Vitronectin Negatively Regulates IGF-II-Induced Mitogenic Signaling and Migration Mediated by The Insulin Receptor Isoform-A
Pierluigi Scalia1,2,*, Emma Heart3, Alison Doughty4, Antonio Giordano1,5, Stephen J Williams1,2
1Sbarro Institute for Cancer Research & Molecular Medicine, Temple University, Philadelphia, PA, 19122, USA
2The ISOPROG-Somatolink Network, Philadelphia, PA, 19102, USA and Caltanissetta, 93100, Italy
3Department of Physiology and Biophysics, Keck School of Medicine of the University of Southern California, Los Angeles, CA, 90033, USA
4Department of Molecular Microbiology & Immunology, Keck School of Medicine of the University of Southern California, Los Angeles, CA, 90033, USA
5Department of Medical Biotechnology, University of Siena, 53100, Italy
*Corresponding Author: Pierluigi Scalia, Sbarro Institute for Cancer Research & Molecular Medicine, Temple University, Philadelphia, PA, 19122, USA; The ISOPROG-Somatolink Network, Philadelphia, PA, 19102, USA and Caltanissetta, 93100, Italy
Received: 04 August 2020; Accepted: 24 August 2020; Published: 27 August 2020
Article Information
Citation: Pierluigi Scalia, Emma Heart, Alison Doughty, Antonio Giordano, Stephen J Williams. Vitronectin Negatively Regulates IGF-II-Induced Mitogenic Signaling and Migration Mediated by The Insulin Receptor Isoform-A. International Journal of Applied Biology and Pharmaceutical Technology 11 (2020): 229-244.
View / Download Pdf Share at FacebookAbstract
Abstract
Vitronectin (VTN) is an extracellular matrix glycoprotein regulating both cell adhesion and spreading via Integrin alpha V-beta 3 and a variety of secreted/membrane anchored factors, however the cell signaling underlying VTN-induced effects is unclear. Here we show VTN to specifically modulate cellular effects of Insulin like growth factor II. Specifically, we find that vitronectin, either by addition or induced cell membrane expression, markedly reduced or neutralized IGF-II-induced proliferation and migration on mouse embryo fibroblasts genetically deprived of the IGF1R and expressing the human insulin receptor isoform (R-IRA) with high affinity for IGF-II. VTN transient expression at increasing amounts in these cells inhibited, accordingly, the IGF-II-mediated Ser473 acute phosphorylation of AKT. Under the same conditions, the IGF-II acute stimuli (5 min) on ERK1/2 (Thr202/Tyr204) activation via the IRA was also inhibited. Higher VTN expression levels in R-IRA cells associated a reproducible delayed increase in AKT S473 phosphorylation following 30 minutes stimulation for both AKT and ERK1/2. Altogether these data demonstrate that VTN, when overexpressed in the extracellular matrix, negatively modulates the IGF-II proliferative signal and biological effects mediated by the insulin receptor short isoform (IRA) and suggest potential new strategies for the modulation of the IGF-II effects in vivo.
Keywords
<p>VTN: Vitronectin; PAI1: Plasminogen Activator Inhibitor 1; uPA: Urokinase-type Plasminogen Activator; IGF-II: Insulin-like Growth Factor-II; VEGF: Vascular Endothelial Growth Factor; MMP: Matrix Metallo-Protease; IGFBP (2/3/7): Insulin-like Growth Factor Binding Protein (2/3/7); ITN: Integrin; IRA: Insulin Receptor, Isoform A; MEF: Mouse Embryo Fibroblast; EC: Extra Cellular</p>
Article Details
Introduction
Vitronectin (VTN), a cell-surface component of the Extracellular matrix, is thought to exert its cell spreading and tissue remodeling activities via interaction with integrin αvβ3 [1] and other secreted and membrane anchored factors such as PAI1 and uPA [2]. Among these, VTN binding with uPA and its soluble receptor form (suPAR) has been found to focally recruit and concentrate PA-dependent proteolytic activity responsible for tissue remodeling [3]. Furthermore VTN has been shown to upregulate MMP2 [4] and to negatively modulate the interaction between ITN αvβ3 and VEGFR2 in which VTN glycation has been found to decrease αvβ3 phosphorylation and downstream signaling along with inhibiting VEGFR2-induced migration and vessel outgrowth in vitro [5]. Altogether, such findings suggest a number of contextual effects conveyed in the modulation of the extracellular matrix and affecting cell/tissue adhesion along with synergizing and/or unleashing the active role of its interacting molecular partners. VTN has also been shown to bind IGF-II [6]. This last finding bears potential implications since IGF-II is a commonly secreted growth factor in development and cancer with established auto-stimulatory effects [7-12]. Such interaction has been shown to affect endothelial-mesenchymal transition (Endo-MT) in chick-embryo [13]. IGF-II has been described to exert its cellular effects through activation of the IGF1R and the insulin receptor isoform-A [14-16]. Nonetheless, recent studies have found qualitative differences in downstream signals mediated by these receptors when activated by related ligands [17,18]. Therefore, any molecular agent modulating IGF-II binding with such membrane receptors under physiological and/or pathological conditions, could bear unexploited value towards fine-tuning IGF-II actions (eg: in the clinical setting). In this context, the specific role of extracellular microenvironment components in affecting the IGF-II intracellular signals both in embryonic and cancer conditions can play a critical biological role. Among these possible novel regulatory checkpoints, the mechanistic and cell signaling events linked to the VTN-IGF-II binding are still unclear. The present study was conceived towards shedding additional light on the existing gap and provide evidence of the effects of VTN on the IGF-II/IRA signaling axis which bears high biological value during embryonal development and in tumorigenesis [9,14].
Materials And Methods
Cell Cultures, Proliferative Assays and Transfection Studies: R- mouse embryo fibroblasts (MEF) were originally established through an igf1r null mutation of mouse embryonic stem cells [19]. R-IRA cells were established though stable transfection of the human insulin receptor exon 11- isoform variant expression plasmid that we previously described [14]. R-IRA mouse embryo fibroblasts used in this study [14,15] were cultured in DMEM supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO) and 0.3 mg/ml Puromycin (Sigma, St. Louis, MO). Human recombinant IGF-II (Sigma, Saint Louis, MO) was used at 10 nM concentration for all the study. For the cell proliferative study cells were cultured to approximately 70% confluency, serum deprived for 12 hours in DMEM serum free, 0.1% bovine serum albumin and stimulated with human recombinant IGFII (10nM, Sigma, St Louis, MO) either in presence or absence of a growing amount of human Vitronectin (Promega, Madison, WI) in the culture media (from 1.5 to 3 micrograms/1ml/ well) for 24 hours prior to transmitted microscopy analysis. For the transfection studies, R-IRA cells were transfected with 2.5 or 5 micrograms, respectively, of the human VTN expression DNA vector (described above) or the corresponding amount of empty vector using Lipofectamine reagent and OptiMEM media (Invitrogen, Carlsbad, CA) for 4 hours and fed overnight with 10% FBS complete DMEM media before 12 hours serum starvation in DMEM, 0.1% Bovine serum albumin followed by growth factor stimulation as described above. Total expression of VTN in transfected R-IRA cells was confirmed by western blot in total cell lysates by anti-His Antibody, Sigma, clone GT359 (not shown).
Wound Streak/Migration Assay: For this assay R-IRA cells were growth in complete medium up to 100% confluence in 6 well plates. Wound streak was applied with a sterile pipet tip to confluent cells followed by serum starvation in 1 ml serum free media (containing BSA 0.1%) in presence or absence of human recombinant IGF-II (10nM, Sigma, St Louis, MO) and human recombinant Vitronectin (2ug/ml/well, Promega, Madison, WI) and migration was monitored and digitally recorded at different time points under inverted optic microscopy for 24 hrs after which time cells were fixed in 4% paraformaldehyde in PBS at 4C for 10 minutes before fixation media removal, replacement with 1ml of PBS (Room Temp.) and inverted digital optic microscopy analysis (DMi1 inverted microscope, Leica Microsystems, Wetzlar, Germany).
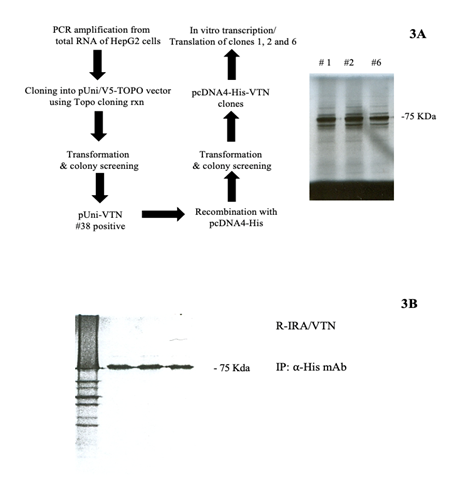
Human Vitronectin Cloning: The human Vitronectin (VTN) gene was obtained from RNA isolated from HepG2 cells (ATCC, Manassas Virginia) using RT-PCR cloning strategy (summarized in figure 3A). cDNA was obtained by retro-transcription of 2 micrograms RNA using Superscript (Invitrogen, Carlsbad, CA). Primers used for VTN gene PCR amplification from cDNA were: 5’-ATGGCACCCCTGAGACCC-3’ (Forward primer) and 5’-CTACAGATGGCCAGGAGCTG-3’ (Reverse primer). The PCR product (1.4 Kb) was cloned into pUni/V5-TOPO vector using a TOPO cloning kit (Invitrogen, Carlsbad, CA) and colonies obtained after transformation of PIR1 competent cells with 2 microliters of the in vitro cloning reaction were screened using SacI. DNA of pUni/V5-TOPO-hVTN obtained from a positive colony was further used for recombination using pcDNA4-His 6x vector (Invitrogen, Carlsbad) using CRE Recombinase (New England Biolabs, Ipswich, MA) according to manufacturer recommendation. Colonies obtained after transformation of Top10F as were screened with SacI and showed 100% correct size. The correct translational product of the pcDNA-His-hVTN construct from three colonies was confirmed via in vitro transcription/translation reaction (Promega, Madison, WI) using S35 a Methionine/Cystidine mix (Perkin Elmer, Waltham, MA). All colonies screened for transcription/translation provided a 72 KDa product consistent with the size of the protein [20] as shown in figure 3A. Efficiency of VTN expression in R-IRA following transient transfection was tested as shown Figure 3B prior using cells for the signaling study.
Membrane-linked VTN-His in R-IRA Cells: The membrane localization of transiently expressed VTN-His construct in R-IRA cells (treated for S35-methionin/cystidine in vivo labeling 8 hrs prior) was assayed in membrane preps obtained by pelleting cells monolayers mechanically scraped from 2 x 150 mm culture dishes in ice-cold Phosphate Saline Buffer, pH 7.4. Cells were collected into propylene tubes, spun down at 3,000 rpm and the dry pellets added with 1 ml cold homogenization buffer (250 mM sucrose, 1 mM EDTA, 10 mM Tris HCl buffer, pH 7.2 plus protease inhibitors). Pellets were resuspended in homogenization buffer, transferred into a glass dounce homogenizer and 20 strokes applied using a Teflon pestle. Samples were transferred to microfuge tubes and sonicated on ice using two 10 second pulses (30 seconds in between pulses) with a sonicator (Branson, Denbury, CT, model 102C). The membrane homogenate was then added with 0.1% NP40 (final C) and for 1hr at 4C in end-over-end rotation. An aliquot of the homogenized prep used for protein measurement (Bradford, Biorad Inc., Hercules, CA). One hundred micrograms of lysed membrane preparation from previously homogenized material was then used for immunoprecipitation using anti-histidine Ab (Sigma, clone GT359) for 1 hr at 4C, end-over-end rotation followed by recovery in prot A/G beads (EMD Millipore, Danvers MA), 30 min, 4C, gently rotating as above. Prot A/G pellets were washed twice with ice cold PBS and denatured 3 minutes at 95C in gel loading buffer (Laemmli) followed by brief microfuge spin. Supernatant was used for SDS PAGE and resolving gel was dried on Whatman paper before undergoing autoradiography.
Antibodies, Western Blotting and Signaling Study: Activation of two downstream targets of the IGF-II/IRA signal, AKT and MAPK, was tested in total cell lysates (RIPA) of VTN transiently-transfected R-IRA cells using monoclonal antibodies against the activated kinases forms. Anti-phospho-AKT1(Ser473), (rabbit monoclonal, clone D9E), anti-phospho ERK1/2 (Thr202/Tyr204) (rabbit polyclonal) and total ERK (monoclonal) were obtained from New England Biolabs (now Cell Signaling Technologies, Ipswich, MA). The Pan-Akt antibody was obtained from Santa Cruz Biotechnologies (Dallas, TX). Thirty micrograms of RIPA total cell lysates were used for SDS-PAGE. Primary antibodies were used at 1:1000 dilution. Horse-radish-conjugated secondary antibody chemo-luminescent signal was obtained with West-PicoPlus kit (Pierce, Waltham, MA) and detected using a LICOR-Odyssey image detection system (Lincoln, NE).
Results
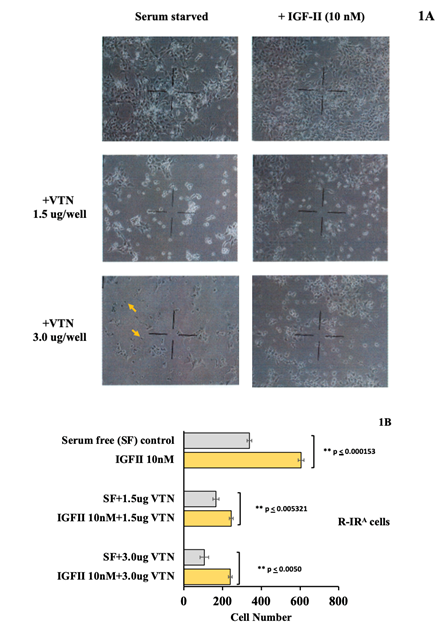
Human recombinant Vitronectin negatively regulates IGF-II-induced cell proliferation in monolayer culture of R-IRA mouse embryo fibroblasts: In order to test a possible influence of Vitronectin on the IGF-II effects in R-IRA, 1.5 micrograms and 3.0 micrograms, respectively, of human recombinant Vitronectin (Promega, Madison, WI) were added to cultured cells following serum starvation with and without IGF-II stimulation (10 nM). Microscopy and cell count were performed at the indicated time. Consistently with our previous studies [14,15], low nanomolar amounts (10 nM) of human recombinant IGF-II (Sigma, St. Louis, MO) triggered proliferation in R-IRA cells through the exclusive activation of the insulin receptor isoform A, due to the genetic knockdown for the IGF1R in this cell line (Figure 1A, compare upper left with upper right picture and 1B upper columns). The effect of human recombinant Vitronectin added post cells-attachment at sub confluency, irrespectively on the amount added to the culture media, displayed a marked anti-proliferative effect on both serum starved and IGF-II stimulated R-IRA cells. In particular, at 1.5 micrograms per well VTN triggered a clear anti-proliferative effect which consensually decreased basal (serum-free) and IGF-II-dependent cell proliferation and markedly reduced the proliferative IGF-II effect (see middle composite pictures in figure 1A and mid columns in figure 1B). The addition of higher amounts of VTN (3.0 micrograms) to serum-starved R-IRA at the end of additional 24 hours had a milder inhibitory effect on cell number compared to exposure to lower amounts of VTN but had a clear effect on cell shape, surface attachment and clustering versus scattering pattern in serum deprived cells (Figure 1A, compare middle left versus lower left picture). Interestingly, “pyknotic” elements (shown with arrows in figure 1 lower left picture) were observed in serum starved R-IRA exposed to 3 ug VTN (this was more evident at higher microscopic magnification). The observed pyknotic elements were still viable as estimated by their ability to incorporate trypan blue dye (not shown). Under the same conditions, 10 nM IGF-II exerted (a) a marked rescue on the VTN scattering pattern and cell morphology (1A, compare left and right pictures for VTN-treated conditions); (b) a statistically significant survival effect (compare mid and lower columns in Figure 1B). Interestingly, IGF-II at the low nanomolar concentration used in the study was able to counteract the VTN spreading activity in these cells.
Figure 1: Effect of human Vitronectin on IGF-II induced proliferation in R-IRA mouse embryo fibroblasts. 1A. Cell proliferative effect of hIGF-II (10 nM) on serum deprived R-IRA cells in absence (1a, top panel) or presence of growing amounts (1a mid panel: 1.5ug, 1a bottom panel: 3 ug) of human recombinant VTN added to the culture media (representative experiment shown). Yellow arrows in 1A bottom panel indicate pyknotic cells (explanation in the text). 1B. Growth and survival effect induced by IGF-II (10 nM) in presence of increasing amount of VTN (n=3, SE and p values are shown).
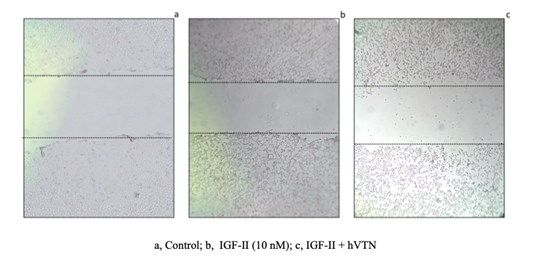
Vitronectin impairs IGF-II-dependent migration in R-IRA cells in vitr: We wished to test whether VTN would affect migration mediated by IGF-II in R-IRA cells, where the IGF-II effects are due solely to the interaction between IGF-II and the stably transfected human insulin receptor isoform A (R-MEF cells are genetically deprived of the mouse igf1r). Therefore we set out a streak/wound healing assay on culture plate as specified under methods which results are summarized in figure 2 and Table 1. The result of three independent assays (Table 1) showed that human recombinant VTN, added to the IGF-II stimulatory medium, significantly interfered with IGF-II-IRA induced migration in an IGF1R-free extracellular context provided by the selected cellular model.
Figure 2: Effect of human Vitronectin on IGF-II-induced migration in R-IRA mouse embryo fibroblasts. Wound streak healing assay was performed on R-IRA cells monolayer. Images in 2a-c, show the neutralizing effect of 2ug VTN (2c) on IGF-II (10 nM n)-induced migration (shown by comparing 2b and 2c) following serum starvation. The starting unstimulated control is shown figure 2a. A representative experiment is shown. Effect quantitation out of three independent experiments is shown in Table 1.
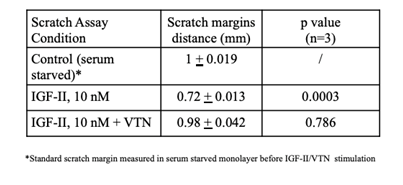
Table 1: Cumulative Effect of human Vitronectin on IGF-II induced migration in R-IRA mouse embryo fibroblasts. The margins distance on 3 independent migration/wound healing experiments performed as in figure 2 were measured per each condition. SE and p values are shown.
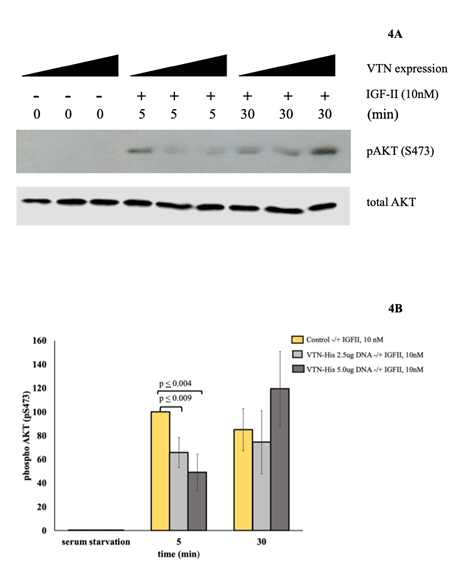
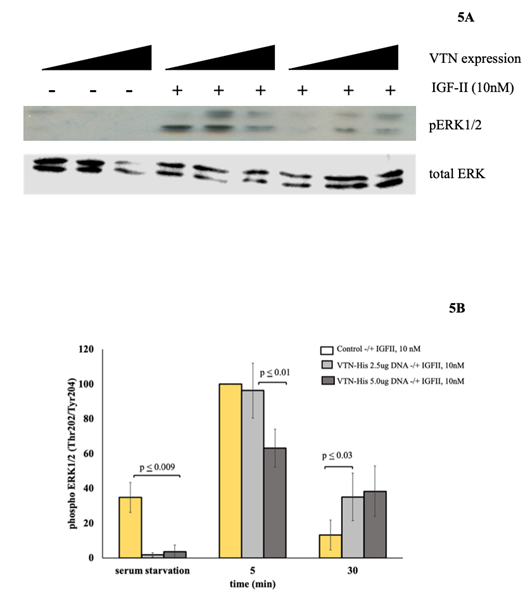
IGF-II mitogenic signaling is differentially modulated in R-IRA cells following transient membrane expression of VTN: In the attempt to establish the effect of hVTN on the IGF-II-IRA ligand-receptor mediated mitogenic signal, we studied the dynamic of phosphorylation of AKT (S473) and ERK1/2 in R-IRA MEF (igf1r-null) cells transiently transfected with increasing amounts of a hVTN expression vector using a previously established protocol [15]. First, we tested the presence of VTN-His in R-IRA cell membranes and found that expressed (His) tagged protein was present in the cell membrane compartment (Figure 3B). Therefore, R-IRA cells were transfected with either 2.5 micrograms or 5.0 micrograms the human VNT-His expression construct described herein (Figure 3) or the corresponding empty vector and stimulated with 10 nM IGF-II for five and thirty minutes, respectively, following 12 hrs serum starvation. As shown in Figure 4A, VTN transfected at 2.5 and 5.0 ug expression vector amounts, respectively, strongly inhibited (from 40 to 60%) the IRA-dependent acute (5 minutes) IGF-II stimulation of AKT (measured by Ser473 phosphorylation), which was previously reported in these cells [14,15]. Interestingly, at 30 minutes post-stimulation the dose-dependent inhibitory effect of VTN on the IGF-II-IRA signal targeting AKT on its Serine 473 was lost, and an unexpected “rebound” phosphorylation was observed on the same phospho Tyrosine site (see blot in figure 4A and column in 4B for the condition R-IRA/VTN5ug + IGF-II, 30 minutes). This result was not completely surprising since phosphorylation on critical intracellular transduction components is usually reinstated by redundant mechanisms when high doses of a steady-state inhibitory signal is applied to the cell. For example, AKT paradoxical activation is observed in response to wild type BRAF inhibition using its PLX4032 inhibitor [21]. In these cases, AKT is thought to be activated through a non-canonical PI3K-pathway downstream of RAS [22]. A similar non-canonical signaling route could be responsible for the observed “escape” to the higher inhibitory levels of VTN on the IGF-II-IRA action following 30 minutes stimulation. The same specimen used for the AKT activation experiments were used to determine ERK1/2 activation by the above ligand/receptor axis via SDS-PAGE and western blotting with a specific phospho-ERK/1/2 (Thr202/Tyr204 Ab). As a result (summarized in figure 5A and 5B), the acute (5 minutes) ERK-1/2 activation peak induced by the IGF-II-IRA signal was significantly decreased in a VTN-expression dose-dependent pattern. ERK1/2 activation was measured by antibody detection of ERK1-p44MAPK Thr202/Tyr204 phosphorylation (corresponding to residues 185/187 on the ERK2-p42MAPK ortholog), (Figure 4B, middle columns) as we previously described [14]. At 30 minutes stimulation with IGF-II, Higher VTN expression in the same cells caused a variable trend of activation (which was reproducible but not statistically significant). Consistently to the findings for AKT1 S473 phosphorylation (that could be also interpreted as a “peak delay”), and if confirmed by further investigation(s), this finding strengthens the hypothesis of a potential rebound mechanism, described in the literature as “MAPK reactivation” caused by a harsh block of an upstream activator (eg: BRAF) [23]. Furthermore, it would further support the role of VTN on the regulation of AKT1 and ERK1/2-mediated signals triggered by the IGF-II-IRA ligand-receptor system under physiological and/or pathological circumstances associated to VTN exuberant production.
Figure 3: Cloning and transient expression of hVTN on R-IRA EC membranes. 3A. Left, strategy used for hVTN PCR cloning from HepG2 cells and subcloning in eukaryotic expression vector (pcDNA4-His tag). 3A, right, cell-free transcription/translation in rabbit reticulocytes (by S35-methyonine authoradiography). 3B. Transient expression of hVTN-His construct in R-IRA cells. Membrane preparations were obtained from Met/Cys-S35 labeled cells and immunoprecipitated VTN (anti-His Ab) resolved on gel and used for autoradiography. Details under methods.
Figure 4: Effect of transiently expressed hVTN on the IGF-II-induced activation of AKT in R-IRA cells (time course). 4A. Western blot. Growing amounts of hVTN expression construct (2.5ug and 5ug plasmidic DNA) were used for transient expression in R-IRA cells as described under methods. Control and transfected cells were serum deprived before IGF-II stimulation (10nM) at the time points shown. Protein normalized total cell lysates were used for western blot for total AKT and phospho-AKT-S473. SE of individual bands densitometric values out of 3 independent experiments is shown in figure 4B. SE and p values are shown.
Figure 5: Effect of transiently expressed hVTN on the IGF-II-induced activation of MAP2K-ERK2/4 in R-IRA cells (time course). Cell extracts from same conditions shown in Figure 4 were used for SDS-PAGE and Western blot analysis of ERK1/2 activation using anti-phospho-specific antibody (5A top panel) and total ERK1/2 (5A bottom panel). A graph of 3 independent experiments is shown in figure 5B (densitometry, SE and p values are shown).
Discussion
Physical and functional interactions of extracellular matrix cellular components and cell adhesion molecules with ligands and growth factor receptors have been previously described [5,24-27]. Vitronectin (VTN) along with Fibronectin contribute to the formation of the extracellular matrix, which is part of the so called “stromal” tissues component on which cellular interactions allow specialized cells (eg. epithelial) to form their parenchymal tissue [28]. Such organization is typically altered during a variety of pathological conditions, such as it occurs in cancer, where “loosening” of the extracellular matrix as that conveyed in quantitative and/or qualitative changes in VTN, cause alterations of tumor microenvironment with detachment and displacement of local cellular components resulting in invasion and distant tissues colonization, also known as metastasis [4,29,30]. IGF-II is a commonly secreted growth factor which has been found in a wide number of solid cancers at advanced stages where it establishes an autocrine stimulatory loop through both the IGFIR and the Insulin receptor isoform A. IGF-II is associated to angiogenic properties in vivo [31,32] and displays angiogenic actions in vitro [33] and we recently found it to be a tight regulator of EphB4 protein expression through activation of the Insulin receptor A in malignant mesothelioma cell lines [11]. Therefore, the control of extracellular levels and bioavailability of IGF-II (either cancer-secreted or by cancer-activated fibroblasts, CAFs) offers a relevant biological topic. In this regard, mature IGF-II (7.5KDa) has been previously shown to interact and/or form complexes with IGFBP3 and 7. Furthermore, IGF-II binds with high affinity with its membrane scavenger receptor (the Igf2/M6P receptor) which, for its IGF-II lowering effect in vivo, has been found to bear anti-tumor suppressor properties [34,35]. IGF-II has also been shown to be present in high molecular weight forms spanning from 10 to ~18 KDa (known as Big-IGF-II) establishing variable MW complexes between 34 to 45 KDa with IGFBPs binding with less affinity compared to its mature form typically found in the bloodstream as a tertiary complex at ~150KDa [36,37]. Such high molecular variants, typically secreted by cancer cells, have been found to have comparable binding and signaling affinities to its mature (7.5KDa) ligand with the IGFIR and Insulin Receptor-A [38]. Interestingly, big-IGF-II secreted by Malignant Mesothelioma which we found complexed in its extracellular 45KDa form (typical of its IGFBP2/3 binding) was a key autocrine regulator of EphB4 ectopic expression in these cancer cells [11], suggesting that the type and binding affinities of IGF-II cancer secreted variants with extracellular binding factors must be taken in consideration when evaluating the resulting biologic effects [18]. Accordingly, other studies have demonstrated a functional interaction between VTN and IGF-II in the extracellular environment [6,13]. However, to date experimental evidences linking the IGF-II and VTN extracellular interaction with specific mitogenic signals activation had been missing although indirectly assumed [13]. AKT1 and p42/44 MAPKs (also known as ERK1 and ERK2) are established molecular cross-roads for growth-factors triggered mitogenic signals mediated by their specific Receptor Tyrosine Kinases (RTKs). They are also activated in response to other extracellular-generated signals mediated by cell-cell contact via both classical junctional molecular clusters as well as by bidirectional kinases like the Eph/Ephrin RTK family. Furthermore, a number of studies have established the role of phosphorylative post-translational modifications (PTMs) on these mitogenic pathways common to the Insulin and IGF receptors signal and canonically activated via the IRS(1/2)-PI3K(p85/110)-mTORC2 pathway for AKT1 and via a IRS1/2-GRB2-SOS1-RAS-BRAF-MEK pathway in the case of ERK1/2 (reviewed in Mendoza MC et al.) [39]. In this context, the role and functional meaning of AKT1Ser473 phosphorylation by mTORC2 has been found to be directly related to its kinase activation (reviewed by Manning & Toker) [40]. Similarly, the role of Thr202 and Tyr204 phosphorylation on ERK2 (corresponding to Thr187 and Tyr189 on ERK1) has been regarded as a reliable indirect method for evaluating ERKs kinase activation upon demonstration of the role of these PTMs for MAPK activation [41]. By making use of widely recognized anti-phospho antibodies raised, respectively against phospho-AKT (Thr473) and ERK1/2 (Thr187/202-Tyr189/204), the present study provides a direct evidence of the effect and modulating potential of hVTN on IGF-II-induced intracellular signaling. Furthermore, the present study suggests that the levels of expression and/or concentration of VTN in the EC microenvironment and possibly the underlying IGF-II/VTN ratio may play a critical as underscored role towards the IGF-II proliferative/survival rescue effect in vivo. Accordingly, the presented findings shed light on the ability of the VTN/IGF-II extracellular interaction to exert its observed biological effects by modulation of AKT1 and ERK1/2 activation dynamics where acute versus sustained post-translational modifications at critical signals cross-roads may justify the formation of differential complexes resulting in the observed effects. Noteworthy, VTN has been found to interacts with alpha5-beta3 in the EC environment [42]. Therefore, studies addressing the specific role of the VTN sub-regions involved in the IGF-II neutralizing/modifying effects on AKT1-ERK1/2 activation should be taken in consideration in cellular models prior to test the practical potential for the use of VTN and/or VTN-derived molecules in the modulation of IGF-II effects at the preclinical level. Ultimately, the present study confirms the role of the extracellular microenvironment and the interaction between EC matrix components and cell-secreted factors as key target for the modulation of biological effects towards potential therapeutic strategies.
Acknowledgments:
P.S. Was a recipient of the Gian-Amico Alessandrini Early Faculty Award in Onco-Genomic Research. E.H. Passed away in 2017. We dedicate this study to her memory.
Author Contributions:
Conceptualization, funding acquisition, investigation, formal analysis, original draft preparation, P.S.; investigation, E.H, A.D; data curation, original draft preparation, S.J.W.; funding acquisition, resources, A.G.
Conflicts of Interest:
“The authors declare no conflict of interest”.
References
- Maile LA, Aday AW, Busby WH, et al. Modulation of integrin antagonist signaling by ligand binding of the heparin-binding domain of vitronectin to the αVβ3 integrin. Journal of Cellular Biochemistry 105 (2008): 437-446.
- Chain D, Kreizman T, Shapira H, et al. Plasmin cleavage of vitronectin Identification of the site and consequent attenuation in binding plasminogen activator inhibitor-1 FEBS Letters (1991): 251-256.
- Chavakis T, Kanse SM, Yutzy B, et al. Vitronectin concentrates proteolytic activity on the cell surface and extracellular matrix by trapping soluble urokinase receptor-urokinase complexes. Blood, The Journal of the American Society of Hematology 91 (1998): 2305-2312.
- Bafetti LM, Young TN, Itoh Y, et al. Intact vitronectin induces matrix metalloproteinase-2 and tissue inhibitor of metalloproteinases-2 expression and enhanced cellular invasion by melanoma cells. Journal of Biological Chemistry 273 (1998): 143-149.
- Wang L, Zhang X, Pang N, et al. Glycation of vitronectin inhibits VEGF-induced angiogenesis by uncoupling VEGF receptor-2–α v β 3 integrin cross-talk. Cell Death & Disease 6 (2015): e1796.
- Upton Z, Webb H, Hale K, et al. Identification of vitronectin as a novel insulin-like growth factor-II binding protein. Endocrinology 140 (1999): 2928-2931.
- Dynkevich Y, Rother KI, Whitford I, et al. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocrine Reviews 34 (2013): 798-826.
- Logie A, Boulle N, Gaston V, et al. Autocrine role of IGF-II in proliferation of human adrenocortical carcinoma NCI H295R cell line. Journal of Molecular Endocrinology 23 (1999): 23-32.
- Louvi A, Accili D, Efstratiadis A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Developmental Biology 189 (1997): 33-48.
- Rogers MA, Kalter V, Strowitzki M, et al. IGF2 knockdown in two colorectal cancer cell lines decreases survival, adhesion and modulates survival-associated genes. Tumor Biology 37 (2016): 12485-12495.
- Scalia P, Pandini G, Carnevale V, et al. Identification of a novel EphB4 phosphodegron regulated by the autocrine IGFII/IR A axis in malignant mesothelioma. Oncogene 38 (2019): 5987-6001.
- Xu WW, Li B, Guan XY, et al. Cancer cell-secreted IGF2 instigates fibroblasts and bone marrow-derived vascular progenitor cells to promote cancer progression. Nature Communications 8 (2017): 14399.
- Arciniegas E, Neves YC, Carrillo LM. Potential role for insulin-like growth factor II and vitronectin in the endothelial–mesenchymal transition process. Differentiation 74 (2006): 277-292.
- Frasca F, Pandini G, Scalia P, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Molecular and Cellular Biology 19 (1999): 3278-3288.
- Scalia P, Heart E, Comai L, et al. Regulation of the Akt/Glycogen synthase kinase-3 axis by insulin-like growth factor-II via activation of the human insulin receptor isoform- Journal of Cellular Biochemistry 82 (2001): 610-618.
- Xu Y, Kirk NS, Venugopal H, et al. How IGF-II Binds to the Human Type 1 Insulin-like Growth Factor Receptor. Structure (2020).
- Morcavallo A, Gaspari M, Pandini G, et al. Research resource: new and diverse substrates for the insulin receptor isoform A revealed by quantitative proteomics after stimulation with IGF-II or insulin. Molecular Endocrinology 25 (2011): 1456-1468.
- Scalia P, Giordano A, Williams SJ. The IGF-II–Insulin Receptor Isoform-A Autocrine Signal in Cancer: Actionable Perspectives. Cancers 12 (2020): 366.
- Sell C, Dumenil G, Deveaud C, et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Molecular and Cellular Biology 14 (1994): 3604-3612.
- Suzuki S, Oldberg A, Hayman EG, et al. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. The EMBO Journal 4 (1985): 2519-2524.
- Shi H, Hong A, Kong X, et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discovery 4 (2014): 69-79.
- Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes & Cancer 2 (2011): 261-274.
- Gibney GT, Messina JL, Fedorenko IV, et al. Paradoxical oncogenesis—the long-term effects of BRAF inhibition in melanoma. Nature reviews Clinical Oncology 10 (2013): 390-399.
- Brooks PC, Klemke RL, Schon S, et al. Insulin-like growth factor receptor cooperates with integrin alpha v beta 5 to promote tumor cell dissemination in vivo. The Journal of clinical investigation 99 (1997): 1390-1398.
- Fujita M, Ieguchi K, Davari P, et al. Cross-talk between integrin α6β4 and insulin-like growth factor-1 receptor (IGF1R) through direct α6β4 binding to IGF1 and subsequent α6β4-IGF1-IGF1R ternary complex formation in anchorage-independent conditions. Journal of Biological Chemistry 287 (2012): 12491-12500.
- Miles FL, Sikes RA. Insidious changes in stromal matrix fuel cancer progression. Molecular Cancer Research 12 (2014): 297-312.
- Zhu CQ, Popova SN, Brown ER, e al. Integrin α11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proceedings of the National Academy of Sciences 104 (2007): 11754-11759.
- Bornstein P, Ash JF. Cell surface-associated structural proteins in connective tissue cells. Proceedings of the National Academy of Sciences 74 (1977): 2480-2484.
- Hurt EM, Chan K, Duhagon Serrat MA, et al. Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells 28 (2010): 390-398.
- Shi K, Lan RL, Tao X, et al. Vitronectin significantly influences prognosis in osteosarcoma. International Journal of Clinical and Experimental Pathology 8 (2015): 11364.
- Björndahl M, Cao R, Nissen LJet al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proceedings of the National Academy of Sciences 102 (2005): 15593-15598.
- Ritter MR, Dorrell MI, Edmonds J, et al. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proceedings of the National Academy of Sciences 99 (2002): 7455-7460.
- Lee OH, Bae SK, Bae MH, et al. Identification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis models. British Journal of Cancer 82 (2000): 385-391.
- De Souza AT, Hankins GR, Washington MK, et al. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nature Genetics 11 (1995): 447-449.
- Hankins GR, De Souza AT, Bentley RC, et al. M6P/IGF2 receptor: a candidate breast tumor suppressor gene. Oncogene 12 (1996): 2003.
- Baxter RC, Daughaday WH. Impaired formation of the ternary insulin-like growth factor-binding protein complex in patients with hypoglycemia due to nonislet cell tumors. The Journal of Clinical Endocrinology & Metabolism 73 (1991): 696-702.
- Oesterreicher S, Blum WF, Schmidt B, et al. Interaction of Insulin-like Growth Factor II (IGF-II) with Multiple Plasma Proteins High Affinity Binding Of Plasminogen To Igf-Ii and Igf-Binding Protein-3. Journal of Biological Chemistry 280 (2005): 9994-10000.
- Marks AG, Carroll JM, Purnell JQ, et al. Plasma distribution and signaling activities of IGF-II precursors. Endocrinology 152 (2011): 922-930.
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends in Biochemical Cciences 36 (2011): 320-328.
- Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell 169 (2017): 381-405.
- Canagarajah BJ, Khokhlatchev A, Cobb MH, et al. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90 (1997): 859-869.
- Schiller HB, Szekeres A, Binder BR, et al. Mannose 6-phosphate/insulin-like growth factor 2 receptor limits cell invasion by controlling αVβ3 integrin expression and proteolytic processing of urokinase-type plasminogen activator receptor. Molecular Biology of the Cell 20 (2009): 745-756.


 Impact Factor: * 3.0
Impact Factor: * 3.0 Acceptance Rate: 76.32%
Acceptance Rate: 76.32%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
