disabled, Oven Program: 5°C/min On 250°C for 1 min, #1 then 30°C/min to 300°C for 0 min, Post Run: 50 °C. Cryo: Off, Front SS Inlet He – Mode: Split. Heater: On 250°C, Pressure: On 11.089psi, Total Flow on 19.204mL/min, Septum Purge Flow: On 3 mL/min, Gas Saver: off, Purge flow to Split vent: on 15mL/min at 0.75min. Holdup time: 1.2386min, Flowrate: 1.0mL/minute.
Maintenance of Experimental Animals
Seventy-five (75) male Swiss albino mice with body weight between 20 and 30g were obtained for this study. Chicken grower’s marsh and water was provided at all time for the experimental mice [17]. The overall maintenance of the experimental animals was in accordance with the procedures approved by the ethical committee of the institution where the experiment was conducted.
Determination of Lethal Dose of Plant Extract
The median lethal dose of the ethanol leaf extract of J. secunda was determined per the Up and Down method described by the Organization for Economic Co-orporation and Development [18].
Evaluation of Antiplasmodial Activity of Plant Extract
The infected experimental mice were inoculated intraperitoneally with 0.2mL of blood from donor mouse infected with P. berghei NK65 (30% parasitaemia) and normal saline (1:9) containing 105 P. berghei parasitized erythrocytes. The seven-day curative test was evaluated seventy-two (72) hours after inoculation and confirmation of parasitemia in thirty (30) mice, and they were randomly assigned into six (6) groups. The suppressive test was conducted, and the inception was precisely three (3) hours after the infection of mice for a four-day duration using thirty (30) mice. These tests were performed using the method of Onyegeme-Okerentaa et al. [19], with a slight modification. Groups 1–3 were the control groups treated with 1 mL of normal saline (the negative control), 25 mg/kg of chloroquine (the positive control), and the uninfected and untreated groups (the normal control), respectively. Groups 4-6 were given 500 mg/kg, 1000 mg/kg, and 1,500 mg/kg of the crude extract based on the LD50 for the ethanol leaf extract of J. secunda determined in this study. The oral treatment was done once a day.
Collection of Blood and Liver Samples
The animals were sacrificed after inhaling vapour from ketamine-damped cotton wool at the end of the experiment. The blood of experimental animals was collected in EDTA bottles for haematological and biochemical analysis through cardiac aspiration. A portion of the liver was placed in cold phosphate buffer for antioxidant and MDA analysis. A small portion of liver of mice was preserved in phosphate-buffered formalin.
Determination of Parasitaemia and Percentage Chemosuppression
The number of parasitized erythrocytes in ten (10) slides prepared with thin blood smears was counted, and the average was computed to give the parasitemia of each mouse [20].
Percentage parasitaemia and chemosuppression was calculated as:
Parasitaemia (%) = (Number of parasitized RBC/Total number of RBC) x 100
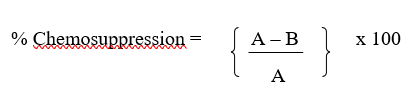
A= mean % parasitaemia in negative control
B= mean % parasitaemia in treated group
Assessment of Haematological Parameters
The Red Blood Cell (RBC), White Blood Cell (WBC), Packed Cell Volume (PCV) and Haemoglobin (HGB) concentration were analyzed using a Biobase Bk6100 haematology analyzer [21] in blood samples collected in Ethylene Diamine Tetracetic Acid (EDTA) bottles.
Assessment of Plasma and Liver Biochemicals
The liver homogenate was used to evaluate the levels of Malondialdehyde (MDA), Superoxide dismutase (SOD), Catalase (CAT) and reduced Glutathione (GSH) using a Sigma Aldrich Assay kit. Blood in EDTA bottles was used to check the concentrations of Alanine aminotransferase (Randox kit), Interleukin-10 and Tumour Necrosis Factor-α using ELISA kits by FineTest.
Liver Histopathological Studies
The liver of mice preserved in phosphate-buffered formalin was prepared for histological study using standard histotechnique and viewed with a microscope at 400 × magnification. The photomicrographs were taken using a digital camera [22].
Statistical Analyses
One-way Analysis of Variance (ANOVA) was used to determine statistical differences in the antiplasmodial activity, haematological and biochemical parameters in the in vivo suppressive and curative test. Tukey HSD test was used to rank the difference between significant parameters at p<0.05. Data were represented in tables and summarized in form of mean ± standard error. Statistical analyses were done using 'R' statistical package (version 4.1.2) for windows.
Results
Phytochemical Characterization and Acute Toxicity
The phytochemical screening detected eight different classes of phytochemicals (Table 1), and the GC-MS technique confirmed the presence of thirty different compounds, as shown in Table 2. Treatment of mice with limit dose of ethanol leaf extract of J. secunda (5000mg/kg) caused no mortality and signs of overtoxication. This suggests that the LD50 of the extract is greater than 5000mg/kg.
Table 1. Phytochemical screening of ethanol leaf extract of Justicia secunda
|
S/No |
Phytoconstituent |
Inference |
|
1. |
Alkaloids |
+ |
|
2. |
Cardiac glycosides |
+ |
|
3. |
Saponin |
- |
|
4. |
Phenolic compounds |
+ |
|
5. |
Tannins |
+ |
|
6. |
Steroids |
+ |
|
7. |
Carbohydrates |
+ |
|
8. |
Flavonoids |
+ |
|
9. |
Terpenoids |
+ |
|
10 |
Anthraquinones |
- |
Keys: (+) = Present, (-) = Absent
Table 2: GC-MS profile of ethanol leaf extract of Justicia secunda
|
Peak |
Retention Time (Minute) |
Library I.D |
Peak area (%) |
Chemical Abstract Service Number |
|
1 |
17.4652 |
17-Pentatriacontene |
0.1691 |
006971-40-0 |
|
2 |
17.64 |
Oxalic acid, cyclobutyl heptadecyl ester |
0.2012 |
1000309-70-7 |
|
3 |
20.0166 |
Hexadecanoic acid, methyl ester |
2.6316 |
000112-39-0 |
|
4 |
21.4568 |
3-Chloropropionic acid, heptadecyl ester |
0.2485 |
1000283-05-1 |
|
5 |
23.0506 |
9,12-Octadecadienoic acid, (linoleic) methyl ester, (E,E)- |
1.1048 |
002566-97-4 |
|
6 |
23.2025 |
cis-13-Octadecenoic acid, methyl ester |
6.3996 |
1000333-58-3 |
|
7 |
23.7984 |
Methyl stearate |
0.8041 |
000112-61-8 |
|
8 |
25.1498 |
Carbonic acid, but-3-en-1-yl hexadecyl ester |
0.1945 |
1000383-23-7 |
|
9 |
25.279 |
Aspidospermidin-17-ol, 1-acetyl-19,21-epoxy-15,16-dimethoxy- |
0.2787 |
002122-26-1 |
|
10 |
28.5241 |
8-Hexadecenal, 14-methyl-, (Z)- |
1.1543 |
060609-53-2 |
|
11 |
26.5546 |
Cyclododecane |
1.5526 |
000294-62-2 |
|
12 |
26.7618 |
9,12-Octadecadienal |
0.6438 |
026537-70-2 |
|
13 |
26.8969 |
p-Menth-8(10)-en-9-ol, cis- |
0.1951 |
015714-13-3 |
|
14 |
26.9881 |
Oxirane, [(dodecyloxy)methyl]- |
0.7356 |
002461-18-9 |
|
15 |
27.3173 |
Oleic Acid |
2.1944 |
000112-80-1 |
|
16 |
27.7978 |
22-Stigmasten-3-one |
3.3243 |
004736-95-2 |
|
17 |
28.2861 |
(S)(+)-Z-13-Methyl-11-pentadecen-1-ol acetate |
3.9133 |
1000130-84-8 |
|
18 |
28.5241 |
8-Hexadecenal, 14-methyl-, (Z)- |
1.2379 |
060609-53-2 |
|
19 |
28.9265 |
3-Cyclohexylthiolane,S,S-dioxide |
1.317 |
071053-08-2 |
|
20 |
28.9874 |
cis-7, cis-11-Hexadecadien-1-yl acetate |
1.3175 |
052207-99-5 |
|
21 |
29.3867 |
cis-9-Hexadecenal |
0.7726 |
056219-04-6 |
|
22 |
29.8609 |
9-Octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester |
3.1331 |
000111-03-5 |
|
23 |
30.1094 |
Z-8-Methyl-9-tetradecenoic acid |
3.1701 |
1000130-84-5 |
|
24 |
30.2394 |
1-Nonadecene |
1.2528 |
018435-45-5 |
|
25 |
30.394 |
Cyclododecanol, 1-ethenyl- |
5.5624 |
006244-49-1 |
|
26 |
30.9115 |
Cyclododecane, ethyl- |
3.3424 |
028981-49-9 |
|
27 |
31.2385 |
2-Methyl-Z,Z-3,13-octadecadienol |
3.9236 |
1000130-90-5 |
|
28 |
31.5878 |
E-9-Tetradecenal |
0.9144 |
1000131-35-7 |
|
29 |
32.575 |
β-Sitosterol |
19.7234 |
000083-46-5 |
|
30 |
34.706 |
Squalene |
4.1521 |
000111-02-4 |
Antiplasmodial Activity of the Crude Extract
The crude extract was tested for curative and suppressive antiplasmodial activity as presented in Table 3 and the result was expressed in the form of percentage parasitaemia and percentage chemosuppression for the infected controls and the treatment groups. The extract significantly (p<0.05) reduced parasitaemia level in infected mice for both curative and suppressive tests compared with the negative control. A dose-dependent chemosuppression was observed for the experimental groups, which was lower than the standard drug with 100% chemosuppression.
Table 3. Antiplasmodial activity of ethanol leaf extract of Justicia secunda against P. berghei in mice
|
Curative test |
Suppressive test |
|||
|
Experimental groups |
% Parasitaemia |
% Chemo-suppression |
% Parasitaemia |
% Chemo-suppression |
|
C- |
44.8 ± 1.61a |
0.00 |
41.5 ± 2.40a |
0.00 |
|
C+ |
0.00c |
100 |
0.00c |
100 |
|
NC |
0.00 |
0.00 |
0.00 |
0.00 |
|
T1 |
21.0 ± 1.29b |
53.13 |
19.8 ± 3.70b |
52.2 |
|
T2 |
17.6 ± 0.959b |
60.71 |
15.7 ± 1.46b |
62.16 |
|
T3 |
13.9 ± 2.81b |
71.21 |
10.3 ± 2.67b |
75.18 |
|
p-value |
0.00 |
0.00 |
NOTE: Data are expressed as mean ± SEM, n=5
Statistical analysis performed amongst Negative control (C-), Positive control (C+), Normal control (NC), 500mg/kg(T1), 1000mg/kg(T2) and 1,500mg/kg(T3) dose of ethanol leaf extract of J. secunda, means with different superscript within a column are statistically significant at p<0.05
Haematological Profile of Experimental Mice
The haematological profile of mice measured at the end of the experiment shows that the extract significantly (p<0.05) prevented the reduction in Packed Cell Volume (PCV), Red Blood Cell (RBC) and Haemoglobin (HGB) for both curative and suppressive test as shown in Figure 2. The group of mice that received chloroquine (4.26±0.189) had a statistically (p<0.05) reduced White Blood Cell (WBC) than and the negative control (5.76±0.356) and experimental groups treated for seven days (4.8±0.362, 4.72±0.252, 4.76±0.218).
Statistical analysis performed amongst negative control (C-), positive control (C+), normal control (NC), 500mg/kg(T1), 1000mg/kg(T2) and 1500mg/kg(T3) dose of ethanol leaf extract of J. secunda respectively, groups with different letters on bars are statistically significant at p<0.05, each point is the mean ± SEM (Standard error of mean) n=5.
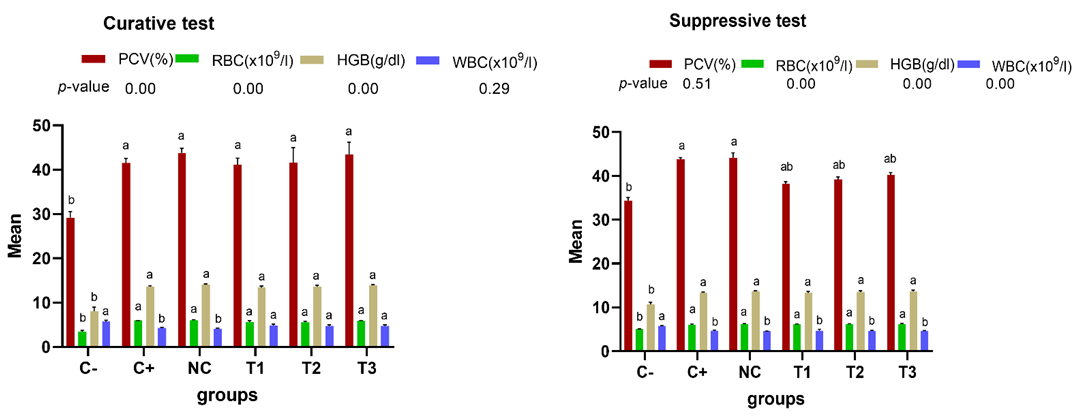
Blood Plasma Biochemicals of Experimental Mice
The immunomodulatory and hepatoprotective potentials of the plant extract was assessed by analysing the biochemical components of plasma such as TNF-α, IL-10 and ALT. The outcome of this biochemical assay for the therapeutic and inhibitory test is presented in Figure 3. The infected mice treated with ethanol leaf extract of J. secunda had significantly higher (p<0.05) concentrations of TNF-α and IL-10 compared to negative control for both curative and suppressive test. Furthermore, the plasma concentration of ALT in negative control was significantly higher (p<0.05) than groups that received plant extract treatment.
Statistical analysis performed amongst negative control (C-), positive control (C+), normal control (NC), 500mg/kg(T1), 1000mg/kg (T2), 1500mg/kg (T3) dose of ethanol leaf extract of J. secunda, groups with different letters on bars are statistically significant p<0.05, each point is the mean ± SEM (Standard error of mean) n=5.
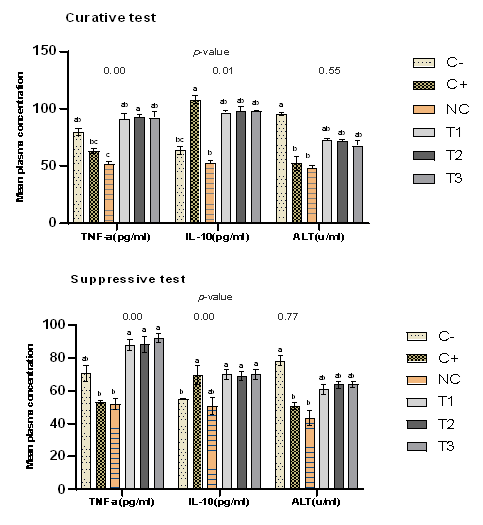
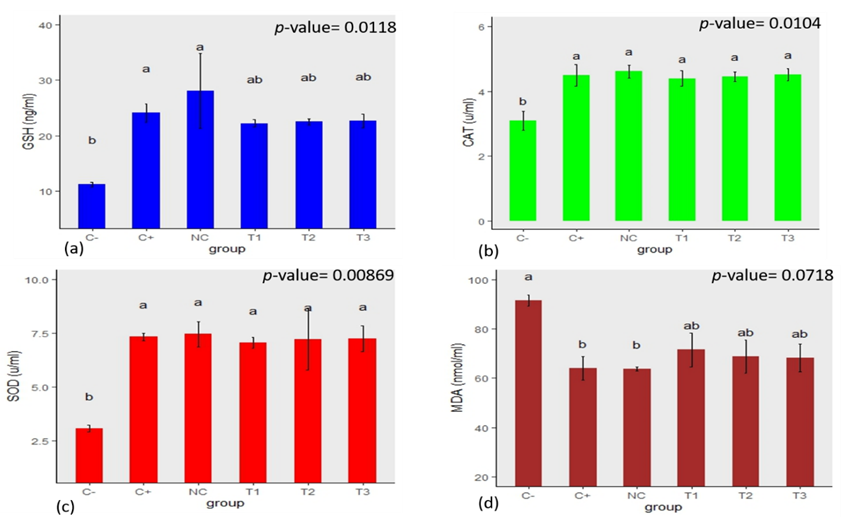
Antioxidant and Lipid Peroxidation Status of the Liver
Figure 4 shows the liver’s antioxidant and lipid peroxidation profile of experimental mice for the curative and suppressive test. The groups treated with plant extract exhibited a significant (p<0.05) increase in the bioactivity of antioxidants, including SOD, CAT, and GSH, compared to the negative control. The SOD concentration was, however, significantly (p<0.05) lower in the extract-treated group (3.32±0.447u/ml, 3.48±0.526u/ml, 3.47±0.579u/ml) and negative control (3.38±0.481u/ml) than in the normal control (7.26±0.462u/ml) and positive control (6.84±0.331u/ml). In addition, the hepatic malondialdehyde (MDA) concentrations were significantly (p<0.05) reduced in treatment groups compared to the negative control.
Statistical analysis performed amongst negative control (C-), positive control (C+), normal control (NC), 500mg/kg(T1), 1000mg/kg (T2), 1500mg/kg (T3) dose of ethanol leaf extract of J. secunda, groups with different letters on bars are statistically significant p<0.05, each point is the mean ± SEM (Standard error of mean) n=5.
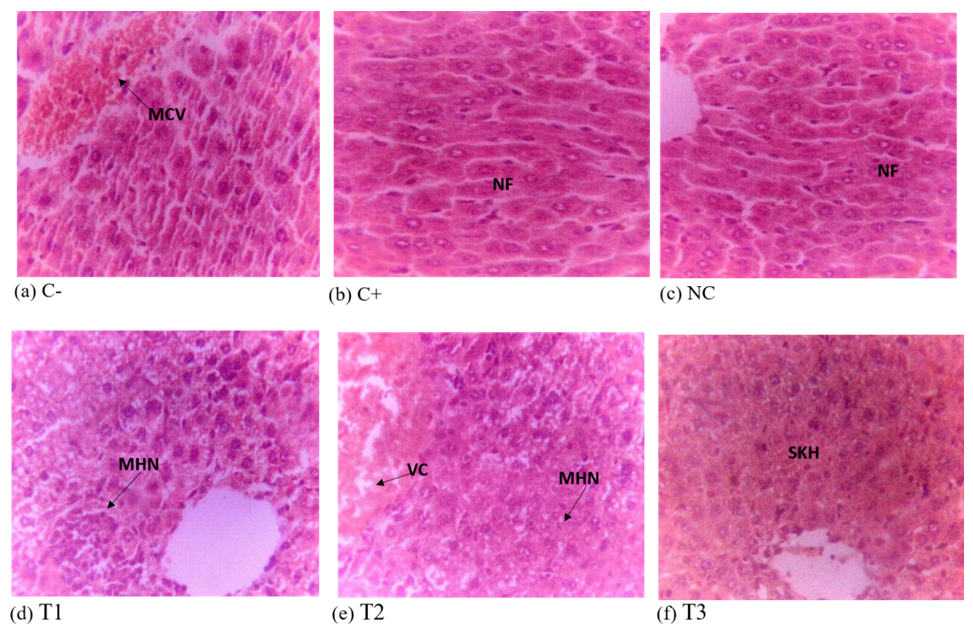
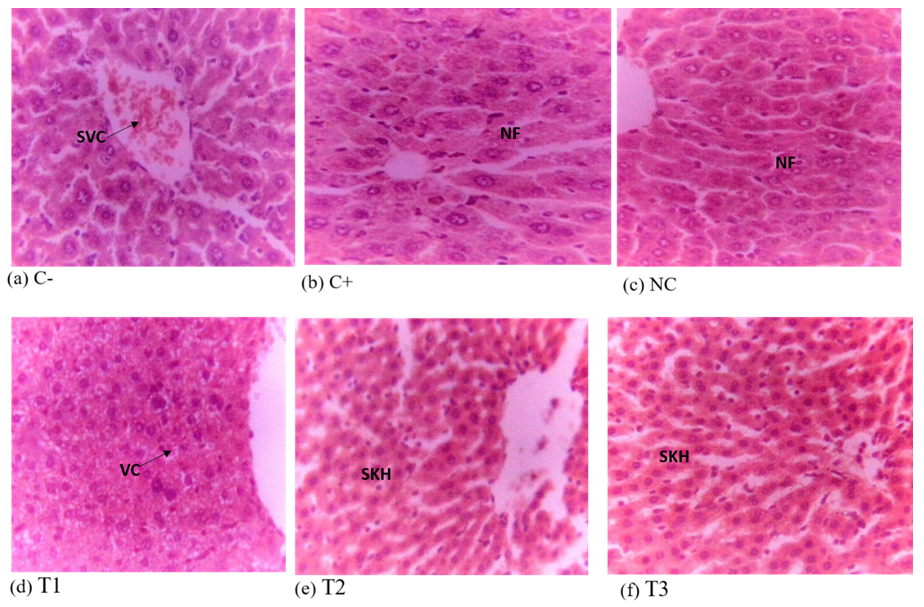
Liver Histology of Experimental Mice
The liver of negative control shows mass vascular congestion when compared to liver sections of other groups for the curative test (Figure 5). The positive control and normal control have normal architecture of a liver photomicrograph, while the 500mg/kg, 1000mg/kg and 1,500mg/kg have a moderate hepatic necrosis, moderate vacuolation and slight Kupffer cell hyperplasia respectively. Comparing the histological sections of the treatment groups to those of the negative control group for the suppressive test (Figure 6), the treatment groups showed slight vacuolation and necrosis (500 mg/kg), slight Kupffer cell hyperplasia (1,000mg/kg and 1,500 mg/kg) and the negative control had slight vascular congestion. Also, the liver photomicrographs of the positive and normal control exhibit normal features in their hepatic histology.
KEYS:
Negative control (C-), positive control (C+), normal control (NC), 500mg/kg(T1), 1000mg/kg (T2), 1500mg/kg (T3) dose of ethanol leaf extract of J. secunda, MVC= Mass Vascular Congestion, NF= Normal Feature, VC= Vacuolation, MHN= Moderate Hepatic Necrosis, MVC= Mass Vascular Congestion, SKH= Slight Kupffer cell Hyperplasia.
KEYS
Negative control (C-), positive control (C+), normal control (NC), 500mg/kg(T1), 1000mg/kg (T2), 1500mg/kg (T3) dose of ethanol leaf extract of J. secunda, SVC= Slight Vascular Congestion, NF= Normal Feature, VC= Vacuolation, SKH= Slight Kupffer cell Hyperplasia.
Discussions
Eight (8) different secondary metabolites were present in the ethanol leaf extract of J. secunda namely: alkaloids, cardiac glycosides, phenolic compounds, tannins, steroids, carbohydrates, flavonoids and terpenoids. In addition, several phytochemical compounds with antioxidant, anti-inflammatory and anti-plasmodial activity were detected in the extract. For instance, β-sitosterol has anti-inflammatory properties [23], Squalene has antiparasitic and anti-inflammatory properties [24], 2-Methyl-Z,Z-3,13-octadecadienol has antimicrobial properties [25], 17-Pentatriacontene has anti-inflammatory properties [26], hexadacenoic acid methyl ester has anti-inflammatory properties [27], 9,12-Octadecanoic (linoleic) acid methyl ester has anti-plasmodial and anti-inflammatory [28], Methyl stearate has anti-inflammatory properties [24], cis-13-Octadecenoic acid, methyl ester has anti-inflammatory activity [29], 16-dimethoxy-aspidospermidin-17-ol has anti-plasmodial activity [30], 8-Hexadecenal 14-methyl-, (Z)- has antioxidant activity [31], Oleic acid has antioxidant activity [32], cis-9-Hexadecenal has anti-inflammatory properties [33] and cyclododecane, ethyl has antioxidant activity [34].
These myriads of phytochemicals may have conferred on this plant its popularity and wide use in African traditional medicine [35]. The phytochemical screening contrasts with the findings of a study conducted on a similar extract of J. secunda leaves collected from a farm in south-east Nigeria that is devoid of steroids and glycosides [36]; however, hexadecenoic acid methyl ester and octadecanoic acid methyl ester was also detected in extract of J. secunda [37]. The difference in phytochemical constituents in both studies could be attributed to ecological variation [38]. According to Hodge and Sterner's [39] toxicity scale, this plant extract is practically non-toxic. A study [40] that processed the leaves of J. secunda for three weeks and macerated them in ethanol for 24 hours reported an LD50 of 3,800 mg/kg body weight in rats due to the cyanide content of the extract. It is very likely that the selection of mature leaves and the longer processing duration of the extract for this present study may be reasons for the reduced toxicity observed [41]. One of the major foci of this study is to include Justicia secunda in the record of antimalarial study, and the crude extract has a percentage chemosuppression of 71.21% (1,500 mg/kg) and 75.18% (1,500 mg/kg) in the suppressive and curative tests, respectively. The result is less than the antimalarial activity of the ethanol leaf extract of Justicia carnea (800 mg/kg dose) with a percentage chemosuppression of 82% [42]. The efficacy of plant extracts in treating malaria disease depends on several factors, such as the Plasmodium species, the dosage, the duration of treatment, and the host immune response [43]. Experimental research on 16-dimethoxy-aspidospermidin-17-ol [31] and linoleic acid-methyl esters [29] suggest that these phytochemicals inhibit the growth of Plasmodium parasites by binding with the Fab-I enzyme responsible for fatty acid biosynthesis. This is to create awareness that J. secunda could be an alternative source of antimalarial compounds.
The ethanol leaf extract of J. secunda prevented PCV, HGB, and RBC reduction in mice, but there was an increase in WBC in the curative test. A different study observed similar trend for these haematological parameters with a normal WBC in curative and suppressive tests on the antioxidant and antimalarial activity of the ethanol stem bark extract of Terminalia macroptera in P.berghei-infectedmice [44]. The haematinic properties of the extract in this study may be due to the presence of flavonoid compounds that have been reported to increase iron absorption and deposition in tissues while reducing iron excretion [45]. In addition, many antimalarial herbal preparations may exert their anti-infective activity not only by affecting the parasite directly but may also stimulate the defensive system of the host through many other mechanisms [46]. The assessment of modulatory and hepatoprotective potentials reveals that TNF-α and IL-10 increased and the concentrations of ALT reduced in treatment groups. This outcome corroborates the TNF and IL-10-increasing potentials observed in P.berghei-infectedmice treated with the ethyl acetate leaf extract of Sonchus arvensis [47] and the hepatoprotective nature of J. secunda in reducing plasma ALT in rats administered carbon tetrachloride [48]. Infection and immunological reactions to the extract may be reasons for the increased level of TNF-α in treated mice. The anti-inflammatory activity of β-sitosterol was harnessed in reducing inflammation in Zebra fish [49] and the presence of this phytochemical in the extract utilized for this research may be the reason for the observed pharmacological effect. This is an indication that J. secunda may be utilized for treatment associated with pathogenesis of malaria infection.
The liver homogenate of the experimental groups sustained an increased bioactivity of antioxidants (SOD, GSH, and CAT). Yet, a reduced level of SOD was observed in mice treated days after infection. The lipid peroxidation biomarker (MDA) is equally reduced in mice treated with crude extract. Hepatic antioxidant and MDA concentrations follow the same pattern as those of experimental mice treated with an extract of Crotonmembranaceusbut differ with an increased SOD level [50]. The infection also caused a reduction in hepatic SOD in mice infected with P. berghei and treated with a stem-bark extract of Terminalia macroptera [51]. Justicia secunda exhibited antioxidant properties in this study, which may be attributed to components of the extract with the relevant pharmacological activity [49, 52, 53]. The ethanol leaf extract of J. secunda reduced vascular congestion in the liver of mice, as the photomicrograph of the treatment groups only shows sections with Kupffer cell hyperplasia, vacuolation, and hepatic necrosis. Ibukunoluwa [54] also reported similar histopathology in the antiplasmodial activity of polyherbal mixtures. The slight changes seen in the liver when used to test the efficacy of the plant extract may be due to the induced infection and the activation of immune cells in the organs. Ayawa et al. [55] also advanced cellular damage by immunological reactions as a reason for histopathological changes in the liver of mice. Pure compounds should be isolated from J. secunda extract to conduct trials on their pharmacological activity in relation to malaria infection and this could potentially uncover another effective antimalarial compound.
Conclusion
In conclusion, the ethanol leaf extract of J. secunda contains phytochemicals with numerous pharmacological activities, and is non-toxic at 5,000mg/kg. It exhibits a dose-dependent percentage of chemosuppression of P. berghei at 500mg/kg, 1,000mg/kg and 1,500mg/kg. The extract prevented the reduction of the studied haematological parameters such as RBC, HGB and PCV. The concentrations of TNF-α and IL-10 increased and ALT concentrations reduced in the plasma of mice treated with extract. Evaluation of liver biochemicals revealed that MDA concentration was reduced with increased bioactivity of the studied antioxidants for the infected mice treated with the plant extract. Vascular congestion was also reduced in the liver histology of mice infected and treated with the plant extract but shows increased infiltration of immune cells and necrosis. This present study infers that ethanol leaf extract of J. secunda contains phytochemicals that have been tested individually to treat malaria and inflammation, which can be isolated for treating malarial pathogenesis.
Abbreviations
ALT Alanine aminotransferase
MDA Malondialdehyde
SOD Superoxide dismutase
CAT Catalase
GSH Reduced Glutathione
IL-10 Interleukin-10
TNF-α Tumour Necrosis Factor-alpha
HGB Haemoglobin
PCV Packed Cell Volume
RBC Red Blood Cell
WBC White Blood Cell
Declarations
Ethical approval
Maintenance and procedures performed involving animals in this study follow the ethical standards of Ahmadu Bello University, Zaria Committee on Animal Use and Care (ABUCAUC) with the approval number ABUCAUC/2023/055.
Consent for publication
Not applicable
Availability of data and materials
All data generated or analyzed in this study are included in this article.
Competing interest
None
Funding
None
Authors’ Contributions
N.A.A, S.J.O, P.I.R, A.Y., and Y.A.W conceptualized the study, conducted the investigation and formal analysis.
N.A.A and M.H. prepared figures and tables. All authors reviewed the manuscript.
Acknowledgements
A sincere gratitude to the Department of Pharmacology, Ahmadu Bello University (ABU), Zaria for providing the animal house and Swiss albino mice used for this research, Department of Pharmacognosy and Drug Development ABU, Zaria for providing the facility to achieve the extraction of the plant and the phytochemical screening, the Multi-User Science Research Laboratory ABU, Zaria for the Gas Chromatography-Mass Spectrometric analysis of the plant extract and the Teaching laboratory, Department of Human Anatomy ABU, Zaria for the successful investigation of histological, haematological and biochemical aspect of this work. We also appreciate Mr. S. Namadi from the Department of Botany, ABU, Zaria for the authentication of the plant (voucher number: ABU06885). A special thank you to the laboratory staff of these departments for their assistance in successfully conducting this experiment.
References
- World Health Organization.World malaria report (2022).
- Okoronkwo IL. Social, cultural, political and economic issues connected to the causes and consequences of malaria.Journal of Public Health Epidemiology 6 (2014): 45-52.
- Ibor U, Okoronkwo E. Demographic and socioeconomic factors influencing malaria incidence in Calabar, Cross River State, Nigeria. Science World Journal 12 (2017):19-24.
- Ukaegbu CI, Ibe SN, Ohaeri OC, Nwosu DC. Prevalence and epidemiology of malaria in Nigeria: a review. Journal of Public Health Epidemiology 6 (2014): 302-307.
- Kar NP, Kumar A, Singh OP, Carlton JM, Nanda N. A review of malaria transmission dynamics in forest ecosystems. Parasite and Vectors 7 (2014): 1-12.
- Wirth DF, Casamitjana N, Tanner M, Reich MR. Global action for training in malaria elimination. Malaria Journal 17 (2018): 1-4.
- Sabina K. Prevalence and epidemiology of malaria in nigeria: a review. Interanational Journal of Research in Pharmaceutical science 4 (2017): 10-12.
- Bekono BD, Ntie-Kang F, Onguono PA, Lifongo LL, Sippl W, Fester K, et al. The potential of anti-malarial compounds derived from african medicinal plants: a review of pharmacological evaluations from 2013 to 2019. Malaria Journal 19 (2020): 1-35.
- Rathmes G, Rumisha SF, Lucas TC, Twohig KA, Python A, Nguyen M, et al. Global estimation of anti-malarial drug effectiveness for the treatment of uncomplicated plasmodium falciparum malaria 1991'2019. Malaria Journal, 19 (2020):1-15.
- Kitadi JM, Lengbiye EM, Gbolo BZ, Inkoto CL, Muanyishay CL, Lufuluabo GL, Tshibangu DS, Tshilanda DD, Mbala BM, Ngbolua K. Justicia secunda Vahl species: phytochemistry, pharmacology and future directions: a mini-review. Discovery Phytomedicine 6 (2019): 157-171.
- Carrington S, Cohall D, Gossell-Williams M, Lindo J. The antimicrobial screening of a barbadian medicinal plant with indications for use in the treatment of diabetic wound infections. West Indian Medical Journal 61 (2012).
- Irinmwinuwa EO, Afonne JO. Investigation into the effect of ethanol leaf extract and fractions of Justicia secunda on platelets and WBC counts in mice. Magna Scientia Advanced Research and Review 6 (2022): 001-007.
- Yamoah A, Adosraku RK, Amenu JD, Baah MK, Abaye DA. Evaluation of the haematinic activities of extracts of Justicia secunda Vahl leaves in red blood cells of laboratory rats. Journal of Bioscience and Medicine 8 (2020): 48-57.
- Onoja SO, Ezeja MI, Omeh YN, Onwukwe BC. Antioxidant, anti-inflammatory and antinociceptive activities of methanolic extract of Justicia secunda Vahl leaf. Alexandria Journal of Medicine 53 (2017): 207-213.
- Edoh SP, Sani D, Mbah CE, Yusuf H, Ishaq AN, Sule TG, Audu KE. Phytochemical evaluation and acute oral toxicity of crude methanol extract of Pleurotus tuber-regium (Fr.) Singer in laboratory mice (Mus musculus).International Journal of Plant Pharmaceutical science 3 (2023): 68–72.
- Evans WC. Trease and Evans' Pharmacognosy (16th). Elsevier Health Sciences (2009).
- Daskum AM, Chessed G. Repository and curative antimalarial activities of Artemisia absinthium in mice experimentally infected with P.berghei (NK65). Journal of Medicinal Plant Studies 10 (2022): 47-53.
- Organization for Economic Coorporation and Development. Acute oral toxicity: up-and-down procedure. Washington DC, USA: Organization for Economic Coorporation and Development (OECD)-(2008).
- Onyegeme-Okerentaa BM, Dickson GE, Amadi BA, Essien EB. The antiplasmodial effects of Senna alata and Dennettia tripetalla on chloroquine-sensitive Plasmodium berghei (NK65).Journal of Medicinal Plant Research7 (2023): 1012-1019.
- Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigorio AM. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infection and Immunity 78 (2010): 4033-4039.
- Awoke N, Arota A. Profiles of hematological parameters in Plasmodium falciparum and Plasmodium vivax malaria patients attending Tercha General Hospital, Dawuro Zone, South Ethiopia. Infection and Drug Resistance 12 (2019): 521-527.
- Slaoui M, Fiette L. Histopathology procedures: from tissue sampling to histopathological evaluation. In J. C. Gautier (Ed.), Drug Safety Evaluation (2011): 55-93.
- Loizou S, Lekakis I, Chrousos GP, Moutsatsou P. Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Molecular Nutrition and Food Research 54 (2010): 551-558.
- Adnan M, Nazim UC, Mostafa KA, Azad MO, Paul A, Uddin SB, et al. Investigation of the biological activities and characterization of bioactive constituents of Ophiorrhiza rugosa prostrata (D. Don) & mondal leaves through in vivo, in vitro, and in silico approaches. Molecules 24 (2019): 1367.
- Mehranian M, Farshbaf PR, Sokhandan BN, Motavalizadehkakhky A. Isolation and identification of cuticular compounds from the mediterranean flour moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae), their antibacterial activities and biological functions. Archives of Phytopathology and Plant Protection 49 (2016): 618–632.
- El ON, Guaouguaou FE, El MN, Benali T, Aanniz T, Chamkhi I, Balahbib A, Taha D, Shariati MA, Zengin G. Phytochemical and biological activities of pinus halepensis, and their ethnomedicinal use. Journal of Ethnopharmcolology 268 (2021): 113661.
- Trinh PT, Chanh NT, Ngoc NT, Tien PQ, Ly BM, Van TT. secondary metabolites from a marine-derived fungus Penicillium chrysogenum 045-357-2. Vietnam Journal of Science and Technology 55 (2017): 65-72.
- Melariri P, Campbell W, Etusim P, Smith P. In vitro and in vivo antimalarial activity of linolenic and linoleic acids and their methyl esters. Advanced Studies in Biology 4 (2012): 333-49.
- Ayoola AA, Ekunseitan DA, Muhammad SB, Oguntoye MA, Adejola YA. Phytochemicals analysis and gc-ms determination of ethanolic extracts of Azadirachta indica and Mangifera indica stem bark and their biological potentials.The Pacific Journal of Science and Technology 21 (2020): 219-22.
- Kane NF, Kyama MC, Nganga JK, Hassanali A, Diallo M, Kimani FT. Expression of the Fab enzymes (Fab I and Fab Z) from Plasmodium falciparum after exposure to Artemisia afra plant extracts and drugs screening. Journal of Parasite Discovery 47 (2023): 46-58.
- Revathi N, Dhanaraj T. Evaluation of bioactive phytochemicals in leaves extract of dodonaea angustifolia using gas chromatography and mass spectroscopic technique. Journal of Pharmacognosy and Phytochemistry 8 (2019): 4406-4409.
- Alabi K, Lajide L, Owolabi B. Biological activity of oleic acid and its primary amide: experimental and computational studies. Journal of Chemical Society of Nigeria, 43(2018).
- Mujeeb F, Bajpai P, Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle mrmelos. BioMedical Research International (2014).
- Joo SS, Kim YB, Lee DI. Antimicrobial and antioxidant properties of secondary metabolites from white rose flower. Plant Pathology Journal 26 (2010): 57-62
- Mpiana PT, Ngbolua KN, Bokota MT, Kasonga TK, Atibu EK, Tshibangu DS, Mudogo V. In vitro effects of anthocyanin extracts from Justicia secunda Vahl on the solubility of haemoglobin s and membrane stability of sickle erythrocytes. Journal of Blood Transfusion 8 (2010): 248.
- Irinmwinuwa EO, Afonne JO. Investigation into the effect of ethanol leaf extract and fractions of Justicia secunda on platelets and WBC counts in mice. Magna Scientia Advanced Research and Review 6 (2022): 001-007.
- Aimofumeh EO, Anyasor GN, Esiaba I. Justicia secunda Vahl leaf fraction protects against acetaminophen-induced liver damage in rats by alleviating oxidative stress and enhancing membrane-bound phosphatase activities. Asian Pacific Journal of Tropical Biomedicine 10 (2020): 479.
- Dhawan D, Gupta J. Research article comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. International Journal for Biological and Chemical Science 11 (2017): 17-22.
- Hodge A, Sterner B. Toxicity classes. In: Canadian Center for Occupational Health and Safety (2005).
- Onochie AU, Oli AH, Oli AN, Ezeigwe OC, Nwaka AC, Okani CO, et al. The pharmaco biochemical effects of ethanol extract of Justicia secunda Vahl leaves in Rattus norvegicus. Journal of Experimental Pharmacology (2020): 423-437
- Panter KE. Cyanogenic glycoside–containing plants. In R. C. Gupta (Ed.), Veterinary Toxicology 3 (2018): 935-940.
- Chidi AS, Mattew WO, Benjamin AA, Peter UA, Uche NC. The modulatory activity of Justicia carnea in Plasmodium infected mice.Trends Journal of Science Research 3 (2018): 151-160
- White NJ. Malaria parasite clearance. Malaria Journal, 16 (2017): 88.
- Haidara M, Haddad M, Denou A, Marti G, Bourgeade-Delmas S, Sanogo R, Bourdy G, Aubouy A. In vivo validation of anti-malarial activity of crude extracts of Terminalia macroptera, a Malian medicinal plant. Malaria Journal 17 (2018): 1-10.
- Carneiro MR, Sallum LO, Martins JL, Peixoto JD, Napolitano HB, Rosseto LP. Overview of the Justicia genus: insights into its chemical diversity and biological potential. Molecules 28 (2023): 1190.
- Arrey TP, Okalebo FA, Ayong LS, Agbor GA, Guantai AN. Anti-malarial activity of a polyherbal product (Nefang) during early and established Plasmodium infection in rodent models. Malaria Journal 13 (2014): 1-11.
- Wahyuni DK, Wacharasindhu S, Bankeeree W, Wahyuningsih S P, Ekasari W, Purnobasuki H, Punnapayak H, Prasongsuk S. In vitro and in vivo antiplasmodial activities of leaf extracts from Sonchus arvensis BMC Complementay Medicine 23(2023): 1-12.
- Anyasor GN, Moses N, Kale O. Hepatoprotective and hematological effects of justicia secunda vahl leaves on carbon tetrachloride induced toxicity in rats. Biotech Histochemistry 95 (2020): 349-359.
- Zhang P, Liu N, Xue M, Zhang M, Liu W, Xu C, et al. Anti-Inflammatory and antioxidant properties of β-sitosterol in copper sulfate-induced inflammation in zebrafish. Antioxidants 12 (2023): 391.
- Afriyie D, Ameyaw EO, Acheampong F, Appiah-Opong R. In vitro and in vivo antioxidant properties of stem extracts of Croton membranaceus. Jounal of Medicinal Plant Studies 9 (2021): 1-8.
- Sidiki NN, Nadia NA, Cedric Y, Guy-Armand GN, Sandra TN, Kevin TD, et al. Antimalarial and antioxidant activities of ethanolic stem bark extract of Terminalia macroptera in swiss albino mice infected with Plasmodium berghei. Journal of Parasitology Research (2023).
- Zhang P, Liu N, Xue M, Zhang M, Xiao Z, Xu C, et al. Anti-Inflammatory and antioxidant properties of squalene in copper sulfate-induced inflammation in zebrafish (Danio rerio). International Journal of Moleular Science 24 (2023): 8518.
- Wei CC, Yen PL, Chang ST, Cheng PL, Lo YC, Liao VHC. Antioxidative activities of both oleic acid and Camellia tenuifolia seed oil are regulated by the transcription factor DAF-16/FOXO in Caenorhabditis elegans. PloS one, 11 (2016): e0157195.
- Ibukunoluwa MR. In vivo anti-plasmodial activity and histopathological analysis of water and ethanol extracts of a polyherbal antimalarial recipe. Journal of Pharmacognosy and Phytotherapy, 9 (2017): 87-100.
- Ayawa NG, Ramon-Yusuf SB, Wada YA, Oniye SJ, Shehu DM. Toxicity study and anti-trypanosomal activities of aqueous and methanol whole plant extracts of Brillantaisia owariensis on Trypanosoma brucei-induced infection in BALB/c mice. Clinical Phytoscience 7 (2021): 39.



 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
