Transcatheter Closure of Traumatic Ventricular Septal Defects: Two Cases and a Review of the Literature
Tai H Pham, Matthew S Glassy, Gagan D Singh, Jason H Rogers*
1University of California, Division of Cardiovascular Medicine, California, USA
*Corresponding author: Jason H Rogers, MD Division of Cardiovascular Medicine, University of California, Davis Medical Center, Sacramento, California 95817, USA.
Received: 04 January 2022; Accepted: 11 January 2022; Published: 14 February 2022
Article Information
Citation: Tai H Pham, Matthew S Glassy, Gagan D Singh, Jason H Rogers. Transcatheter Closure of Traumatic Ventricular Septal Defects: Two Cases and a Review of the Literature. Cardiology and Cardiovascular Medicine 6 (2022): 42-50.
View / Download Pdf Share at FacebookKeywords
<p>Traumatic Ventricular Septal Defects; Transcatheter Closure</p>
Article Details
1. Introduction
Traumatic ventricular septal defects (VSDs) compli-cate 1-5% of penetrating cardiac injuries and can occur from direct lacerations (i.e. stab or gunshot) or contusions [1, 2]. Although any cardiac structure can be involved, most cases of traumatic cardiac injuries involve the ventricular chambers with more than 50% occurring on the right ventricular (RV) free wall given its anterior location [3, 4]. Only 10-25% of patients with a penetrating cardiac injury survive to reach hospital care and only 20% have stable hemo-dynamics on arrival [4, 5]. At presentation, stabili-zation of penetrating cardiac injuries is the priority and sternotomy or thoracotomy with exploration should be considered when there is hemodynamic instability [6]. Immediate treatment involves repair of free wall lacerations and a thorough cardiac exami-nation is often deferred if the causative lesion is identified and repaired [7]. VSDs can be diagnosed immediately or weeks after the initial injury and may not be noted on initial surgical exploration [8]. Mana-gement of these traumatic VSDs are challenging since patients can have numerous other injuries. Although some small VSDs may close spontane-ously, they can enlarge over time as a result of remodeling and turbulent flow. This can result in significant left to right shunting with signs and symptoms of congestive heart failure that are often preceded by enlarging cardiac chambers and eleva-tion in right ventricular systolic pressure (RVSP) [9, 10]. Although open surgical repair with sternotomy can be performed, transcatheter repair is less invasive and can be effective. Technical challenges include the heterogeneous nature of these defects in terms of size, location, irregularity, and potential proximity to surrounding structures. We herein present two illustr-ative cases and review of the literature.
2. Case Reports
2.1 Case 1
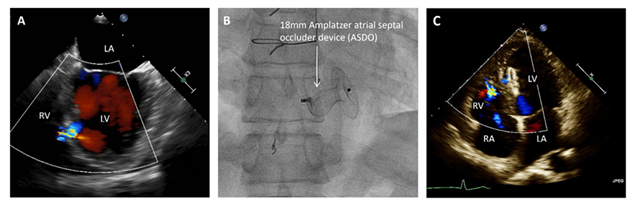
A 25 year-old man suffered an anterior stab wound during a physical altercation. Emergent surgical exp-loration revealed no evidence of penetrating cardiac injury at that time. After discharge he was found to have a mid-muscular VSD on transthoracic echocar-diogram (TTE). He was asymptomatic and was followed for many years with clinical monitoring of symptoms and serial TTEs. Cardiac catheterization 19 years after the initial injury revealed a left to right shunt with a pulmonary to systemic flow ratio (Qp: Qs) of 1.7. On TTE, RV and left ventricular (LV) chamber sizes were normal, left ventricular ejection fraction (LVEF) was normal, and the RVSP was estimated at 32 mm Hg. At 52 years-old (27 years after his initial injury), he began to develop symptoms of decreased exercise capacity as well as worsening dyspnea on exertion (DOE) with NYHA class II symptoms. Subsequent TTE revealed increasing LV and RV diameters. He was again referred for cardiac catheterization which revealed a Qp:Qs of 2.4, prompting a plan for transcatheter VSD closure. A TEE demonstrated a 0.7 x 0.7 cm muscular inferoseptal VSD (Figure 1a).
An Amplatzer muscular VSD occluder (VSD-MUSC) was planned for closure. The technique of transcatheter closure is similar to the common technique for closure of congenital VSDs. The defect is crossed from the LV with a wire which is snared in the pulmonary artery and externalized through the femoral vein creating an AV loop wire. Over this, a delivery sheath is advanced over the wire from the RV into the LV and the occluder device is deployed in the usual fashion with deployment of the proximal disc, waist, and distal disc in sequence. A safety “buddy” wire may be left in place to allow ease in recrossing the defect in situations where the occluder device must be recaptured or prolapses due to unstable seating. In this case, there were multiple failed attempts to deploy 12, 18, 20 and 22-mm VSD occluder devices due to inability to span the muscular septum and obtain optimal positioning under transe-sophageal echocardiogram (TEE) and fluoroscopic guidance. An 18-mm Amplatzer septal occluder device (ASO) was then deployed with stable position and minimal residual shunt (Figure 1b). The techni-que used was to deploy the distal disc in the LV, and have the proximal disc constrained within the defect (Figure 1b). On post-operative day one, TTE showed a left ventricular internal diameter in diastole (LVIDd) of 6.2 cm (4.1 - 5.8 cm) and mid right ventricular dimension (RVd) of 3.8 cm (2.7 – 3.3 cm). There was no evidence of hemolysis and he was discharged with aspirin and clopidogrel. One month later on follow up, he reported resolution of his symptoms and noted improved exercise tolerance. A follow up TTE revealed a minimal residual shunt with a decrease in LVIDd to 5.6 cm, a decrease in RVd to 3.0 cm, and an estimated RVSP of 27 mm Hg (Figure 1c).
2.2 Case 2
A 32-year-old inmate at a local prison presented after a stab wound to the left anterior chest with a left-sided pneumothorax requiring a chest tube for decompression. Due to persistent hypotension, the patient was taken to the operating room emergently. Upon opening the pericardium, clot and blood were noted with immediate improvement in blood pressure after evacuation. Further inspection revealed a single small laceration on the free wall at the apex of the heart. The laceration was repaired, a right pericardial window was performed, and a Swan-Ganz catheter was placed for continued hemodynamic monitoring.
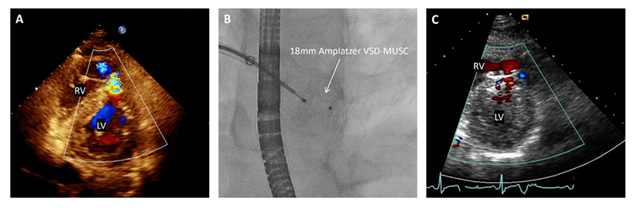
Post-operative TTE revealed an antero-apical 0.7 x 1.1 cm muscular VSD with a Qp:Qs of 2.0 (Figure 2a). After discussion with cardiothoracic surgery, he was considered at increased surgical risk and percutaneous transcatheter Amplatzer occlusion was planned. Subsequent pre-operative cardiac catheteri-zation revealed a Qp:Qs of 1.5 with variability in pulmonary artery saturation measurements, sugges-ting either sampling variability or a dynamic nature to the VSD. Unfortunately, his hospital course was complicated by methicillin-sensitive staphylococcus aureus (MSSA) bacteremia from an antecubital abscess, requiring intravenous (IV) antibiotics. He remained hemodynamically stable during the rest of his hospitalization and was discharged back to prison with 4 weeks of IV nafcillin and plans for trans-catheter VSD closure after blood culture clearance and completion of his antibiotic course. He returned two months later and pre-procedure TTE showed an LVIDd of 5.5 cm and a mid RVd of 3.11 cm. Closure of VSD was first attempted with a 14-mm VSD-MUSC device. This was removed without deployment as secure positioning under TEE and fluoroscopy was not achieved. An 18-mm Amplatzer VSD-MUSC device was then deployed with stable position and significant reduction in left to right shunt (Figure 2b). He was discharged in stable condition with aspirin and clopidogrel. On follow up one month later the patient reported no cardiac or respiratory symptoms and his follow up TTE demonstrated an LVIDd of 4.9 cm and a mid RVd of 2.8 cm with a trivial left to right shunt (Figure 2c).
Figure 1: (A) Pre-operative transesophageal echocardiogram demonstrating left to right shunting through the VSD in the 4-chamber view. (B) Fluoroscopic still image of the successfully deployed 18mm ASO. (C) An apical 4-chamber view from the one-month post-operative TTE which shows minimal residual shunting around the ASO.
Figure 2: (A) An off-axis apical view from the pre-operative echocardiogram demonstrating a left to right shunt through the traumatic VSD. (B) Fluoroscopic still image of the successfully deployed 18mm Amplatzer VSD-MUSC device. (C) A parasternal short view from the 1-month post-operative transthoracic echocardiogram with a small residual shunt around the device.
3. Discussion
3.1 Timing
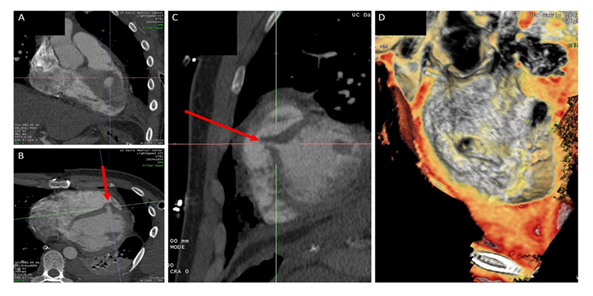
Traumatic VSDs usually arise with deep penetrating chest trauma (typically anterior or left chest) with subsequent creation of an injury tract that involves the free wall of the RV and interventricular septum (Figure 3). Despite improvements in resuscitative efforts, blunt force and penetrating chest trauma complicated by structural cardiac injuries remain a management challenge [1]. Surgical relief of obstructive causes of acute shock and direct repair of free wall injuries take priority [5, 11, 12]. Patients in extremis from intracardiac injuries should undergo acute surgical closure of traumatic VSDs [8]. In cases where VSD closure is not emergent, percutaneous transcatheter intervention with occluder devices is a worthwhile alternative to surgical patch repair [13]. Furthermore, time may allow for development of granulation tissue and fibrosis, which can result in spontaneous closure or permit the tissue to be more amenable to stabilization with surgical sutures or a transcatheter occluder device [8, 14]. The patient’s comorbidities may also influence the timing and preferred approach (surgical vs. transcatheter). As illustrated in our second case, the development of MSSA bacteremia precluded definitive closure until completion of his antibiotic course. When a delayed approach is decided, close follow up in clinic is important to assess for any new or worsening symptoms of heart failure. Serial TTEs can be done to assess for structural or hemodynamic changes. Closure should be considered if there is a significant left to right shunt with Qp:Qs >1.5, chamber dilation, elevated pulmonary artery pressures, congestive heart failure, and/or attributable symptoms [13]. Of note, left to right shunting can be difficult to accurately measure and can be grossly underestimated with echocardiography [1, 15]. In our second case, the Qp:Qs was influenced by dynamic VSD closure as well as hyperdynamic cardiac output from sepsis.
Figure 3: (A) Coronal view from the pre-procedure CT chest showing the defect along the muscular ventricular septum. (B) Axial cross-section view from the same CT study with the red arrow showing the medial to lateral trajectory of the penetrating object. (C) Sagittal view of the defect with red arrow demonstrating the superior to inferior angle of the penetrating object. (D) CT reconstruction with an en face view from the left ventricle of the VSD.
3.2 Anatomy
Anatomical considerations also influence the decision regarding the optimal approach. Surgical repair has inherent risks of operative and re-operative morbidity and mortality. The choice and type of surgical repair can have consequences of ventricular scarring, injury to underlying conduction, damage to surrounding valvular structures, and even lead to coronary vessel impingement [8, 16, 17]. Transcatheter Amplatzer VSD-MUSC devices have been widely implemented in the adult congenital population to avoid complex and recurrent surgical operations [18]. Transcatheter treatment of muscular adult congenital VSDs have seen excellent results with up to 97% successful closure rates and low rates of complications. Complex VSDs near papillary muscles or chordae, and VSDs very low in the apex or high in the perimembranous septum may not be amenable to transcatheter device closure [17]. Specifically, perimembranous VSDs lie in proximity to the tricuspid and aortic valves as well as the cardiac conduction system. Placing a percutaneous closure device in this location is generally prohibitive but can be possible in select cases [18, 19].
3.3 Device selection
The inaccuracy of echocardiographic measurement, friability of tissue, septal location, irregular course of traumatic injuries, and the structures in proximity all play a role [8]. Some operators have reported choosing a device size according to echo-derived measurements, while others have reported success with oversizing the device by 1-2 mm larger than the VSD [13, 20]. Yet others have been successful with oversizing by at least 50%, similar to a post-MI VSD [16]. In approximately half the cases reviewed, balloon sizing was used to mitigate the inherent difficulties with sizing on echocardiogram but our review did not demonstrate any benefit in reduction of devices used compared to cases that did not utilize balloon sizing. Availability of specific devices and sizes also affected which devices were ultimately used to close the defect. Several case reports noted using an available ASDO despite operator preference for VSDOs of the same size due to availability issues [21]. For several decades, novel use of other occluder devices has been utilized for traumatic VSD closure to address the heterogeneity of these injuries. In one case series, Amplatzer patent ductus arteriosus occluders (ADO device) were used in three patients who developed VSDs from acute chest trauma [22]. In their experience, use of the PDA occluders offered several advantages over the Amplatzer VSDO or ASDO’s. The smaller singular distal disc is held in place by the higher pressures of the left heart. The PDA occluder may also decrease the risk of ventricular outflow tract obstruction as compared to the two-disc systems of the Amplatzer septal occluder devices. The main limitation of the ADO device is relatively smaller waist diameters of 5 to 12 mm and lack of a right ventricular retention disc.
Occluder devices carry their own set of complications that need to be considered, including prolapse into the left or right ventricular cavities, outflow tract obstruction, embolization, thrombus formation, ero-sion, conduction block from mechanical compress-ion, and papillary muscle entrapment with impair-ment of valvular function. Understanding these risks and proper device selection is critical. There are commercially available muscular VSD occluder devi-ces with waist diameter ranging 4 to 24 mm, waist length 7 mm, and both discs the same size, 5 to 8 mm larger than the waist diameter. Larger Amplatzer post-infarct muscular VSD occluders (VSD-MUSCPI) are also available in waist diameters 16 to 24 mm, with the main differences being a larger waist length of 10 mm and both discs 10 mm larger than the waist diameter. Finally, ASOs are available in very large waist sizes up to 38 mm with the main limitation being a very short waist length of 3 or 4 mm. These ASO devices can be used to close VSDs as well [13, 16, 23]. The technique involves deplo-ying the larger distal disc flush against the LV septal wall and the proximal disc within the defect, as with the first case we presented. As a note of caution, ASO devices are prone to hemolysis as their construction with Nitinol mesh and polyester is not designed for the higher pressures of the LV and RV, and clinical monitoring for hemolysis after implant-tation is recommended. Since ASOs have a waist thickness of 4 mm compared to 7 mm in VSDO devices the thicker septum may experience higher compression forces with the use of an ASO, resulting in conduction block or compromised intramyocardial circulation. Additionally, the thicker muscular inter-ventricular septum may deform a thinner ASO device during systole and result in incomplete occlusion and continued shunting as was seen by Suh et al. [17].
In summary, the selection of a compatible and appropriately sized device can be challenging. We have reviewed prior published reports and summar-ized the findings in Table 1. These published cases have demonstrated the feasibility of transcatheter occluder devices for traumatic VSD closure and its safety profile in the perioperative and post-operative periods. All but three cases reviewed resulted in successful deployment of occluder devices. One case required surgical removal and patch repair secondary to bilaterally deformed discs and persistent high velocity shunting. Another case required surgical repositioning of distal disc after it was shown to have partially prolapsed into the right atrium across a disrupted tricuspid valve. Residual shunts were common, but none were noted to be hemodyna-mically compromising. The most common complica-tion was hemolysis, with several cases significant enough to require blood transfusions. Questions remain regarding the long-term durability of these devices, and there have been no clinical trials evaluating the non-inferiority of percutaneous trau-matic VSD closure over surgical repair.
4. Conclusions
The optimal timing of traumatic VSD closure should focus on hemodynamic and structural consequences of the defect, attributable symptoms, cardiac chamber size, and the degree of left to right shunting. VSD device selection depends on several key factors as listed above, and optimal sizing may prove to be difficult given the heterogeneity of traumatic defects. Nevertheless, transcatheter VSD closure can be a safe and effective alternative to surgical closure.
References
- Sugiyama G, Lau C, Tak V, Lee DC, Burack J. Traumatic ventricular septal defect. Ann Thorac Surg 91 (2011): 908-910.
- De'Ath HD, Vulliamy PE, Davies C, Uppal R. A large ventricular septal defect compli-cating resuscitation after blunt trauma. J Emerg Trauma Shock 5 (2012): 350-352.
- Ivantury RR, Rohman M, Steichen FM, et al. Penetrating cardiac injuries: twenty-year experience. Am Surg 53 (1987): 310-317.
- Kulshrestha P, Das B, Iyer KS, Sampath KA, Sharma ML, Rao IM, et al. Cardiac injuries-a clinical and autopsy profile. J Trauma 30 (1990): 203-207.
- Brathwaite CEM, Rodriguez A, Turney SZ, Dunham CM, Cowley RA. Blunt traumatic cardiac rupture. Ann Surg 212 (1990): 701-704.
- Asensio JA, Soto SN, Forno W, Roldan G, Petrone P, Pereira B, et al. Penetrating cardiac injuries: a complex challenge. Injury 32 (2001): 533-543.
- Stein E, Daigle S, Weiss SJ, Desai ND, Augoustides JG. CASE 3–2011: Successful management of a complicated traumatic ventricular septal defect. J Cardiothorac Vasc Anesth 25 (2011): 547-552.
- Harling L, Ashrafian H, Casula RP, Athanasiou T. Late surgical repair of a traumatic ventricular septal defect. J Cardiothorac Surg 9 (2014): 145.
- Juneau D, Hermann D, Wells GL. Penetrating trauma resulting in ventricular septal defect. World J Cardiovasc Surg 4 (2014): 77-80.
- Thandroyen FT, Matisonn RE. Penetrating thoracic trauma producing cardiac shunts. J Thorac Cardiovasc Surg 81 (1981): 569-573.
- Ivatury RR, Rohman M, Steichen FM, Gunduz Y, Nallathambi M, Stahl WM. Penetrating cardiac injuries: twenty-year experience. Am Surg 53 (1987): 310-317.
- Knott-Craig CJ, Dalton RP, Rossouw GJ, Barnard PM. Penetrating cardiac trauma: management strategies based on 129 surgical emergencies over 2 years. Ann Thorac Surg 53 (1992): 1006-1009.
- Ali TA, Fatimi SH, Hasan BS. Transcatheter closure of a traumatic ventricular septal defect using an Amplatzer atrial septal occluder device. Catheter Cardiovas Interv 82 (2013): 569-573.
- Pedra CA, Pontes SC Jr, Pedra SR, Salerno L, Sousa JB, Miaira MA, et al. Percutaneous closure of postoperative and post-traumatic ventricular septal defects. J invasive Cardiol 19 (2007): 491-495.
- Cha EK, Mittal V, Allaben RD. Delayed sequel of penetrating cardiac injury. Arch Surgery 128 (1993): 836-841.
- Suh WM, Kern MJ. Transcatheter closure of a traumatic VSD in an adult requiring an ASD occluder device. Catheter Cardiovasc Interv 74 (2009): 1120-1125.
- Wilson W, Osten M, Benson L, Horlick E. Evolving trends in interventional cardiology: endovascular options for congenital disease in adults. Can J Cardiol 30 (2014): 75-86.
- Bentham JR, Thomson JD. Current state of interventional cardiology in congenital heart disease. Arch Dis Child 100 (2015): 787-792.
- Saurav A, Kaushik M, Mahesh Alla V, White MD, Satpathy R, Lanspa T, et al. Comparison of percutaneous device closure versus surgical closure of peri-membranous ventricular septal defects: a systematic review and meta-analysis. Catheter Cardiovasc Interv 86 (2015): 1048-1056.
- Bauriedel G, Redel DA, Schmitz C, Welz A, Schild HH, Lüderitz B. Transcatheter closure of a posttraumatic ventricular septal defect with an Amplatzer occluder device. Catheter Cardiovas Interv 53 (2001): 508-512.
- Tang L, Tang JJ, Fang ZF, Hu XQ, Shen XQ, Zhou SH. Severe mechanical hemolysis after Transcatheter closure of a traumatic ventricular Septal defect using the Amplatzer atrial Septal Occluder. Int Heart J 57 (2016): 519-521.
- Xi EP, Zhu J, Zhu SB, Yin GL, Liu Y, Dong YQ, et al. Percutaneous closure of a post-traumatic ventricular septal defect with a patent ductus arteriosus occlude. Clinics 67 (2012): 1281-1283.
- Alidoosti M, Hoseini SK, Shafiee A. Managing a traumatic ventricular septal defect with atrial septal defect occluder device. Cardiol in the Young 23 (2013): 436-439.
- Dehghani P, Ibrahim R, Collins N, Latter D, Cheema AN, Chisholm RJ. Post-traumatic ventricular septal defects–review of the literature and a novel technique for per-cutaneous closure. J Invasive Cardiol 21 (2009): 483-487.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 