Rejection of corneal transplant after administration of SARS-CoV-2 messenger RNA vaccine
Hisataka Fujimoto1,2*
1Department of Ophthalmology, Hyogo College of Medicine, Mukogawa-cho 1-1, Nishinomiya, Hyogo 663-8501, Japan
2Department of Ophthalmology, Kawasaki Medical School, 577 Matsushima, Kurashiki, Okayama 701-0192, Japan
*Corresponding author: Hisataka Fujimoto, Department of Ophthalmology, Hyogo College of Medicine Mukogawa-cho 1-1, Nishinomiya, Hyogo 663-8501, Japan
Received: 02 January 2024; Accepted: 08 January 2024; Published: 12 January 2024
Article Information
Citation: Hisataka Fujimoto. Rejection of corneal transplant after administration of SARS-CoV-2 messenger RNA vaccine. Journal of Ophthalmology and Research. 7 (2024): 13-15.
DOI: 10.26502/fjor.2644-00240089
View / Download Pdf Share at FacebookAbstract
Purpose: This study reports the frequency of corneal allograft rejection following vaccination with a messenger RNA (mRNA) vaccine for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and describes the rate of rejection.
Methods: This retrospective and observational study included 64 patients who underwent keratoplasty, and the rates of rejection corrected for the observation period were analyzed. Results: Nine cases of rejection were observed following mRNA vaccination, with an observational period of 804 days, while the no-vaccination control group had two cases of rejection during an observational period of 923 days. The overall rate ratio of rejection related to vaccination, compared to that in the control group, was 5.74. Notably, the second vaccination correlated with the most prominent rate ratio 27.
Conclusion: Corneal transplant rejection is suspected to be associated with SARS-CoV-2 mRNA vaccination. Rejection, believed to be induced by vaccination, is primarily observed in the early stages of the vaccination process.
Keywords
<p>Severe Acute Respiratory Syndrome Coronavirus-2 (SARSCoV- 2) messenger RNA (mRNA) Vaccine, Rejection, Keratoplasty, Corneal Transplantation</p>
Article Details
1. Introuduction
Corneal transplantation is a commonly employed ophthalmic procedure. Despite the cornea being an immune-privileged organ, allogeneic rejection is the leading cause of graft failure [1]. Rejection is reported in various transplantation methods, including penetrating keratoplasty (PK), Descemet’s stripping automated endothelial keratoplasty (DSAEK), anterior lamellar keratoplasty (ALK), deep anterior lamellar keratoplasty (DALK), and most frequently following corneal limbal transplantation (LT) [2]. While treatable, rejection causes irreversible harm to the donor, particularly to the endothelial cells responsible for maintaining corneal transparency [3]. The loss of endothelial cells leads to decompensation and stromal edema, necessitating retransplantation. Despite the immune privilege of the cornea, immune-mediated corneal allograft rejection can occur, especially after LT and PK, in eyes with a heightened risk of rejection [4].
Recently, several case reports have revealed rejection induced by BNT162b2 vaccination [5,6]. A quantitative study has also reported on the rejection properties induced by SARS-CoV-2 mRNA vaccination [7]. Here, we describe the chronological characteristics of rejection following the COVID-19 immunization.
2. Methods
2.1 Study Design
This retrospective observational study included 64 patients who underwent PK, DSAEK, ALK, DALK, or LT keratoplasty. This study was approved by the Institutional Review Board of Kawasaki Medical School Hospital (approval number 3572-00). Informed consent was obtained from all study participants, in adherence to the tenets of the Declaration of Helsinki; the study was performed according to Good Clinical Practice (GCP). This study did not involve patients or the public in its design, recruitment, or conduct.
2.2 Data collection
This retrospective observational study included 64 patients who underwent keratoplasty. Data on the incidence of rejection, rejection onset date, transplantation date, vaccination date of the SARS-CoV-2 mRNA vaccine, number of vaccinations, and background data such as age, sex, and operative procedure (PK, DSAEK, DALK, LT, or a combination of these) were collected. On-site training was conducted to ensure uniform data collection, assessment, and compliance with the GCP. Patient data records were selected from April 1, 2001to December 1, 2023, and were included in the study.
2.3 Participants
The clinical records, including imaging records, of 64 patients who underwent keratoplasty were retrospectively reviewed at a single center, Kawasaki Medical School, in Japan. The study included patients without active ocular surface disorders, such as epithelial disorders, infections, conjunctivitis, ocular inflammatory disease, or nasolacrimal duct obstruction.
2.4 Statistical analysis
The observation period for the control group spanned from the day of keratoplasty to the first vaccination day. For the first vaccination, the observation period was between the first and second vaccination days. Similarly, the observation periods for the second, third, and subsequent vaccinations were set between the corresponding vaccination days. This was extended up to the sixth vaccination (N=6). The rejection rate was defined as the number of rejections divided by the observation period. The rate ratio against the control was defined as the rate of the Nth observation period divided by the rate of the control period.
All the analyses were conducted using SPSS version 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, version 25.0. Armonk, NY: IBM Corp. USA).
3. Results
The study included 35 males and 29 females with an age of 67.95 ± 17.89 years (maximum, 95; minimum, 30). The average observation period in the control group was 923 days. For the first to sixth vaccination, the average observation periods were 21, 120, 175, 132, 216 days, and 140 days respectively.
Rejection following mRNA vaccination was observed in nine cases, with an overall observational period following vaccination of 804 days. In the non-vaccination control group, rejection was observed in two cases, with an overall observational period of 923 days. The overall rate ratio of rejection related to vaccination compared with that in the control group was 5.74.
The control rejection rate was 3.4 x 10-5 (rejection/day). The rejection rates of the first to sixth vaccination were 7.4 x 10-4, 9.1 x 10-4, 1.9 x 10-4, 0, 0, and 0 (rejections/day) respectively.
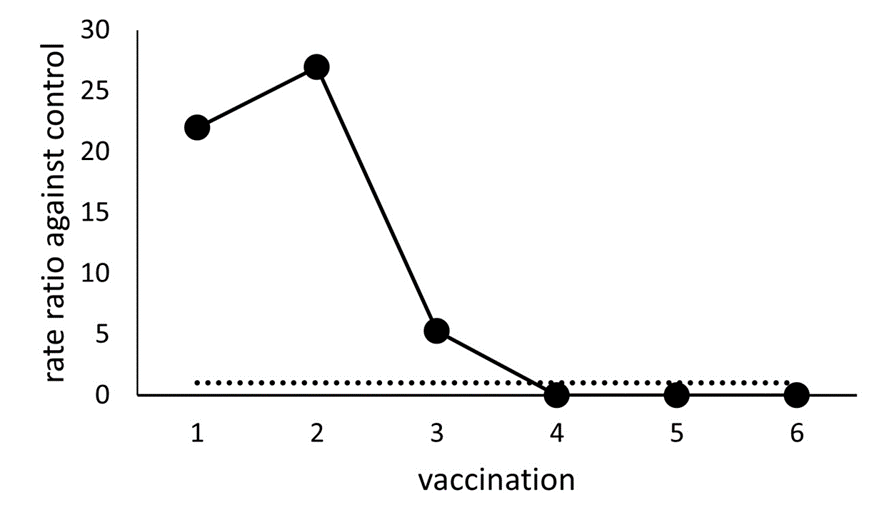
The rejection rate ratios of the first to sixth vaccination compared to those in the control were 21.98, 26.93, 5.28, 0, 0, and 0, respectively (Figure 1). During the control period, the operative procedure for the two cases with rejection and the operative procedure for the rejection case during the first vaccination (one case) was PK. In the rejection cases during the second vaccination, four cases underwent PK, two cases underwent DSAEK, and one case underwent PK+LT. The surgical procedure for the third vaccination rejection in the one case was PK.
4. Discussion
The rejection rate ratios of the first to the third vaccinations were also high. The rejection cases were particularly noticeable during the first and second vaccinations, exhibiting rate ratios exceeding 20. Traditionally, corneal graft rejection associated with vaccination is considered a rare occurrence [[8]. Only a few cases reporting corneal graft rejection following influenza, hepatitis B, and tetanus toxoid immunization have been reported [8,9,10]. The COVID-19 pandemic has accelerated the introduction of immunization against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to reduce morbidity and mortality [11]. Recent studies have brought attention to the potential for rejection induced by BNT162b2 vaccination [5,6,7].
In this study, instances of rejection were primarily observed during the first or second vaccination, with no occurrences after the fourth vaccination. This could be attributed to high-risk rejection patients experiencing rejection in the early stages of vaccination. Another possibility is that ophthalmologists, suspecting rejection after mRNA vaccination, opted for stringent monitoring and control measures against rejection.
One of the limitations of this study is its retrospective design and relatively small sample size. Further research should aim for larger case enrollments to enhance robust findings.
5. Conclusion
In conclusion, there appears to be an association between SARS-CoV-2 mRNA vaccination and corneal transplantation rejection. Ophthalmologists and patients should consider the possibility of rejection following vaccination. However, the risk of rejection tends to decrease with the number of vaccinations administered.
Acknowledements:
I thank J. Kiryu and N. Okamoto for their discussions, advice, and criticism, which greatly benefited this project. This study was supported by the Taiju Life Social Welfare Foundation (to H.F.)
Conflicts of Interests: The author declares no conflicts of interest regarding the publication of this article.
References
- Niederkorn JY, Larkin DFP. Immune privilege of corneal allografts. Ocul Immunol Inflamm 18 (2010): 162-171.
- Price DA, Kelley M, Price FW, et al. Five-Year graft survival of Descemet membrane endothelial keratoplasty (EK) versus Descemet stripping EK and the effect of donor sex matching. Ophthalmol 125 (2018): 1508-1514.
- Anshu A, Price MO, Price FW. Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. Ophthalmol 119 (2012): 536-540.
- Hos D, Matthaei M, Bock F, et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog Retin Eye Res 73 (2019): 100768.
- Wasser LM, Roditi E, Zadok D, et al. Keratoplasty rejection after the BNT162b2 messenger RNA Vaccine. Cornea 40 (2021): 1070-1072.
- Phylactou M, Li J-PO, Larkin DFP. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. British J Ophthalmol 105 (2021): 893-896.
- Fujimoto H, Kiryu J. Incidence of corneal transplant rejection following BNT162b2 SARS-CoV-2 messenger RNA vaccination. J Ophthalmol Res 4 (2021): 279-288.
- Lockington D, Lee B, Jeng BH, et al. Survey of corneal surgeons’ attitudes regarding keratoplasty rejection risk associated with vaccinations. Cornea 40 (2021): 1541-1547.
- Solomon A, Frucht-Pery J. Bilateral simultaneous corneal graft rejection after influenza vaccination. American J Ophthalmol 121 (1996): 708-709.
- Vignapiano R, Vicchio L, Favuzza E, et al. Corneal graft rejection afteryellow fever vaccine: a case report. Ocul Immunol Inflamm 30 (2021): 1-4.
- Cook TM, Roberts JV. Impact of vaccination by priority group on UK deaths, hospital admissions and intensive care admissions from COVID-19. Anaesthesia 76 (2021): 608-616.


 Impact Factor: * 1.2
Impact Factor: * 1.2 Acceptance Rate: 79.45%
Acceptance Rate: 79.45%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
