Tacrolimus Improved Reproductive Outcomes of Women with Recurrent Pregnancy Loss (PRL) Showing Elevated T Helper 1 (Th1) /Th2 Cell Ratios
Koji Nakagawa1*, Joanne Kwak-Kim2, Keiji Kuroda1, Takashi Horikawa1, Satoru Takamizawa1, Michi Hisano3, Yoshimitsu Kasahara4, Rikikazu Sugiyama1, Koushi Yamaguchi3
1Center for Reproductive Medicine and Implantation Research, Sugiyama Clinic Shinjuku, Tokyo
2Reproductive Medicine and Immunology, Obstetrics and Gynecology, Clinical Sciences Department, Chicago Medical School at Rosalind Franklin University of Medicine and Science, Vernon Hills, Illinois, USA
3Department of Maternal-Fetal Biology, National Center for Child Health and Development, Tokyo
4Division of Obstetrics, Sugiyama Clinic, Tokyo
*Corresponding author: Koji Nakagawa, Center for Reproductive Medicine and Implantation Research, Sugiyama Clinic Shinjuku, 1-19-6, Nishi-Shinjuku, Shinjuku-ku, 160-0023, Tokyo, Japan.
Received: 07 November 2022; Accepted: 11 November 2022; Published: 02 December 2022
Article Information
Citation: Koji Nakagawa, Joanne Kwak-Kim, Keiji Kuroda, Takashi Horikawa, Satoru Takamizawa, Michi Hisano, Yoshimitsu Kasahara, Rikikazu Sugiyama, Koushi Yamaguchi. Tacrolimus Improved Reproductive Outcomes of Women with Recurrent Pregnancy Loss (PRL) Showing Elevated T Helper 1 (Th1) /Th2 Cell Ratios. Journal of Women’s Health and Development 5 (2022): 264-270.
DOI: 10.26502/fjwhd.2644-28840098
View / Download Pdf Share at FacebookAbstract
Purpose: Elevated T helper 1 (Th1)/Th2 cell ratio has been associated with recurrent pregnancy losses (PRL). In this study, the clinical efficacy of tacrolimus, a calcineurin inhibitor, was investigated in women with consecutive four or more recurrent pregnancy losses (RPL) and elevated Th1/Th2 (CD4+IFN-γ+/ CD4+IL-4+) cell ratio. The live-birth rate (LBR) was prospectively investigated in women with RPL who received tacrolimus treatment.
Methods: A total of 100 pregnant women with PRL with elevated Th1/Th2 cell ratios (≥10.3) were recruited from November 2013 to December 2019. Seventy-one women received tacrolimus between 1 mg and 4 mg daily (treatment group) and 29 women did not receive tacrolimus (control group).
Results: In the treatment group, the LBR was 70.4% (50/71), which was significantly higher than that of the control group (48.2%, p<0.05). According to the tacrolimus doses, the treatment group was divided into three subgroups. The LBRs of women with tacrolimus 1mg, 2mg, and ≥3mg daily were 52.2%, 72.3%, and 93.3%, respectively. In women with tacrolimus ≥3mg daily, the LBR was significantly higher than women with tacrolimus 1mg daily (P<0.05). Conclusion: In conclusion, the LBR of RPL women with increased Th1/Th2 cell ratios was significantly improved with tacrolimus treatment.
Keywords
<p>Immunosuppressive Agent; Immunological Rejection; Recurrent Pregnancy Loss; Tacrolimus; Th1/Th2 Ratio</p>
Article Details
1. Introduction
The establishment and maintenance of pregnancy is an enigmatic immunological phenomenon, which has been investigated over several decades [1]. In particular, pregnancy is considered semi-allograft transplantation; however, there is no immunological rejection at the maternal-fetal interface during implantation and pregnancy. In the 1990s, the T helper (Th)1/Th2 hypothesis was proposed that the dominance of Th2 cells is critical to establishing and maintaining a pregnancy [2, 3]. Based on this hypothesis, multiple clinical investigations have been made in women with reproductive failures. Ng et al, reported that shifted Th1/Th2 immune balance by increased Th1 and decreased Th2 immune response was associated with repeated implantation failures (RIF) after in-vitro fertilization and embryo transfer (IVF-ET) and recurrent pregnancy losses (RPL) [4]. Indeed, Th2 cell count in patients with RPL was significantly lower than normal fertile controls. After this study, multiple studies have validated the findings [5]. Previously, we reported for the first time that tacrolimus, a calcineurin inhibitor, could improve the clinical pregnancy rate in women with RIF and elevated Th1/Th2 cell ratios [6]. Moreover, the safety of tacrolimus for pregnant women and newborns was documented [7]. Based on the previous study of women with RIF, we hypothesized that the immunosuppressive therapy using tacrolimus could effectively prevent RPL since increased Th1/Th2 ratios were also associated with RPL [4]. In 2017, we reported the first case with 12 consecutive RPLs, who received tacrolimus treatment and delivered a live-born female infant [8]. This patient had a history of various treatments for unexplained RPLs, including anti-coagulation and intravenous immunoglobulin G administration, but failed to have a liveborn infant. The live-birth rate (LBR) and pregnancy outcomes were analyzed among the women with RPL and elevated Th1/Th2 cell ratio, who received various doses of tacrolimus during pregnancy and compared to women without tacrolimus treatment.
2. Material and Methods
2.1 Study Population
The study is designed as a prospective cohort study. The institutional review board of Sugiyama Clinic Shinjuku and National Center for Child Health and Development (NCCHD) approved the study, and all participants signed a consent form prior to entering the study. A total of 293 women, who are 26 to 44 years old, with a history of 4 or more consecutive RPL were recruited in Sugiyama Clinic Shinjuku, Tokyo, Japan and NCCHD, Tokyo, Japan, from November 2013 to December 2019 (Figure 1). Women with a history of or active autoimmune disease (n=3), anti-phospholipid syndrome (n=4), and endocrine etiologies such as diabetes mellitus (n=1), hyperprolactinemia (n=1), and thyroid dysfunction (n=11) were excluded from the study. All participants received a vaginal ultrasound, hysterosalpingography, hysteroscopy, and endometrial biopsy to evaluate underlying etiologies for PRL. Patients with uterine anomaly (n=2), uterine myoma (n=3) endometrial polyps (n=5), chronic endometritis (n=12), and intrauterine adhesion (n=2) were also excluded from the study. Finally, a total of 100 women with PRL met the selection criteria.
All had Th1/Th2 ratio testing, factor XII and protein C and S tests between cycle day (CD) 5-10 of an ovarian cycle prior to any infertility treatment. 100 out of 249 women (40.2 %) with PRL had elevated Th1/Th2 cell ratios (≥10.3). After thorough counseling with couples, 71 women chose tacrolimus treatment (treatment group), and others (n=29) opted out for the treatment (control group) (Figure 1). Women with thrombophilia were treated with low-dose aspirin 81 mg per day starting after the confirmation of pregnancy.
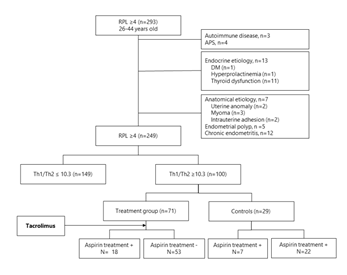
Figure 1: Flowchart of patient selection. Of 293 women, who are 26 to 44 years old, with a history of 4 or more consecutive RPL, 100 women showing elevated Th1/Th2 cell ratios (≥10.3) were recruited. AS shown in this figure, 71 women receive tacrolimus treatment (treatment group), and others (n=29) opted out for the treatment (control group). Women with thrombophilia were treated with low-dose aspirin 81 mg per day starting after the confirmation of pregnancy.
PRL: Repeated Pregnancy Losses, APS: Anti-Phospholipid Syndrome; DM: Diabetes Mellitus, Th: T Helper
2.2 Conception Cycle
All treatment and control groups achieved pregnancy via either natural intercourse (n=36), intrauterine insemination (IUI) (n=3), fresh embryo transfer (fresh ET) (n=9), or thawed embryo transfer (thawed ET) (n=52, Table 1). Women who conceived naturally or by IUI did not receive luteal support. In women who underwent a fresh ET or thawed ET with a natural cycle, dydrogesterone 30mg/day (Duphaston®, MyLan EPD, Tokyo) was given for 11 days before ET and hydroxyprogesterone caproate 125mg (PROGE DEPOT®, Mochida Pharmaceutical, Tokyo) on the day of ET. In women with the hormone replacement cycle (HRC) for thawed ET, conjugated estrogens (Premarin® 0.625mg, Wyeth, Tokyo) and transdermal estradiol (Estorana TAPE® 0.72mg, Hisamitsu Pharmaceutical, Tokyo) were given from CD3 to the day of a pregnancy test. Vaginal progesterone and dydrogesterone were started CD12 day of the conception cycle [9]. In women who received embryo transfer, one or two embryos were transferred. The transferred embryos were either cleavage-stage embryos or blastocysts. In fresh ET, all transferred embryos were cleavage-stage embryos, and only blastocysts were used for thawed ET. Embryo transfer was performed transcervically under transabdominal or transvaginal ultrasound with a sterile soft catheter (Kitazato ET catheter, Kitazato Supply, Shizuoka, Japan) [10].
|
Treatment group |
Control group |
||
|
Patients, n |
71 |
29 |
- |
|
Age, years * |
36.2 ± 3.6 |
36.5 ± 4.9 |
0.85 |
|
BMI |
21.2 ± 3.0 |
21.1 ± 4.2 |
0.82 |
|
Previous pregnancy loss, times** (range) |
4.8 (4-9) |
4.9 (4-11) |
0.43 |
|
Previous parity, times** (range) |
0.3 (0-1) |
0.2 (0-1) |
0.08 |
|
Living children, n** (range) |
0.3 (0-1) |
0.1 (0-1) |
0.08 |
|
Primipara, n |
40 |
13 |
- |
|
History of IUFD, n |
6 |
1 |
- |
|
Anti-coagulation treatment, n |
18 |
7 |
0.89 |
|
factor XII deficiency, n |
13 |
5 |
|
|
Protein C deficiency, n |
3 |
1 |
|
|
Protein S deficiency, n |
2 |
1 |
|
|
Methods of pregnancy, |
|||
|
TI/IUI, n |
30-Mar |
6/0 |
- |
|
Fresh ET, n |
7 |
2 |
- |
|
Thawed ET in natural cycle, n |
22 |
9 |
- |
|
Thawed ET in HRC, n |
9 |
12 |
- |
|
Th1 cell value, %* |
25.1 ± 2.1 |
26.3 ± 7.5 |
0.37 |
|
Th2 cell value, %* |
2.1± 0.9 |
1.7 ± 0.6 |
0.58 |
|
Th1/Th2 cell ratio* |
14.6 ± 10.0 |
16.2 ± 5.6 |
0.44 |
|
*mean ± SD, **mean, # range IUFD- Intrauterine Fetal Death; TI- Timed Intercourse; IUI- Intrauterine Insemination; ET- Embryo Transfer; HRC- Hormone Replacement Cycle; Th1 cell- Type 1 Helper T Cell; Th2 cell- Type 2 Helper T Cell |
|||
Table 1: The background of the participants.
2.3 Analyses of the Th1 and Th2 Cells
The analysis of peripheral blood Th1 and Th2 cells was previously described [6]. Briefly, Th1 and Th2 cells were evaluated by detecting the intracellular interferon (INF)-ϒ and IL-4 production. The red blood cells (RBCs) were removed by lysis (FACS Lysing Solution; Becton Dickinson, BD Biosciences, Franklin Lakes, NJ, USA), and the lymphocytes were analyzed using flow cytometry (FACSCalibur; Becton Dickson). The specific staining of lymphocytes was done by incubating whole blood with anti-CD4-PC5 or anti-CD8-PC5-conjugated monoclonal antibodies (mAbs) (Beckman Coulter, Fullerton, CA, USA). After surface staining of the activated whole-blood samples with anti-PC5-conjugated mAbs, specific intracellular staining using FastImmuneTM INF-ϒ-FITC/IL-4-PE (Becton Dickinson) was subsequently performed according to the manufacturer’s instructions. Th1 cells were defined as CD4+ lymphocytes with intracellular INF-ϒ without IL-4. In contrast, Th2 cells were defined as CD4+ lymphocytes with intracellular IL-4 but without INF-ϒ. The ratio of INF-ϒ to IL-4 positive Th cells was expressed as the Th1/Th2 ratio. Previously we determined the normal range for Th1/Th2 cell ratios using healthy women who had a history of normal delivery by either natural conception or IUI. The Th1/Th2 cell ratio < 10.2 was normal, and 10.3 or above was determined elevated [6].
2.4 Tacrolimus Treatment
The daily dose of tacrolimus was decided according to the Th1/Th2 cell ratios; patients with a mild (≥10.3 and <13.0), moderate (>13.0 and <15.8), and high levels of Th1/Th2 ratio (>15.8) were treated with 1 (n=23), 2 (n=33), and 3 (n=11) or 4 mg (n=4) of tacrolimus daily (Table 2). According to our previous report, patients (n=4) with increased Th1/Th2 ratio due to high Th1 level (≥28.8) received 4 mg of tacrolimus [7]. Patients started tacrolimus administration either 2 days before ET (n=25) or with the confirmation of a positive pregnancy test (n=46). The average daily dose of tacrolimus was 1.9 ± 0.7 mg, ranging from 1 to 4 mg daily.
|
Treatment group |
Control |
||||
|
Total |
Tacrolimus dose, daily |
||||
|
1mg |
2mg |
≥3mg |
|||
|
Patients, n |
71 |
23 |
33 |
15 |
29 |
|
Biological pregnancy, n (%) |
3 (4.2) |
2 |
1 |
0 |
8 (27.6)* |
|
Clinical pregnancy, n (%) |
68 (95.8) |
21 |
32 |
15 |
21 (72.4)** |
|
Clinical miscarriage before FHB, n |
9 |
6 |
3 |
0 |
4 |
|
Embryo miscarriage after FHB, n |
9 |
3 |
5 |
1 |
3 |
|
Clinical miscarriage rate |
29.60% |
47.80% |
27.30% |
6.70% |
55.20% |
|
Delivery, n |
50 |
12 |
24 |
14 |
13 |
|
Live birth rate |
70.40% |
52.2 %** |
72.70% |
93.30% |
44.8 %# |
|
GS; gestational sac *p<0.05 between the total treatment group and controls; **p<0.05, between 1mg and ≥3mg treatment groups. |
|||||
Table 2: The outcomes of the tacrolimus treatment for the patients with multiple pregnancy losses.
2.5 Evaluation Points and Statistical Analysis
Statistical analysis was made by using SPSS Statistics (IBM Japan Ltd, Tokyo, Japan). Continuous variables were analyzed via Wilcoxon signed-rank test. Categorical variables were analyzed by chi-square analysis or Fisher’s exact test as indicated. A probability of <.05 was considered to be statistically different.
3. Results
3.1 Patient Characteristics
The characteristics of the study groups, treatment during the index cycle, and laboratory evaluations are summarized in Table 1. The average age (mean ± SD) of the treatment group (36.2 ± 3.6 years) was not different from that of controls (36.5 ± 4.9 years) (p>0.05). The average body mass index (BMI) of the treatment group (21.2 ± 3.0) was comparable to the control group (21.2 ± 4.2) (p>0.05). The mean numbers of gravidities, parity, and living children of the treatment group (4.8, 0.3, and 0.3, respectively) were comparable to those of controls (4.9, 0.2, and 0.1) (P>0.05, respectively). The numbers of past spontaneous pregnancy losses were 4.8 (mean; ranged 4-9 times) in the treatment group and 4.9 (mean; 4-11 times) in the control group (p>0.05). The numbers of primipara in the treatment and control groups were 40 and 13, respectively (p>0.05). Six patients (8.5%) in the treatment group and 1 (3.4%) in the control group had a history of unexplained intrauterine fetal death (IUFD) (p>0.05). A higher proportion of patients in the treatment group got pregnant after natural conception (n=30) than control (n=6) (p<0.05). There were no differences in the proportions of conception cycle types such as IUI with husband sperm, fresh ET, and thawed ET between the treatment group and controls.
3.2 Th1/Th2 Cell Ratios and Thrombophilia Workup
In the treatment group, the mean Th1 and Th2 cell values (mean ± SD) were 25.1 ± 2.1% and 2.1 ± 0.9%, respectively, and Th1/Th2 cell ratio was 14.6 ± 10.0, while those of the control group were 26.3 ± 7.5% and 1.7 ± 0.6%, and 16.2 ± 5.6, respectively. There were no significant differences between the treatment and control groups (p>0.05) (Table 1). In 13 out of 71 women (72.2%) in the treatment group showed factor XII deficiency, and this was comparable to that in the control group (5 out of 29; 71.4%) (p>0.05). The proportions of protein C and S deficiencies in the treatment group were 16.7% and 11.1%, respectively, without significant difference compared to those in the control group (14.3% and 14.3%, respectively) (p>0.05) (Table 1).
3.3 Reproductive Outcomes
3.3.1 Live Birth Rate: The reproductive outcomes of the treatment and control groups were summarized in Table 2. In the treatment group, three women (4.2%) had biochemical pregnancies, and the others (n=68) had clinical pregnancies, which were documented by the detection of a gestational sac (GS) using ultrasound. By contrast, the biochemical pregnancy rate of the control group was 27.6%, which was significantly higher than the treatment group (p<0.05). In the treatment group, 9 women (12.7%) lost a pregnancy before the confirmation of fetal heartbeats (6-8 weeks gestation). Fetal heartbeats were confirmed in 59 women in the treatment group, but 9 women (12.7%) miscarried after confirmation of fetal heartbeats. Finally, 50 women gave birth. The live-birth rate (LBR) of the treatment group was 70.4%. On the other hand, 13 out of 29 women in the control group gave birth (44.8%), which was significantly lower than that of the treatment group (p<0.05). When the LBR was analyzed based on the tacrolimus dosing, the tacrolimus 1 mg daily group had 52.2% (12/23) of LBR, and the 2 and ≥3 mg daily groups had 72.7% (24/33) and 93.3% (14/15) of LBRs, respectively (Table 2). The LBR of the tacrolimus 1mg group was significantly lower than that of the ≥3mg groups (p<0.05).
3.3.2 Obstetrical Outcomes: The perinatal and obstetrical outcomes of women with tacrolimus are shown in Table 3. In the treatment group, 42.0 % delivered a liveborn infant via cesarean delivery, which was similar to the control group. Preterm birth (delivery before 37 weeks of gestation) occurred in one woman (2.0%) who delivered a liveborn infant at 29 weeks of gestation due to intrauterine fetal growth restriction (IUFR). Birth weights and height of newborns (mean ± S.D) in the treatment group were 3003.6 ± 497.6 gm and 48.8 ± 3.3 cm, respectively. Apgar score at 1 and 5 minutes (mean ± S.D) were 8.3 ± 1.1 and 9.3 ± 0.8, respectively, and pH of umbilical cord gas analysis was 7.30 ± 0.7. These were comparable to those of the control group (p>0.05, respectively). One infant in the treatment group was small-for gestational (SGA) infant whose weight were 2,360 gm despite being born at 38 weeks of gestational age. Another infant was very low-birthweight (VLBW) in the treatment group, which was associated with preterm birth (29 weeks of gestational age). Due to VLBW and congenital heart anomaly (VSD), she was admitted to NICU. Otherwise, both groups had no placenta abruptio, stillbirth, and hypertensive disorder of pregnancy (HDP).
|
Treatment group |
Control |
|
|
Patients, n |
50 |
13 |
|
Age*, years |
37.2 ± 3.6 |
36.9 ± 4.0 |
|
Mode of Delivery (TV /CS), n |
29/21 |
08-May |
|
Percentages of CS, % |
42 |
|
|
Live newborns, n |
50 |
13 |
|
Still Birth, n |
0 |
0 |
|
Gestational age*, days |
271.0 ± 12.1 |
273.0 ± 13.2 |
|
Preterm delivery, n (%) |
1 |
0 |
|
Birthweight*, g |
3003.6 ± 497.6 |
3094 ± 521.2 |
|
Birth height*, cm |
48.8 ± 3.3 |
49.1 ± 3.3 |
|
SGA infant, n (%) |
1 (2.0) |
0 (0) |
|
VLBW infant, n (%) |
1 (2.0) |
0 (0 |
|
Boy/Girl, n |
24/26 |
07-Jun |
|
Apgar score (1 min)* |
8.3 ± 1.1 |
8.3 ± 1.0 |
|
Apgar score (5 min)* |
9.3 ± 0.8 |
9.4 ± 1.1 |
|
pH of cord gas analysis* |
7.30 ± 0.7 |
7.32± 0.6 |
|
NICU administration, n (%) |
1 (2.0) |
0 |
|
Obstetrical complication, n (%) |
1 (2.0) |
0 |
|
Congenital abnormalities, n |
0 |
0 |
|
*mean ± S.D., Abbreviations: TV- Transvaginal Delivery; CS- Cesarean Section; SGA- Small For Gestational Age; VLBW- Very Low-Birthweight; NICU- Neonatal Intensive Care Unit |
||
Table 3: The perinatal and obstetrical outcomes of the patients treated with tacrolimus.
4. Discussion
In women with RPL, as the number of previous spontaneous miscarriages increased, the LBR was decreased even though the prevalence of genetically normal miscarriages was increased [11]. By the time of five or more spontaneous miscarriages, a live birth rate without any treatment is lower than 50%. In other words, more than half of them would miscarry without any treatment. Several risk factors for RPL have been reported, including thyroid dysfunction, antiphospholipid syndrome, and thrombophilia, such as decreased Factor XII, low protein C or S activities [12]. Indeed, 25.0 % of the participants in this study received anti-coagulation treatment using low-dose aspirin due to thrombophilia. Ford et al. stated that 40 - 50 % of women with PRL are unexplained [13]. Since not all pregnancy losses underwent genetic study, the known etiology might include fetal chromosomal abnormalities. According to the previous report [11], the prevalence of miscarriages with a euploid pregnancy might be as high as 50% of women who experienced four spontaneous miscarriages. Hence, half of the women who miscarried a euploid pregnancy might have other underlying etiologies, such as immune etiologies. It is well-known that the conceptus is considered a semi-allograft to a mother. Transplantation of a semi-allogenic solid organ might be immunologically challenging to the recipient. However, there is no immunological rejection in the maternal-fetal interface during implantation. The mechanism of this paradoxical immune phenomenon has been explored by the sophisticated regulation of Th1, Th2, Th17, and Treg cells, in normal pregnancy [14]. Contrarily, dysregulated Th1 and Th2 cells and Th1/Th2 cell ratios have been detected in both PRL and RIF patients compared to normal pregnant women [5, 6]. Tacrolimus is an immunosuppressive agent commonly utilized to reduce the immunological rejection of solid allogenic organ transplantation, such as kidney or liver, in transplant recipients [15, 16]. Tacrolimus, a macrolide antibiotic calcineurin inhibitor, inhibits calcineurin action through binding to FKBP12 in lymphocyte [17]. Consequently, T cell activation is blocked, and cytokine production is reduced, including INF-ϒ, IL-2, IL-4, and TNF-α. Previously, we reported that tacrolimus could improve the clinical pregnancy rate among the RIF patients with elevated Th1/Th2 cell ratios [6]. The increased Th1/Th2 cell ratio can be two reasons: the high level of Th1 cell and low level of Th2 cell. The RIF patients with elevated Th1/Th2 cell ratios usually showed a high level of Th1 cells, immunologically reacting against the transferred embryos. In our study, intracellular INF-ϒ+/ IL-4- CD4+ Th1 cells were defined as Th1 cells. Therefore, tacrolimus might inhibit the secretion of INF-ϒ from the T cells and improve embryonic implantation. Previous reports indicated that the RPL patients showed higher Th1 cells, such as INF-ϒ or TNF-α secreting Th cells, resulting in elevated Th1/Th2 cell ratios [5]. Higher Th1 cell levels indicate an increase of INF-ϒ and TNF-α secretion from the Th cells, which might interfere with placental development. Hence, a similar immune mechanism underlines implantation failure and PRL. The immune effector can be regulated by the interaction between uterine extravillous trophoblasts and immune cells, including T cells, and the dysregulation of immune effectors can lead to adverse results, such as spontaneous miscarriage. The tacrolimus might modulate dysregulated T cells by inhibiting the release of inflammatory cytokines, such as INF-ϒ and TNF-α. According to the previous report [11], the LBR among the RPL patients who experienced 4-6 spontaneous miscarries was 56.6% (175/309). In this paper, the miscarriage rate in the control group was 55.2%, which was comparable to the previous report. The RPL patients in the previous study did not receive any medical treatments before and after the establishment of pregnancy even though they experienced more than four times of miscarriages. By contrast, the RPL patients with elevated Th1/Th2 cell ratios in the treatment group of this study received tacrolimus like our previous study of the RIF patients with similar immunological conditions. As a result of this intervention, 70.4 % of the women gave birth (LBR=70.4%), and this was statistically higher than that of the controls (44.8%, p<0.05). Hence, the intervention of underlying immunopathology improved the outcome of patients with the evidence of immunopathology. Moreover, we evaluated the relationship between the LBR and the tacrolimus dosage in this study. The LBR in the 1mg daily dose group (52.2%) was significantly lower than that of the ≥3mg daily group (93.3%, p<0.05). This result showed a similar trend to our previous report, which indicated that the ongoing pregnancy rate of 1mg daily dose group for the RIF patients was lower than that of the 3mg daily dose group.18 We report that 1mg daily dose of tacrolimus might not be adequate for the maintenance of pregnancy, and higher dosages are needed for women with RPL and Th1/Th2 cell ratio between 10.3 and 13.0. In conclusion, the immunosuppressive agent, tacrolimus, improved the live-birth rate of women who experienced four or more RPL with elevated Th1/Th2 cell ratios than those without tacrolimus treatment. In women with unexplained RPL, immunological evaluation including Th1/Th2 cell proportions and ratios should be considered. If Th1/Th2 cell-related immune etiologies are defined, tacrolimus treatment can be considered. Currently, the guideline about COVID-19 and immunomodulation treatment for women with PRL and RIF was published [19]. The low dose tacrolimus treatment can be utilized in women with RPL and elevated Th1/Th2 cell ratios during the COVID-19 pandemic with careful follow-up. This study investigated the clinical efficacy of tacrolimus, a T cell activation inhibitor, for women who experienced consecutive PRLs (≥4) with elevated Th1/Th2 cell ratios. However, the study was limited since it was an open-label, prospective cohort study but not a randomized trial. Another limitation was that the number of subjected is still small. A further study is needed on larger scale to confirm these findings.
Acknowledgements
The authors would like to thank Sayaka Kitagawa (Sugiyama Clinic Shinjuku, Tokyo) and Noriko Nakayama (National Center for Child Health and Development Tokyo) for their help with data analysis.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Koji Nakagawa, Keiji Kuroda, Takashi Horikawa, Satoru Takamizawa, Michi Hisano, Yoshimitsu Kasahara, Rikikazu Sugiyama and Koushi Yamaguchi. The first draft of the manuscript was written by Koji Nakagawa and Joanne Kwak-Kim and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Statements and Declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Human Rights Statements and informed Consent
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, it was approved by the institutional review board of Sugiyama Clinic (2020-002) and Child Health and Development (2020-043). All patients signed an informed written consent form before entering the study, and they were informed that they could terminate their cooperation with us whenever they wanted without any consequences.
Informed Consent
Informed consent was obtained from all individual participants included in the study. The authors affirm that human research participants provided informed consent for publication of the images in Figure 1, Table 1, 2, and 3.
References
- Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol 7 (1953): 320-337.
- Wegmann TG. Bidirectional cytokine interaction in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 14 (1993): 353-356.
- Makhseed M, Raghupathy R, Azizieh F. Mitogen-induced cytokines responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am J Reprod Immunol 42 (1999): 273-281.
- Ng SC, Gilman-Schs A, Thaker P. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortions, implantation failures after IVF/ET or normal pregnancy. Am J Reprod Immunol 48 (2002): 77-86.
- Kwak-Kim JY, Chung-Bang HS, Ng SC, et al. Increased T cell 1 cytokines responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod 18 (2003): 767-773.
- Nakagawa K, Kwak-Kim J, Ota K, et al. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood Th1/Th2 cell ratios. Am J Reprod Immunol 73 (2015): 353-361.
- Nakagawa K, Kwak-Kim J, Hisano M, et al. Obstetric and perinatal outcome of the women with repeated implantation failures or recurrent pregnancy losses who received pre- and post-conception tacrolimus treatment. Am J Reprod Immunol 82 (2019): e13142.
- Nakagawa K, Kuroda K Sugiyama R. After 12 consecutive miscarriages, a patient received immunosuppressive treatment and delivered an intact baby. Reprod Med Biol 16 (2017): 297-310.
- Nakagawa K, Kaneyama M, Nishi Y, et al. Clomiphene citrate affects the receptivity of the uterine endometrium. Reprod Med Biol 14 (2015): 73-78.
- Nakagawa K, Takahashi C, Nishi Y, et al. Hyaluronan-enriched transfer medium improves outcome in patients with multiple embryo transfer failures. J Assist Reprod Genet 29 (2012): 679-685.
- Ogasawara M, Aoki K, Okada S. Embryonic karyotype of abortuses in relation to the number of previous miscarriage. Fertil Steril 73 (2000): 300-304.
- Saito S, Sugiura M, Tanaka T, et al. Study on risk factor of the infertility in this country and prognosis. J Jpn Soc of Perin Neon Med 45 (2009): 1144-1148.
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2 (2009): 76-83.
- Wang W, Sung N, Gilman-Sachs A, et al. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front Immunol 11 (2020): 2025.
- Abou-Jaoude MM, Najm R, Shaheen J, et al. Tacrolimus (FK506) versus cyclosporin microemulsion (Neoral) as maintenance immunosuppression therapy in kidney transplant recipients. Transpl Proc 37 (2005): 3025-3028.
- Rath T. Tacrolimus in transplant rejection. Expert Opin Pharmacother14 (2013): 115-122.
- Liu J, Farmer JD Jr, Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66 (1991): 807-815.
- Nakagawa K, Kwak-Kim J, Kuroda K, et al. Immunosuppressive treatment using tacrolimus promotes pregnancy outcome in infertile women with repeated implantation failures. Am J Reprod Immunol 78 (2017).
- Kwak-Kim J, Ota K, Sung N, et al. COVID-19 and immunomodulation treatment for women with reproductive failures. J Reprod Immunol 141 (2020).


 Impact Factor: * 3.4
Impact Factor: * 3.4 Acceptance Rate: 78.89%
Acceptance Rate: 78.89%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 