The Safety of Heparin-Free Strategy in Patients Supported by Venoarterial Extracorporeal Membrane Oxygenation
Dong Jung Kim1, Jae Hang Lee1, Jun Sung Kim1, Cheong Lim1, Kay-Hyun Park1, Hyoung Woo Chang1*
1Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Gyeonggi-do, Republic of Korea.
Corresponding author:Hyoung Woo Chang, MD, PhD. Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, Seoul National University Bundang Hospital, 82, Gumi-ro 173beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do, 13620, Republic of Korea.
Received: July 25, 2023; Accepted: August 01, 2023; Published: August 07, 2023
Article Information
Citation: Dong Jung Kim, Jae Hang Lee, Jun Sung Kim, Cheong Lim, Kay-Hyun Park, Hyoung Woo Chang. The safety of heparin-free strategy in patients supported by venoarterial extracorporeal membrane oxygenation. Cardiology and Cardiovascular Medicine. 7 (2023): 205-301.
View / Download Pdf Share at FacebookAbstract
Background: The necessity of heparinization during venoarterial extracorporeal membrane oxygenation (VA-ECMO) is well documented. However, heparinization can increase the risk of bleeding in certain situations. The aim of this study was to investigate the safety of a heparinfree strategy in patients on VA-ECMO.
Methods: Data for 90 adult patients on VA-ECMO, wherein cannulation and maintenance were performed by cardiothoracic surgeons and support was provided for >24 h (2018–2021), were retrospectively reviewed. Patients were divided into two groups: heparin-free group, without heparinization for ≥ 24 h during VA-ECMO support (n = 66), and control group (n = 24). Clinical outcomes including hemorrhagic and thromboembolic complications were compared between the two groups.
Results: The reasons for VA-ECMO support included post-cardiotomy cardiogenic shock in 37 patients (41.1%), and extracorporeal cardiopulmonary resuscitation in 44 patients (48.9%). The total duration of VA-ECMO was not significantly different between the two groups (132.3±106.1 vs. 141.6±117.9 h, P=0.734). In the heparin-free group, the duration of VA-ECMO without heparinization was 79.8±60.7 h, and 26 patients (39.4%) were completely heparin-free during the support period. No significant difference was found in the frequency of oxygenator changes due to thrombosis between the two groups (8.3 vs. 10.6%, P>0.999). Pump malfunction was not observed in any group. The overall incidence of thromboembolic complications was not significantly different between the two groups.
Conclusion: No additional risk of thromboembolic complications was observed with the use of a heparin-free strategy during VA-ECMO support. Appropriate discontinuation of heparinization could be a safe strategy for VA-ECMO patients with active bleeding or a high hemorrhagic risk.
Keywords
Extracorporeal Membrane Oxygenation; Heparin; Anticoagulants
Extracorporeal Membrane Oxygenation articles; Heparin articles; Anticoagulants articles
Article Details
The importance of continuous heparin infusion during venoarterial extracorporeal membrane oxygenation (VA-ECMO) support is well established. In most cases, adequate anticoagulation with heparin is mandatory during VA-ECMO support. However, heparinization can increase the risk of bleeding in certain cases, such as patients who have undergone open cardiac surgery. At our institution, heparinization is liberally discontinued during VA-ECMO support after consideration of the risks and benefits of anticoagulation. On the basis of our experience with VA- ECMO in post-cardiotomy patients, we believe that the unconditional use of heparin is not appropriate, regardless of the patient’s bleeding status. We applied the same heparin-free strategy for patients undergoing extracorporeal cardiopulmonary resuscitation (ECPR) because they had a high risk of bleeding due to undetected chest wall trauma and, intrathoracic or intraabdominal hemorrhage. The aim of this study was to determine the safety of this heparin-free strategy in patients on VA-ECMO.
Materials and Methods
Study population and outcome measures
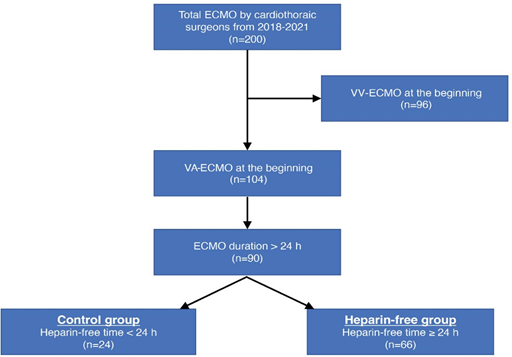
Between January 2018 and December 2021, there were 200 adult cases of ECMO wherein cannulation and maintenance were performed by cardiothoracic surgeons at our institution. From these patients, 96 cases of venovenous- ECMO were excluded. From the 104 patients undergoing VA- ECMO support, 14 patients with an ECMO duration of ≤24 h were excluded. Finally, a total of 90 adult VA-ECMO cases were included, and their medical records were retrospectively reviewed (Figure 1). Demographic and ECMO-related data (reasons for ECMO insertion, support type according to access site, location of cannulation, type of circuit, duration, flow, and successful weaning rate), heparinization (bolus injection during cannulation and continuous infusion during ECMO support), clinical outcomes (intensive care unit [ICU] stay, hospital stay, and survival to discharge), and complications (hemorrhagic and thromboembolic) were recorded. Patients were divided into two groups: heparin-free group, with no heparin infusion for ≥24 h during support, and control group. Clinical outcomes including hemorrhagic and thromboembolic complications were compared between the two groups. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB approval no. B-2305-827-107).
Anticoagulation strategy during VA-ECMO support
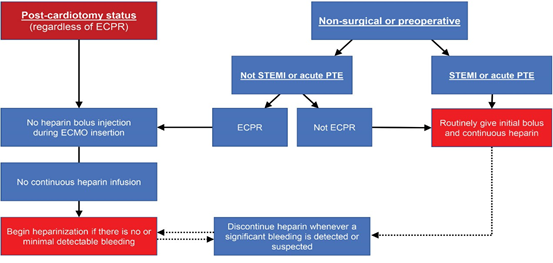
In cases of post-cardiotomy ECMO, wherein cannulation was performed in the operating room (OR), heparinization was not routinely initiated immediately after transfer to the ICU. Continuous infusion of heparin was initiated only after it was confirmed that there was no evidence or possibility of postoperative bleeding. Heparin was not used during VA- ECMO support in patients with active bleeding immediately after surgery. The same strategy was applied to cases of post- cardiotomy cardiogenic shock in the ICU. For patients who required VA-ECMO support preoperatively or due to non- surgical reasons, the use of heparin was based on the reason for ECMO insertion (ECPR or not). If ECPR was the reason for ECMO insertion, bolus heparin was not administered during ECMO cannulation. Moreover, continuous infusion was not started before any possibility of major bleeding was ruled out. In non-ECPR situations, bolus as well as continuous heparin infusions were routinely implemented. Heparinization was also routinely initiated in patients with conditions requiring anticoagulation, such as ST-elevation myocardial infarction (STEMI) and acute pulmonary thromboembolism (PTE) (Figure 2).
VA-ECMO indications, circuit, cannulation, management, and weaning
Indications for VA-ECMO included failure to wean from cardiopulmonary bypass (CPB) in the OR, refractory low cardiac output syndrome despite adequate inotropic support in the ICU, and recurrent cardiac arrest due to reversible causes such as STEMI and acute PTE in the emergency room and catheterization laboratory. Two types of ECMO circuits (GETINGE PLS® and CAPIOX EBS®) were used depending on the availability of equipment. Although cannulation was performed peripherally through the femoral artery and vein in principle, in the OR, some cases were centrally cannulated in the right atrium and the ascending aorta at the operator’s discretion. All patients underwent invasive hemodynamic monitoring during VA-ECMO support in the ICU. Distal perfusion was not routinely performed. According to the results of near-infrared spectroscopy (NIRS) monitoring, a distal perfusion catheter was selectively inserted under ultrasound guidance only in patients with a risk of limb ischemia. Oxygenator function and the circuit status were assessed daily using blood gas analysis and visual inspection. Weaning from ECMO was determined based on improvements in hemodynamic parameters and cardiac function confirmed by echocardiography. ECMO flow was progressively reduced, and the cannulae were surgically removed if the patient was hemodynamically stable on minimal flow.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as number (%). Statistical comparisons between the two groups were made using Student’s t-test for continuous variables and the χ2 test for categorical variables. SPSS 25 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. A P-values of <0.05 were considered statistically significant.
Results
In total, 66 patients had a heparin-free period of ≥24 h during VA-ECMO support (73.3%, heparin-free group). Bleeding was the only reason for not using or intermittently discontinuing heparin during VA-ECMO support in all patients. There were no significant differences in demographic and clinical characteristics between the two groups. The reasons for VA-ECMO insertion were post-cardiotomy cardiogenic shock in 37 patients (41.1%), and ECPR in 44 patients (48.9%). There was no significant difference in the rate of post-cardiotomy cardiogenic shock and ECPR between the two groups. Clinical features associated with ECMO, including the support type according to access site, location of cannulation, and the proportion of patients using the PLS system, were not significantly different between the two groups (Table 1). Although the total duration of VA-ECMO was not significantly different between the two groups, the duration of ECMO without heparinization was significantly longer in the heparin-free group (12.0±7.9 vs 79.8±60.7 h, P<0.001). In the heparin-free group, 26 patients (39.4%) were completely heparin-free including initial bolus administration and continuous infusion throughout the VA-ECMO support period. For these patients, the duration of VA-ECMO was 73.8±40.8 h. Although the number of patients who were successfully weaned from ECMO was significantly greater in the control group (87.5 vs. 60.6%, P=0.021), the rate of survival to discharge was not significantly different between the two groups. Although the incidence of hemorrhagic complications was not significantly different between the two groups, the rate of re-exploration for bleeding control was significantly higher in the heparin-free group. Although
Table 1: Demographical characteristics of patients and clinical features associated with ECMO.
|
Variables |
Control group (n=24) |
Heparin-free group (n=66) |
P value |
|
Age (years) |
58.9±17.1 |
60.3±16.6 |
0.726 |
|
Male |
17 (70.8%) |
42 (63.6%) |
0.620 |
|
DM |
9 (37.5%) |
20 (30.3%) |
0.606 |
|
Preoperative LVEF (%) |
47.0±18.4 |
41.4±18.0 |
0.288 |
|
Indication |
|||
|
Post-cardiotomy |
6 (25.0%) |
31 (47.0%) |
0.089 |
|
ECPR |
15 (62.5%) |
29 (43.9%) |
0.154 |
|
Cannulation |
0.391 |
||
|
Central |
3 (12.5%) |
5 (7.6%) |
|
|
Peripheral |
21 (87.5%) |
56 (84.8%) |
|
|
Mixed |
0 |
5 (7.6%) |
|
|
Location |
0.175 |
||
|
OR |
9 (37.5%) |
31 (47.0%) |
|
|
ICU |
14 (58.3%) |
24 (36.4%) |
|
|
ER |
0 |
7 (10.6%) |
|
|
Cath lab |
1 (4.2%) |
4 (6.0%) |
|
|
Circuit |
|||
|
PLS® system |
16 (66.7%) |
34 (51.5%) |
0.237 |
Values are expressed as mean ± standard deviation or number (%).
DM, diabetes mellitus; LVEF, left ventricular ejection fraction; ECPR, extracorporeal cardiopulmonary resuscitation; OR, operating room; ICU, intensive care unit; ER, emergency room; Cath lab, catheterization laboratory; ECMO, extracorporeal membrane oxygenation.
Table 2: Comparison of outcomes including complications between control group and heparin-free group.
|
Variables |
Control group (n=24) |
Heparin-free group (n=66) |
P value |
|
Heparinization |
|||
|
All-time infusion |
4 (16.7%) |
||
|
All-time no infusion |
26 (39.4%) |
||
|
Initial bolus |
4 (16.7%) |
9 (13.6%) |
0.740 |
|
ECMO related outcomes |
|||
|
Total duration (h) |
132.3±106.1 |
141.6±117.9 |
0.734 |
|
Heparin-free duration (h) |
12.0±7.9 |
79.8±60.7 |
<0.001 |
|
Heparin-free duration (%) |
19.9±27.3 |
69.9±31.1 |
<0.001 |
|
Flow (L/min) |
2.6±0.5 |
2.7±0.7 |
0.258 |
|
Weaning success |
21 (87.5%) |
40 (60.6%) |
0.021 |
|
Clinical outcomes |
|||
|
ICU stay (d) |
13.9±8.7 |
12.3±8.3 |
0.418 |
|
Hospital stay (d) |
30.6±24.7 |
26.7±31.6 |
0.586 |
|
Survival to discharge |
12 (50%) |
26 (39.4%) |
0.470 |
|
Hemorrhagic complications |
|||
|
Blood loss (L) |
3.7±4.3 |
5.2±3.7 |
0.167 |
|
RBC transfusion (units) |
13.6±10.9 |
18.1±14.4 |
0.173 |
|
Re-exploration for bleeding control |
2 (8.3%) |
26 (39.4%) |
0.004 |
|
Pulmonary hemorrhage |
0 |
1 (1.5%) |
>0.999 |
|
Thromboembolic complications |
|||
|
Oxygenator change |
2 (8.3%) |
7 (10.6%) |
>0.999 |
|
Pump malfunction |
0 |
0 |
|
|
Lower limb ischemia |
|||
|
Distal perfusion |
6 (25.0%) |
17 (25.8%) |
>0.999 |
|
Thrombectomy |
1 (4.2%) |
0 |
0.267 |
|
Fasciotomy |
0 |
1 (1.5%) |
>0.999 |
|
Amputation |
1 (4.2%) |
1 (1.5%) |
0.464 |
|
Stroke |
2 (8.3%) |
3 (4.6%) |
0.605 |
|
AKI requiring CRRT |
1 (4.2%) |
2 (3.0%) |
>0.999 |
|
Overall incidence |
11 (45.8%) |
25 (37.9%) |
0.627 |
seven (10.6%) patients in the heparin-free group required oxygenator changes due to thrombosis, there was no significant difference in the frequency of oxygenator changes between the two groups. ECMO pump malfunction was not observed in either group. The frequency of distal perfusion for the management of limb ischemia was not significantly different between the two groups. In the heparin-free group, there was only one case of critical limb ischemia requiring both fasciotomy and limb amputation. In the control group, one patient underwent limb amputation due to ischemia, while another underwent femoral arterial thrombectomy due to thrombosis. The overall incidence of thromboembolic complications was not significantly different between the two groups (45.8 vs. 37.9%, P=0.627, Table 2).
Discussion
Systemic anticoagulants should be routinely administered to patients on VA-ECMO with the primary goal of minimizing circuit thrombosis and preventing thromboembolic complications. The interaction between blood and the artificial surfaces of the ECMO circuit, including the oxygenator, activates both coagulation and inflammatory cascades, inducing a systemic pro-thrombotic state [1]. ECMO technology has been developed with a focus on the management of these inflammatory responses to non- biological surfaces of the ECMO circuit. Newly developed non-thrombogenic substances such as phosphorylcholine and 2-methoxyethyl acrylate are commonly used to coat ECMO circuits [2]. These coatings reduce clot formation in the ECMO circuit, resulting in lower levels of systemic anticoagulation requirement for the patients on ECMO [3]. With the development of these ECMO circuit coating, the incidence of circuit thrombosis is decreasing [4]. Given these technological advances, the use of conventional strict heparinization during ECMO support becomes questionable.
Several researchers have investigated the possibility of using a mild heparinization regimen. Buscher et al. reported a low complication rate despite low-dose heparinization and frequent discontinuation of anticoagulation in patients on ECMO [5]. Muellenbach et al. also demonstrated that long- term, non-heparinized ECMO support was feasible even in patients who initiated ECMO support without heparinization and received heparin 5 days after the initiation of mechanical support [6]. Some researchers have showed that ECMO support could be successfully performed even in patients who are not receiving anticoagulants at all [7,8]. However, most studies involved patients with trauma or patients on VV- ECMO, and there were only few reports involved patients on VA-ECMO for post-cardiotomy cardiogenic shock or ECPR.
At our institution, heparin infusion is liberally discontinued if deemed necessary during VA-ECMO support, after consideration of the risks and benefits of anticoagulation. In post-cardiotomy patients transferred to ICU with initiation of VA-ECMO in the OR, heparinization was not routinely initiated. Considering the residual effects of systemic heparinization during CPB, heparin administration was not prioritized within 24 h of completion of open cardiac surgery, and it was initiated only after confirmation of the absence of postoperative bleeding. For patients with active bleeding immediately after surgery, heparin was not used throughout the period of VA-ECMO support. We applied the same principle to cases of VA-ECMO initiated in the ICU because of post-cardiotomy cardiogenic shock. In cases of ECPR in particular, no bolus heparin was initially administered, and continuous infusion was not routinely initiated either. Heparinization was initiated only after the possibility of active bleeding due to CPR-related injury was completely ruled out. When this strategy was applied, the amounts of blood loss and RBC transfusion in the heparin-free group were not significantly different from those in the control group. Given that there was a significantly larger number of patients who needed re-exploration due to active bleeding in the heparin- free group, it would be reasonable to interpret that application of the heparin-free strategy effectively reduced the amount of blood loss in patients with massive bleeding in the heparin- free group.
A major concern when applying this strategy is sudden ECMO system malfunction requiring replacement of system components due to clots in the circuit, pump head, and oxygenator. The reported incidence of circuit thrombosis in patients on VA-ECMO is 15.6%, with oxygenator thrombosis accounting for 8.2% cases [9]. The frequency of VA-ECMO malfunction due to circuit thrombosis during support without heparinization has been reported by only a few investigators. Lamarche et al. showed an oxygenator change rate of 9.4% in 32 patients with post-cardiotomy cardiogenic shock who were supported by VA-ECMO without anticoagulation for an average of 46.3 h [10]. Although Fina et al. demonstrated that oxygenator failure did not occur even without systemic anticoagulation, they included only six patients, and their mean ECMO duration was only 10 h [11]. In the present study, the mean VA-ECMO duration without heparinization was 79.8 h, and the rate of oxygenator change due to thrombosis was 10.6% in the heparin-free group. No pump malfunction occurred during VA-ECMO support in the heparin-free group. To the best of our knowledge, this study demonstrated that VA-ECMO could be safely maintained without heparinization for the longest period in the largest number of patients to date.
Another concern with the application of our heparin-free strategy is that it might increase the chance of developing thromboembolic complications such as limb ischemia and stroke. In the present study, only one patient (1.5%) in the heparin-free group experienced significant limb ischemia requiring surgical intervention; this is significantly lower than the 70% incidence of limb ischemia reported by Muehrcke et al [12]. Lamarche et al reported a limb ischemia of 16% [10], which was lower than that reported in previous studies; however, the incidence in our study was considerably lower. These results might be because our institution has been using NIRS monitoring for early detection and timely management of limb ischemia [13]. Our findings demonstrate that the incidence of limb ischemia does not significantly increase in patients on VA-ECMO, even after the long-term application of a heparin-free strategy with appropriate management of limb ischemia. The incidence of stroke was also comparable between the two groups in our study. The overall incidence of thromboembolic complications was relatively high in both groups (45.8 vs. 37.9%, P=0.627), although this was probably because all cases of distal perfusion were considered as cases of limb ischemia. With the exclusion of distal perfusion, the proportion of patients with thromboembolic complications was 25.0% in the control group and 19.7% in the heparin-free group (P=0.572).
This study has several limitations, and the results should be interpreted with caution. The relatively small number of patients and the retrospective design did not allow us to reach a definitive conclusion regarding the heparin-free strategy. A randomized study with a larger sample size is necessary to confirm our findings. In addition, because brain imaging is very difficult to perform in patients on VA-ECMO, the incidence of stroke may be underestimated. Moreover, no autopsy was performed in the mortality cases at our institution. Given that approximately 50% of undiagnosed thromboembolic complications are found during the autopsy of patients who died while supporting by ECMO [14], it can be assumed that the probability of underestimation is high, particularly in the mortality cases.
Conclusion
We demonstrated that a heparin-free strategy during VA- ECMO support could be feasible and did not increase the risk of thromboembolic complications. We suggest that appropriate discontinuation of heparinization during VA-ECMO support could be a safe strategy for patients with active bleeding or a high hemorrhagic risk. Although anticoagulation during VA- ECMO support is necessary in principle, it should be adjusted according to the patient’s clinical situation.
Funding
No funding sources.
Conflict of Interest
The authors have no conflict of interest.
References
- Sniecinski RM, Chandler Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 113 (2011): 1319-1333.
- MacLaren G, Combes A, Bartlett Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intens Care Med 38 (2012): 210-220.
- Ranucci M, Isgro G, Soro G, et al. Reduced systemic heparin dose with phosphorylcholine coated closed circuit in coronary Int J Artif Organs 27 (2004): 311- 319.
- Pappalardo F, Della Valle P, Crescenzi G, et Phosphorylcholine coating may limit thrombin formation during high-risk cardiac surgery: a randomized controlled trial. The Annals of thoracic surgery 81 (2006): 886-891.
- Buscher H, Vukomanovic A, Benzimra M, et Blood and anticoagulation management in extracorporeal membrane oxygenation for surgical and nonsurgical patients: A Single-Center Retrospective Review. J Cardiothorac Vasc Anesth 31 (2017): 869-875.
- Muellenbach RM, Kredel M, Kunze E, et al. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain J Trauma Acute Care Surg 72 (2012): 1444-1447.
- Wen PH, Chan WH, Chen YC, et al. Non-heparinized ECMO serves a rescue method in a multitrauma patient combining pulmonary contusion and nonoperative internal bleeding: a case report and literature World J Emerg Surg 10 (2015): 15.
- Reynolds HN, Cottingham C, McCunn M, et Extracorporeal lung support in a patient with traumatic brain injury: the benefit of heparin-bonded circuitry. Perfusion 14 (1999): 489-493.
- Thiagarajan RR, Barbaro RP, Rycus PT, et Extracorporeal life support organization registry international report 2016. ASAIO J 63 (2017): 60-67.
- Lamarche Y, Chow B, Bedard A, et al. Thromboembolic events in patients on extracorporeal membrane oxygenation without anticoagulation. Innovations (Phila) 5 (2010): 424-429.
- Fina D, Matteucci M, Jiritano F, et al. Extracorporeal membrane oxygenation without systemic anticoagulation: a case-series in challenging conditions. J Thorac Dis 12 (2020): 2113-2119.
- Muehrcke DD, McCarthy PM, Stewart RW, et Complications of extracorporeal life support systems using heparin-bound surfaces. The risk of intracardiac clot formation. J Thorac Cardiovasc Surg 110 (1995): 843-851.
- Kim DJ, Cho YJ, Park SH, et Near-infrared spectroscopy monitoring for early detection of limb ischemia in patients on veno-arterial extracorporeal membrane oxygenation. ASAIO J 63 (2017): 613-617.
- Rastan AJ, Lachmann N, Walther T, et Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (ECMO). Int J Artif Organs 29 (2006): 1121-1131.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 