The Safety and Efficacy of Ultrasound-Accelerated-Catheter-Directedthrombolysis with Urokinase in Patients with Intermediate-High Risk Pulmonary Embolism
Hani Al-Terki1*, Tobias Paulus2, Michael Gotzmann1, Andreas Mügge1
1University Hospital St. Josef-Hospital Bochum, Cardiology and Rhythmology, Ruhr University Bochum, Bochum, Germany
2University Hospital St. Josef-Hospital Bochum, Department of Interventional radiology, Ruhr University Bochum, Bochum, Germany
*Corresponding author: Hani Al-Terki, University Hospital St. Josef-Hospital Bochum, Cardiology and Rhythmology, Ruhr University Bochum, Bochum, Germany.
Received: 17 Febraury 2023; Accepted: 01 March 2023; Published: 17 March 2023
Article Information
Citation: Hani Al-Terki, Tobias Paulus, Michael Gotzmann, Andreas Mügge. The Safety and Efficacy of Ultrasound-Accelerated-Catheter- Directed-thrombolysis with Urokinase in Patients with Intermediate-High Risk Pulmonary Embolism. Cardiology and Cardiovascular Medicine. 7 (2023): 79-85.
View / Download Pdf Share at FacebookAbstract
Background: Ultrasound-accelerated-thrombolysis (USAT) represent a safe and effective therapeutic modality in patients with intermediate-tohigh risk pulmonary embolism. The tested thrombolytic agent in all studies investigating USAT in PE patients is alteplase (Actilyse). Actually, there is a lack of supply of alteplase (alteplase®, Boehringer Ingelheim) in Europe.
Methods: Nine patients with intermediate-to-high risk pulmonary embolism were treated with USAT and urokinase (300,000 units per catheter over 10 hours) at our institution. Systolic pulmonary artery pressure, right ventricle function and right ventricle/left ventricle ratio were measured at baseline and after therapy. The primary outcome was the difference in the RV/LV ratio from baseline to 24 hours. Safety outcomes included in-hospital death, major and minor bleeding according to GUSTO bleeding score.
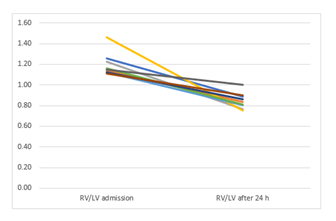
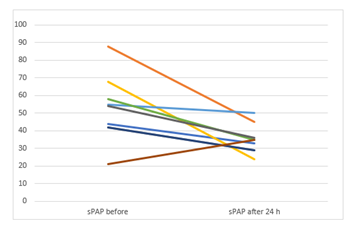
Results: The mean RV/LV ratio was reduced from 1.19±0.11 at baseline to 0.75±0.29 at 24 hours (p=0.003); the systolic pulmonary artery pressure decreased from 52.4±18.7 to 35.11±8.0 mmHg (p=0.021). There was 1 major access-site bleeding and no in-hospital deaths. During a follow-up of 117.8±72.5 days, no recurrent venous embolism occurred.
Conclusion: USAT with Urokinase seems to be safe and effective in patients with intermediate-to-high risk pulmonary embolism with favourable clinical, echocardiographic and laboratory results.
Keywords
<p>Catheter-Directed-Thrombolysis; Pulmonary Embolism (PE); Right Ventricle; Urokinase</p>
Article Details
Abbreviations:
CDT- Catheter-Directed-Thrombolysis; dPAP- Diastolic Pulmonary Artery Pressure; ESC- European Society of Cardiology; LVEDD- Left Ventricular End-Diastolic Diameter; mPAP- Mean Pulmonary Artery Pressure; PA- Pulmonary Artery; PE- Pulmonary Embolism; rt-PA- Recombinant Tissue-Type Plasminogen Activator; RV- Right Ventricle; RVEDD- Right Ventricular End-Diastolic Diameter; sPAP- Systolic Pulmonary Artery Pressure; TTE- Transthoracic Echocardiography; TAPSE- Tricuspid Annular Plane Systolic Excursion; USAT- Ultrasound-Accelerated-Thrombolysis; UK- Urokinase
1. Introduction
Pulmonary embolism (PE) is the third most common cardiovascular disease, after myocardial infarction and stroke with an annual incidence of 39 to 115 per 100,000 individuals yearly. The 2019 European Society of Cardiology (ESC) guidelines classified PE into 4 groups: high-risk, intermediate-to-high risk, intermediate-to-low risk and low risk. Intermediate-to-high risk PE is defined as the presence of both elevated cardiac laboratory biomarkers and right ventricular (RV) dysfunction without systemic hypotension [1]. In intermediate-to-high risk patients, ultrasound-accelerated thrombolysis (USAT) may be an appropriate therapeutic modality with superiority to anticoagulation alone in terms of improving RV-strain [2,3]. The thrombolytic substance used for USAT is recombinant tissue-type plasminogen activator (rt-PA). The optimal dosage and duration of rt-PA delivery has been investigated by the OPTALYSE trial [4]. Currently, there is lack of supply of rt-PA (alteplase®, Boehringer Ingelheim) in Europe. An alternative therapeutic approach may be the use of Urokinase (UK). A previous study suggests that the systemic application of UK in patients with intermediate-to-high risk PE is non-superior to the systemic application of rt-PA [5]. Furthermore, the local intrapulmonary artery infusion of UK may be a safe and efficient treatment modality with significant and rapid resolution of PE [6]. Data about the use of USAT with UK in PE patients does not exist. The current study aimed to investigate the clinical efficacy and safety of USAT with low-dose UK in patients with intermediate-to-high-risk PE.
2. Methods
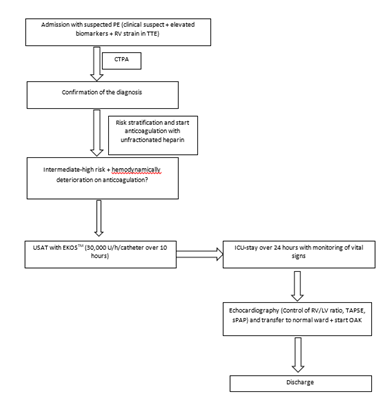
Between August 2022 and February 2023, Nine patients with intermediate-to-high-risk PE received USAT using the EKOSTM Endovascular System at St. Josef Hospital Bochum and were included in this retrospective registry study. The confirmation of the diagnosis in all patients was based on contrast-enhanced-computer tomography. The evaluation of the RV function/strain was carried out with transthoracic echocardiography (TTE) and by measuring cardiac biomarkers (troponin T and NTproBNP). All PE patients were classified according to the ESC guidelines as low, intermediate-to-low-, intermediate-to-high- or high-risk PE. High-risk PE is characterized by overt hemodynamic instability, which makes systemic thrombolytic necessary. Intermediate-to-low-risk PE is defined as either echocardiographic-confirmed RV dysfunction or elevated troponin levels without hemodynamic instability. Low-risk-PE is defined as the absence of RV-strain or elevated laboratory parameters in hemodynamic stable patients. Intermediate-to-high- risk PE is defined as the presence of both right ventricular (RV) dysfunction and elevated cardiac laboratory biomarkers, without systemic hypotension. Patients with intermediate-to-high-risk PE were included in this study and were suitable candidates for USAT with EKOSTM, which was done in the cardiac catheterization lab within 24 hours after admission. The EKOSTM system involved positioning one (for unilateral PE) or two pulmonary arterial infusion catheters, one into each main pulmonary artery (PA), under fluoroscopic guidance via percutaneous transvenous access. The procedure was performed with continuous haemodynamic and electrocardiogram (ECG) monitoring. Over 10 hours, 300,000 Units of UK were continuously administered, with a rate of 30,000 Units/h/catheter. Heparin was administered concomitantly, with doses determined using a hospital-defined nomogram that protocolizes dosing to target partial thromboplastin of 60 seconds. After 10 hours, the system was stopped, and the infusion catheters were removed, followed by compression of the femoral vein for four hours. After USAT with UK, all patients received unfractionated heparin for further two hours and were then switched to oral anticoagulants and transferred to a normal ward. Twenty-four hours after therapy, TTE was conducted to measure RV function (tricuspid annular plane systolic excursion (TAPSE)), systemic pulmonary artery pressure (sPAP), RV end-diastolic diameter (RVEDd) and the RV/LV ratio. In addition, creatinine, troponin T and NTproBNP were monitored 24 hours after the therapy. Vital parameters (respiratory rate, heart rate and blood pressure) were regularly monitored from admission to transfer to the normal ward.
PE- Pulmonary Embolism; TTE- Transthoracic Echocardiography; ICU- Intensive Care Unit; OAK- Oral Anticoagulation; TAPSE- Tricuspid Annular Plane Systolic Excursion; sPAP- Systolic Pulmonary Artery; USAT- Ultrasound-Accelerated-Thrombolysis.
2.1 Efficacy Measures
Compared to baseline, the surrogates for USAT efficacy were the drop of sPAP, the improvement of RV-function and RV/LV ratio measured by TTE.
2.2 Safety Measures
Safety outcomes included minor and major bleeding according to the Global Use of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO score), all-cause- in-hospital mortality.
2.3 EKOSTM System
EKOSTM (Boston Scientific, Marlborough, MA) is an approach combining low-power ultrasound energy with low-dose thrombolytic agents to achieve clot dissolution. Ultrasound uses cavitation-induced microstreaming, which loosens the fibrin strands within the clot, increasing its surface area. In addition, ultrasound enhances the transport of the thrombolytic agent across the clot and makes it more permeable [7,8]. The EKOSTM catheters were placed into the pulmonary arteries in the cath-lab. After ultrasound-guided puncture of femoral vein, a 6-French sheath was used to introduce the EKOS-catheter into the pulmonary arteries under continuous hemodynamic and electrocardiographic monitoring and fluoroscopic guidance.
2.4 Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables as percentages. All statistical analysis was performed using SPSS Version 25.0 for Windows. The comparison between the variables was performed with 2-tailed t-test. A p-value < 0,05 was considered as statistically relevant.
2.5 Follow-up
The patients were contacted via their physicians to ask about recurrent venous thromboembolism or any bleeding complication related to the procedure.
3. Results
3.1 Study Population
Nine patients with intermediate-high-risk PE who underwent USAT with EKOSTM were included in this study and retrospectively analysed. Four of them were women (44%). The mean age was 75.3 years (SD: 8.0), and the mean body mass index (BMI) was 28.9 kg/m2 (SD: 4.46). Three patients (33%) had chronic heart failure, 7 (77 %) had arterial hypertension and 3 (33%) had diabetes mellitus. Four (44%) had been previously diagnosed with PE. Three subjects (33%) had an immobility as a trigger. One patient (11%) had postoperative PE. Baseline characteristics are summarized in Table 1.
|
Patient characteristics |
N=9 |
|
|
Baseline Characteristics |
Age (years), mean (SD) |
75.3 (8.0) |
|
Sex (female), n (%) |
4 (44%) |
|
|
BMI (kg/m2), mean (SD) |
28.9 (4.46) |
|
|
Comorbidities |
Systemic Hypertension, n (%) |
7 (77%) |
|
Diabetes mellitus, n (%) |
3 (33%) |
|
|
Chronic heart failure, n (%) |
3 (33%) |
|
|
Coronary artery disease, n (%) |
3 (33%) |
|
|
Chronic lung disease |
2 (22%) |
|
|
Cancer, n (%) |
0 (0%) |
|
|
Previous PE, n (%) |
4 (44%) |
|
|
Immobility, n (%) |
3 (33%) |
|
|
COVID, n (%) |
1 (11%) |
Table 1: Baseline characteristic. BMI- Body Mass Index; PE- Pulmonary Embolism.
3.2 Initial Presentation, Clinical and Echocardiographic Parameters at Admission
The most common primary complaint was dyspnoea (7 patients, 77%). One patient presented a syncope 24 hours after cholecystectomy. One patient presented with angina pectoris. The median heart rate at admission was 96.3 bpm (SD: 11.4), the median systolic blood pressure was 127.5 mmHg (SD: 19.28), and the median respiratory rate was 24.4 per minute (SD: 6). The initial RV/LV ratio measured by TTE was 1.19 (SD: 0.11). The median RV/LV ratio measured by CT was 1.22 (SD: 0.12). The mean sPAP was 52.4 mmHg (SD 18.7). The mean TAPSE was at 14.7 mm (SD: 3.2). The median initial creatinine value was 1.15 (SD: 0.16), the mean initial NTproBNP was 7256.33 pg/ml (SD: 5762) (normal value < 125 pg/ml). Table 2 summarizes the clinical, chemical laboratory and echocardiographic parameters at admission.
|
Patient characteristics |
N=9 |
|
|
Initial presentation |
Dyspnoea, n (%) |
7 (77%) |
|
Syncope, n (% |
1 (11%) |
|
|
CPR, n (%) |
0 (0%) |
|
|
Angina pectoris, n (%) |
1 (11%) |
|
|
Clinical parameter at admission |
Heart rate (bpm), mean (SD) |
96.3 (11.4) |
|
Systolic pressure (mmHg), mean (SD) |
127.5 (19.28) |
|
|
Respiratory rate, mean (SD) |
24.4 (6) |
|
|
Oxygen saturation, mean (SD) |
91.4% (4.1) |
|
|
Echocardiographic parameter at admission |
RVEDD (mm), mean (SD) |
46.57 (6.39) |
|
LVEDD (mm), mean (SD) |
39.33 (5.8) |
|
|
sPAP (mmHg), mean (SD) |
52.4 (18.7) |
|
|
TAPSE (mm), mean (SD) |
14.7 (3.2) |
|
|
RV/LV ratio, mean (SD) |
1.19 (0.11) |
|
|
Laboratory-chemical values |
Creatinine (mg/dl), mean (SD) |
1.15 (0.16) |
|
NTproBNP (pg/ml), mean (SD) |
7526.33 (5762) |
|
|
Troponin T (pg/ml), mean (SD) |
0.07 (0.05) |
|
|
Lactat (mg/dl), mean (SD) |
2.83 (2.84) |
|
|
Invasive hemodynamic measurements |
Right sPAP (mmHg), mean (SD) |
58.7 (12.8) |
|
Right dPAP (mmHg), mean (SD) |
24.0 (12.3) |
|
|
Right mPAP (mmHg), mean (SD) |
36.8 (10.4) |
|
|
Left sPAP (mmHg), mean (SD) |
56.8 (15.8) |
|
|
Left dPAP (mmHg), mean (SD) |
27.28 (12.18) |
|
|
Left mPAP (mmHg), mean (SD) |
36 (11.04) |
Table 2: clinical, chemical laboratory and echocardiographic parameters at admission. CPR- Cardiopulmonary Resuscitation; RVEDD- Right Ventricle End-Diastolic Diameter; LVEDD- Left Ventricle End-Diastolic Diameter; sPAP- Systolic Pulmonary Artery Pressure; dPAP- Diastolic Pulmonary Artery Pressure; mPAP- Mean Pulmonary Artery Pressure; TAPSE- Tricuspid Annular Plane Systolic Excursion.
3.3 Procedural Data
One patient had right-sided PE and only one EKOSTM catheter was placed into the right pulmonary artery. The remaining eight patients had bilateral PE and two catheters were placed in both pulmonary arteries. The median procedural time was 25.7 min (SD: 11.0), and the mean fluoroscopy time was 7.4 min (SD: 1.6). Table 3 summarizes the procedural data and the post-USAT stay. 25.75 ±11.059
|
N=9 |
||
|
Procedural data |
Procedural time (min), median (SD) |
25.7 (11.0) |
|
Contrast agent (ml), median (SD) |
4.4 ml (6.2) |
|
|
Fluoroscopy dose (Gy), median (SD) |
935.6 cGY/cm2 (418) |
|
|
Fluoroscopy time (min), median (SD) |
7.4 Min (1.6) |
|
|
Post-procedural stay |
ICU-Stay (days), median (SD) |
1.4 (0.72) |
|
Total-Stay (days), median (SD) |
5.8 (1.3) |
Table 3: The procedural data and postinterventional stay. ICU- Intensive Care Unit.
3.4 Postprocedural Data (Clinical Parameters, Echocardiography and Laboratory)
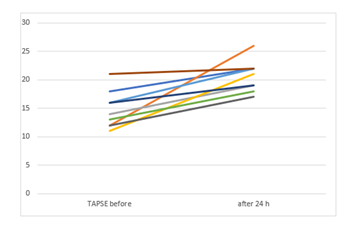
The RV/LV ratio, sPAP and TAPSE decreased significantly after the therapy. NTproBNP and creatinine also significantly decreased. Table 4 summarizes the mean values before and 24 hours after therapy.
|
Before USAT |
after USAT |
Mean absolute change, 95% CI |
t |
p-value |
|
|
Heart rate (bpm) |
96.33±11.42 |
80.33±10.6 |
16.0 (7.7-24.3) |
4.45 |
0.002 |
|
Respiratory rate |
24.44±6.0 |
19±4.1 |
5.4 (1.81-9.0) |
3.46 |
0.009 |
|
Systolic blood pressure (mmHg) |
127.6±19.3 |
132.6±19.5 |
-5.1 (-23.8-13.6) |
-0.62 |
0.54 |
|
Diastolic blood pressure (mmHg) |
81.6±12.3 |
75.44±11.9 |
6.11 (-6.1-18.4) |
1.14 |
0.28 |
|
TAPSE (mm) |
14.78±3.3 |
20.7±2.7 |
-5.8(-8.8 to -2.9) |
4.54 |
0.002 |
|
sPAP (mmHg) |
52.44±18.8 |
35.11±8.0 |
17.33(3.43-31.2) |
2.87 |
0.02 |
|
RVEDD (mm) |
46.57±6.4 |
38.14±4.14 |
8.43 (4.6-12.2) |
5.41 |
0.002 |
|
RV/LV ratio |
1.19±0.11 |
0,75±0.29 |
0.44(0.19-0.68) |
4.12 |
0.003 |
|
NTproBNP (pg/ml) |
7526±5762 |
5164±4669 |
2362(898.5-3826) |
3.7 |
0.006 |
|
Creatinine (mg/dl) |
1.15±0.26 |
0.99±0.25 |
0.15(0.07-0.24) |
4.3 |
0.003 |
Table 4: The mean values before and after the therapy. USAT- Ultrasound-Accelerated-Thrombolysis; CI- Confidence Interval; TAPSE- Tricuspid Annular Plane Systolic Excursion; sPAP- Systolic Pulmonary Artery Pressure; RVEDD- Right Ventricle End-Diastolic Diameter.
3.5 Complications
There was no device failure in our study. No in-hospital deaths occurred. One patient had severe access-related bleeding, which made blood-transfusion of 3 units necessary. This patient had massive thrombus load in the femoral vein on both sides, so that the punction had to be performed very high, making the compression of the puncture very difficult.
3.6 Follow-up
During a follow-up time of 117.8±72.5 days, there were no recurrent venous thromboembolism or any complication related to the procedure. All patients were on oral anticoagulation. No deaths occurred.
4. Discussion
The main finding of the current study is that USAT with UK represents an effective therapeutic modality in patients with intermediate-to-high risk PE with favourable hemodynamic, clinical and laboratory outcomes. PE is the third leading cause of cardiovascular mortality [1]. Normotensive Patients with large PE and RV dysfunction are classified as intermediate-to-high risk and are at high risk of in-hospital and latent mortality [9]. PEITHO Trial aimed to investigate the effectiveness and safety of systemic fibrinolysis in this group [10]. The study compared the use of anticoagulation and systemic fibrinolysis with anticoagulation alone in 1005 patients. Systemic fibrinolysis significantly reduced death and hemodynamic decompensation by 56%, albeit at the expense of a four-fold increase in the rate of severe extracranial bleeding (6.3% vs. 1.2%) and a 12-fold increase in the rate of intracranial bleeding haemorrhagic strokes (2.4% vs. 0.2%). As a consequence, the ESC guidelines do not recommend the routine use of systemic thrombolysis for treating these patients. Since the Therapy with heparin alone can often take more than 3 weeks to completely resolute major PE [11], a more aggressive approach such as catheter-directed-thrombolysis (CDT) has been recommended in such entities [1]. USAT with the EKOSTM system is one of the CDT systems, which combines local delivery of thrombolytic substances together with ultrasound, which increases the permeability of the thrombus to thrombolytic drug [3]. In clinical studies, USAT leads to an improvement in RV-function, RV/LV ratio and to a decrease in sPAP in PE patients, with a low rate of procedural complications [2,3,12,13]. Of note, all these studies were performed using thrombolysis with rt-PA. The effectiveness of other thrombolytics in combination with USAT in PE patients has not been investigated. Ouriel K et.al. compared the efficacy of different thrombolytic agents in an in vitro venous model and reported that streptokinase was the agent associated with the slowest rate of clot lysis. Urokinase was associated with an intermediate rate of clot lysis, but appeared to be the agent with the greatest degree of fibrinolytic specificity. Although rt-PA was associated with improved efficacy early in the perfusion, the differences between rt-PA and UK dissipated after 30 min [14]. The study concluded, that UK may be the most beneficial thrombolytic agent. Since the efficacy rates of UK in the Pilot trial were equally effective to rt-PA [14], we thought that the use of UK with USAT in intermediate-to-high risk PE patiens may be an alternative to the use of rt-PA in the time of its supply bottleneck. Our study demonstrates, that the use of low-dose UK combined with USAT significantly improved sPAP, RV/LV ratio and TAPSE in all patients with intermediate-to-high risk PE 24 hours after the therapy. The improvement of RV/LV ratio from baseline to 24 hours (0.44±0,32) exceeds the difference of RV/LV ratio in the Ultima trial (0.30±0.20) [3] and exceeds the postinterventional mean difference of RV/LV ratio in all four arms of the OPTALYSE Trial [4]. USAT with UK significantly reduced the heart rate and the respiratory rate, but did not significantly affect the blood pressure in our study. This could be due the fact, that all our patients were hemodynamically stable. It remains unclear, if USAT combined with UK could be beneficial in unstable PE patients.
One severe bleeding (according to GUSTO score) occurred. This was due to very high puncture and subsequently difficult compression of the puncture site. We think, that this complication does not correlate with USAT or UK. Nevertheless, our sample size is too small to draw firm conclusions about the safety of USAT with UK in comparison to USAT with rt-PA. One patient with postoperative PE was treated in our study with USAT and UK. Postoperative PE is a serious condition with high mortality [15]. Its management remains challenging, since the use of systemic thrombolysis is here contraindicated due to high bleeding risk. According to our experience, we think that USAT combined with UK could be an alternative therapeutic approach, although safety data are lacking.
5. Conclusion
USAT with UK (300,000 U per catheter over 10 hours) seems to be an effective and safe treatment for patients with intermediate-to-high-risk PE and can improve clinical and echocardiographic parameters.
6. Limitations
The main limitation of our study is the small sample size and the lack of a control group, randomization and echocardiographic follow-up. Large randomized studies with long-term follow-up are needed to validate our findings and to determine the optimum dosage needed to achieve the best outcomes.
References
- Konstantinidis S, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European Heart Journal, Volume 41 (2020): 543-603.
- Piazza G, Hohlfelder B, Jaff M, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC cardiovascular intervention 8 (2015): 1382-1392.
- Kucher N, Boekstegers P, Müller O, et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 129 (2014): 479-486.
- Tapson V, Sterling K, Jones N, et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC cardiovascular Intervention 11 (2018): 1401-1410.
- Zhao T, Ni J, Hu X, et al. The Efficacy and Safety of Intermittent Low-Dose Urokinase Thrombolysis for the Treatment of Senile Acute Intermediate-High-Risk Pulmonary Embolism: A Pilot Trial. Clinical and applied thrombosis and hemostasis 24 (2018): 1067-1072.
- McCotter CJ, Chiang KS, Fearrington EL. Intrapulmonary artery infusion of urokinase for treatment of massive pulmonary embolism: a review of 26 patients with and without contraindications to systemic thrombolytic therapy. Clin Cardiol 22 (1999): 661-664.
- Owens CA. Ultrasound-enhanced thrombolysis: EKOS EndoWave infusion catheter system. Semin Intervent Radiol 25 (2008): 37-41.
- Kim J, Lindsey BD, Chang WY. Intravascular forward-looking ultrasound transducers for microbubble-mediated sonothrombolysis. Sci Rep 7 (2017): 3454.
- Grifoni S, Olivotto I, Cecchini P, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 101 (2000): 2817-2822.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. N Engl J Med 370 (2014): 1402-1141.
- Konstantinides S, Tiede N, Geibel A, et.al. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardial 82 (1998): 966-970.
- Kuo W, Banerjee A, Kim P, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest 148 (2015): 667-673.
- Ouriel K, Welch EL, Shortell CK, et al. Comparison of streptokinase. urokinase, and recombinant tissue plasminogen activator in an in vitro model of venous thronibolysis. J VascSurg 22 (1995): 593-597.
- Zhao T, Ni J, Hu X, et al. The Efficacy and Safety of Intermittent Low-Dose Urokinase Thrombolysis for the Treatment of Senile Acute Intermediate-High-Risk Pulmonary Embolism: A Pilot Trial. Clinical and applied thrombosis and hemostasis 24 (2018): 1067-1072.
- Hall D. Perioperative Pulmonary Embolism. American Journal of Therapeutics 20 (2013): 67-72.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 