Do Post-Operative Electrocardiographic (ECG) Changes Indicate any Underlying Graft Problem after CABG? Correlation of Early Post-Operative ECG Changes with CT Coronary Angiography (CTCA)
Abhishek Rajendra Potnis1*, Sushrut Suhas Potwar2, Balaji Aironi3, Dwarkanath Vivekanand Kulkarni4, Uday Eknath Jadhav5, Gaurish Omprakash Sawant6
1Seth GS Medical college and KEM hospital, Mumbai, Maharashtra, India
2Additional Professor, Seth GS Medical college and KEM hospital, Mumbai, Maharashtra, India
3Additional Professor, Seth GS Medical college and KEM hospital, Mumbai, Maharashtra, India
4Professor, Seth GS Medical college and KEM hospital, Mumbai, Maharashtra, India
5Professor and Head of Department, Seth GS Medical college and KEM hospital, Mumbai, Maharashtra, India
6Seth GS Medical college and KEM hospital, Mumbai, Maharashtra, India
*Corresponding author: Abhishek Rajendra Potnis, Seth GS Medical college and KEM hospital, Mumbai, Maharashtra, India.
Received: 5 May 2023; Accepted: 12 May 2023; Published: 19 June 2023
Article Information
Citation: Abhishek Rajendra Potnis, Sushrut Suhas Potwar, Balaji Aironi, Dwarkanath Vivekanand Kulkarni, Uday Eknath Jadhav, Gaurish Omprakash Sawant. Do Post-Operative Electrocardiographic (ECG) Changes Indicate any Underlying Graft Problem after CABG? Correlation of Early Post- Operative ECG Changes with CT Coronary Angiography (CTCA). Cardiology and Cardiovascular Medicine. 7 (2023): 162-168.
View / Download Pdf Share at FacebookAbstract
Purpose: Identifying occult and potentially life-threatening problems in the absence of hemodynamic instability and elevation of cardiac markers has always remained a diagnostic challenge in patients after coronary artery bypass graft (CABG). A study to identify these threats by comparing ECG changes to CTCA changes is thus essential to isolate and evaluate highrisk cases.
Methods: First 45 post-CABG patients having ECG changes at the 0th, 4th, 18th hour, and follow-up were subjected to computed tomography coronary angiography (CTCA), and results were evaluated using the Chisquare test to establish a correlation.
Results: Absolute high-risk and relative high-risk profiles of patients were established in the present study. Patients with EF 50-60%, with native right coronary involvement and graft to posterior left ventricular (PLV) artery are at absolute risk of having ECG changes translated into CTCA changes.
Conclusion: The study addresses the quintessential question ‘which ECG changes in the postoperative period should be given immediate attention and which ones can be safely ignored?’
Keywords
<p>CABG; CT coronary angiography; ECG changes; Graft patency</p>
Article Details
1. Introduction
An electrocardiogram (ECG) is the first choice of test in many cardiovascular diseases; however, interpretation of normal and abnormal findings in ECG is difficult [1,2]. Correlation between 12 lead ECG and affected coronary territory is already established. S-T elevation in contiguous leads is highly sensitive for the diagnosis of early myocardial infarction (MI). The etiologies of MI during coronary artery bypass graft (CABG) include coronary vasospasm, occlusion, complications of anastomosis, cardioplegia, and aortic cross-clamping. This diagnosis is difficult because the accompanying changes are often attributed to normal postoperative changes[3,4] .Thus, in a post-operative CABG, the association between ECG changes in a particular coronary territory and its translation into graft patency/ occlusion has not been established. This was revealed with the use of computerized tomography (CT) coronary angiography in early period post-surgery to check for graft patency in the territory which had shown ECG changes. The study aimed to identify the ECG changes, in the absence of hemodynamic instability or deranged cardiac markers, which need to be addressed immediately and the ones that can be safely ignored in the immediate post-operative period.
2. Materials and Methods
The first forty-five patients having undergone CABG surgery at our institution were analyzed for post-operative significant ECG changes (S-T changes, new q waves, new onset left bundle branch blocks) at 0, 4, and 18 hours and at follow-up and were included in the current study. Table 1 shows the contiguous leads used to identify the coronary territory involved. Only these patients underwent CT coronary angiography after 1 month and the results correlated with ECG changes. The graft evaluation was done as per Fitzgibbon classification of grafts5: A (excellent), B(stenosed), and C(occluded). The patients were analyzed on the basis of age groups, presence of comorbidities, ejection fraction, original disease, on-pump v/s off-pump surgery performed, native coronary territory involved, limb and precordial lead changes noted, and grafts performed. The statistical significance of these results was established using Chi-square test.
|
Leads with ECG changes |
Affected myocardial area |
Occluded coronary artery |
|
V1-V2 |
Septal |
Proximal LAD |
|
V3-V4 |
Anterior |
LAD |
|
V5-V6 |
Apical |
Distal LAD, LCX or RCA |
|
I, aVL |
Lateral |
LCX |
|
II, III, aVF |
Inferior |
90% RCA, 10% LCX |
|
V7-V9 (reciprocal ST depression) |
Posterolateral |
RCA or LCX |
LAD, left anterior descending; LCX, left circumflex artery; RCA, right coronary artery
Table 1: ECG representation of various coronary territories
2.1 Statistical Analysis:
The statistical software used was SAS version 6.09 on the Unix platform. For dichotomous variables, the chi-squared test or Fischer’s exact test was used. Two tailed p <0.05 was considered significant. Odds ratio and 95% confidence interval based on standard error of logistic coefficient and two tailed probability value are presented. Forty five patients developed significant ECG changes and were subjected to CTCA. The results were then compared to patient profiles to identify correlation using Spearman’s correlation coefficient.
3. Results
The results obtained in the study were as follows:
3.1 Age and correlation between ECG and CTCA:
|
Age |
ECG changes at 0 hrs |
Changes at 4 hrs |
Changes at 18 hrs |
Follow up ECG |
% persistent changes |
|
40-50 |
6 |
9 |
9 |
3 |
33.33 |
|
51-60 |
18 |
18 |
15 |
12 |
60 |
|
61-70 |
15 |
15 |
9 |
9 |
100 |
|
71-80 |
3 |
0 |
0 |
0 |
0 |
|
total |
42 |
42 |
33 |
24 |
72.72 |
p-value, 0.1704
Table 2: Comparison of age of patients with ECG changes
Table 2 shows that the maximum number of ECG changes are seen in the age group 51-60 years; however, most of the changes persisted even at follow-up in the age group 61-70 yrs. Overall, no statistically significant difference was obtained between age group and ECG changes.
|
Age |
Follow up ECG |
CTCA changes |
% |
|
40-50 |
3 |
0 |
0 |
|
51-60 |
12 |
6 |
50 |
|
61-70 |
9 |
6 |
66.66 |
|
71-80 |
0 |
0 |
0 |
|
total |
24 |
12 |
50 |
p-value, 0.4066; CTCA, computed tomographic coronary angiography
Table 3: Comparison of age, ECG, and CTCA changes
As seen from Table 3, patients in the age group 61-70 years showed the maximum percentage of CT coronary angiographic changes corresponding to electrocardiographic changes. However, the results showed no statistical significance, thus indicating no correlation of age groups to electrocardiographic or corresponding CTCA changes.
3.2 Original disease and correlation with ECG and CTCA:
|
Original disease |
ECG changes at 0 hr |
Changes at 4 hr |
Changes at 18 hr |
Follow-up ECG changes |
% persistent changes |
|
Triple vessel disease 21 |
21 |
15 |
18 |
15 |
83.33 |
|
Left main double vessel disease 3 |
3 |
3 |
3 |
0 |
0 |
|
Left main triple vessel disease 21 |
21 |
18 |
9 |
6 |
66.66 |
p-value, 0.3596
Table 4: Comparison of original disease and ECG changes
Table 4 indicates that most non-Left-main triple vessel disease patients show persistence of ECG changes however, the p-value was statistically insignificant indicating no correlation between the original disease and the appearance of ECG changes
|
Original disease |
Follow-up ECG changes |
Follow-up CTCA changes |
% |
|
Triple vessel disease 21 |
15 |
6 |
40 |
|
Left main double vessel |
0 |
0 |
0 |
|
disease 3 |
|||
|
Left main triple vessel disease 21 |
6 |
6 |
100 |
p-value, 0.2188; CTCA, computed tomographic coronary angiography
Table 5: Comparison of the original disease, ECG and CTCA changes
Table 5 shows that all left main disease patients showing ECG changes had corresponding CTCA changes; however, these changes were statistically insignificant. Hence, in the current study, no correlation between the original disease and ECG and CTCA changes could be observed.
3.3 Ejection fraction and correlation with ECG and CTCA:
|
EF |
ECG changes at 0 hr |
ECG changes at 4 hr |
ECG changes at 18 hr |
CTCA changes at follow up |
% persistent changes |
|
30-40 9 |
6 |
9 |
6 |
6 |
100 |
|
40-50 18 |
15 |
18 |
18 |
12 |
66.66 |
|
50-60 18 |
18 |
15 |
9 |
6 |
66.66 |
|
total 45 |
39 |
42 |
33 |
24 |
72.72 |
p-value, 0.1639; CTCA, computed tomographic coronary angiography
Table 6: Comparison of ejection fraction and ECG changes
Table 6 demonstrates that patients with a low ejection fraction (EF) showed maximum percentage of persistence of ECG changes at follow-up, though such results were statistically insignificant.
|
EF (%) |
Frequency |
Follow-up ECG changes |
CTCA changes |
P value |
|
30-40 |
9 |
6 |
0 |
0.00621 |
|
40-50 |
18 |
12 |
3 |
0.00342 |
|
50-60 |
18 |
6 |
9 |
0.2495 |
|
Total |
45 |
24 |
12 |
- |
p-value, 0.01111; CTCA, computed tomographic coronary angiography
Table 7:Comparison of EF, ECG and CTCA changes
Comparison of ECG and CTCA results showed statistically significant differences in p values for EF 30-40 and 40-50, thereby indicating no correlation between ECG and CTCA for these EF groups, but in EF group 50-60 years ECG changes corresponded to CTCA changes. ECG changes in a patient with EF 50-60 are more indicative of an underlying problem than for lower EF groups as indicated in Table 7.
3.4 Comorbidities and relation with CTCA:
|
Comorbidities |
N |
CTCA changes |
% |
|
DM |
24 |
3 |
12.5 |
|
HTN |
27 |
9 |
33.33 |
|
CVA |
3 |
0 |
0 |
|
HC |
3 |
0 |
0 |
p-value, 0.1642; DM, diabetes mellitus; HTN, hypertension; CVA, cerebrovascular accident; HC, hypercholesterolemia
Table 8:Comparison of comorbidities and CTCA changes
No correlation was obtained between comorbid patients having ECG changes and CTCA changes seen from Table 8.
3.5 On pump v/s Off-pump CABG and its relation to CTCA changes :
|
Type |
ECG changes |
CTCA changes |
% |
|
on pump |
15 |
6 |
60 |
|
off pump |
30 |
6 |
20 |
p-value, 0.1528; CABG, coronary artery bypass graft; CTCA, computed tomography coronary angiography
Table 9: Comparison of on-pump vs off-pump CABG, ECG and CTCA changes
No statistically significant difference was obtained between on-pump and off-pump cases with respect to ECG changes and CTCA changes, as shown in Table 9.
3.6 Native territory involvement and correlation with ECG and CTCA changes
|
Native territory involved |
ECG changes at 0 hr |
ECG changes at 4 hr |
ECG changes at 18 hr |
Follow up changes |
% |
|
LAD 39 |
27 |
30 |
21 |
18 |
46.15 |
|
LCX 48 |
27 |
33 |
21 |
15 |
31.25 |
|
RCA 36 |
15 |
15 |
9 |
12 |
33.33 |
|
Total 123 |
69 |
78 |
51 |
45 |
35.43 |
p-value, 0.006584; CTCA, computed tomography coronary angiography; LAD, left anterior descending; LCX, left circumflex artery; RCA, right coronary artery
Table 10:Comparison of native territory involvement and persistence of ECG changes
Table 10 shows a significant correlation (p=0.006) between the appearance of ECG changes in any coronary territory and its persistence at follow-up.
|
Native Territory involved |
Initial ECG changes |
Follow-up ECG changes |
CTCA changes |
P value |
|
LAD |
39 |
18 |
0 |
2.5E-06 |
|
LCX |
48 |
15 |
3 |
0.00201 |
|
RCA |
36 |
12 |
9 |
0.302 |
|
Total |
123 |
45 |
12 |
p-value, 0.00405; CTCA, computed tomography coronary angiography; LAD, left anterior descending; LCX, left circumflex artery; RCA, right coronary artery
Table 11:Comparison of native territory involvement, ECG and CTCA changes
Table 11 elucidates that there is a significant difference in the ECG changes and CTCA changes in native lesions of left anterior descending (LAD) and left circumflex artery (LCA) territories, while native right coronary artery territory involvement showing ECG changes translates into CTCA changes and thus, necessitates vigilance even in a hemodynamically stable patient.
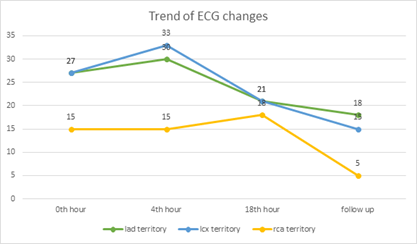
Figure 1 shows the trend of ECG changes in comparison with coronary territories. Further, 46.1% of patients have persistent changes in the LAD territory, 31.25% in the LCX territory, and 33.33% in the RCA territory.
3.7 Grafted vessels and correlation with changes in the corresponding territory in ECG and CTCA status:
|
Grafts |
No of changes |
ECG changes |
CTCA block |
P value |
|
LAD grafts |
45 |
33 |
0 |
<0.0000001 |
|
OM grafts |
48 |
18 |
6 |
0.00476 |
|
diagonal grafts |
15 |
12 |
0 |
2.1E-05 |
|
PDA grafts |
21 |
9 |
3 |
0.04384 |
|
PLV grafts |
9 |
6 |
6 |
0.3085 |
|
Distal RCA grafts |
6 |
3 |
0 |
0.02275 |
p-value, 0.0005135; OM, obtuse marginal arteries; LAD, left anterior descending; PLV, posterior left ventricular graft
Table 12:Comparison of grafts, ECG and CTCA changes
PDA, posterior descending artery; RCA, right coronary artery.
The study shows that ECG changes post-surgery in posterior LVA graft territory translate into CTCA changes. However, grafts to the posterior descending artery, distal right coronary artery, obtuse marginal arteries, diagonal and left anterior descending arteries show a statistically significant difference in ECG changes and CTCA in descending order of importance, i.e. ECG changes in LAD showing the least correlation to CTCA changes while PDA shows relatively higher correlation (Table 12).
3.8 Type of conduit and changes in CTCA:
|
CTCA changes |
Arterial grafts |
Vein grafts |
|
Fitzgibbon A |
66 |
66 |
|
Fitzgibbon B |
0 |
3 |
|
Fitzgibbon C |
0 |
12 |
|
Total |
66 |
81 |
CTCA, Computed tomography coronary angiography
Table 13:Grading of grafts (Arterial v/s Venous) on CTCA
Table 13 shows that the CTCA findings show excellent graft patency (Fitzgibbon A) with arterial grafts as compared to vein grafts, thus showing its importance as an essential graft in a CABG unless contraindicated. Other unconventional findings like apical ballooning (n=1) and focal ventricular outpouching in basal inferior segments (n=3) have also been noted in the CTCA (Table 13)
4. Discussion
Graft occlusion is one of the major determinants of post-operative prognosis in CABG patients. In the early postoperative period, ECG monitoring is routinely performed to check for the involvement of certain territories and their correlation with specific grafts. In the early postoperative period, days to weeks after surgery technical problems and thrombotic activation lead to thrombotic occlusion of venous grafts in approximately 5-10% of cases. Within the 1st year after surgery, the causes are intimal hyperplasia and thrombosis in 10-15% of cases. After 1st year, atherosclerosis predominates at 40-60%[5,6,7] . If arterial grafts are patent after 1 week of surgery, they have a 10-year patency rate of 88%. Despite limitations to CTCA studies due to the presence of metallic clips in arterial grafts that interfere with the visualization of lesions, severe coronary calcifications in native vessels and reduction of imaging quality due to premature atrial or ventricular contractions, CTCA has proven to be more sensitive and specific than echocardiography and magnetic resonance imaging (MRI) in imaging of coronaries and bypass grafts [8,9] . These grafts are accepted by patients due to low risk of complications as compared to invasive angiography. CTCA is rightly termed as a ‘potential gatekeeper’ in a previous meta-analysis[10] . Donkol et al and Hendel et al suggested that indications for CTCA in post-CABG patients were persistent symptoms, suspected graft failure, suspected disease progression in native coronary arteries or grafts; and an understanding of the topography of graft anatomy is needed before attempting to engage graft ostia and evaluate graft body[11,12] . However, literature regarding which cardiac profiles are definitive candidates for CTCA has been mentioned in prospects by Donkol et al[11] which has been the main prospect of the current study. Cardiac enzymes have not been used for postoperative monitoring as there are neither uniform recommendations as to which enzymes to analyze nor any cut-off levels prescribed indicating a need for further diagnostic interventions[13] .CTCA evaluation was performed at 1 month to rule out edema at the graft anastomotic site and to avoid late graft attrition unlike the Surgical Management of Arterial Revascularization Therapies (SMART) trial[14] ; however, the results of the present study correspond to the SMART trial with no difference in patency rates between on and off-pump surgeries. The marginally lower graft patency rates (89.5%) could be attributed to complex coronary anatomy, smaller size coronary vessels, and the inclusion of low ejection fraction patients. Glineur et al[15] documented similar patency rates for sequential grafts to distal right coronary artery (RCA) and posterior descending artery (PDA). Persistent ECG changes and subsequent CTCA changes were noted in our study in EF 50-60% group highlighting the importance of ECG monitoring in patients with normal ejection fraction. A total of 35.4% of grafts showed persistent ECG changes of which 46.1% belonged to the LAD artery. Comparison with CTCA showed no LAD graft abnormality; thus, our study maintains coherence with the results of arterial grafts in previous literature. The RCA territory instead showed a significant correlation between persistent ECG changes and subsequent CTCA abnormality, probably owing to higher venous conduit usage and sequential pattern of anastomosis to PDA and posterolateral vessel (PLV) arteries; This finding corroborates earlier one by Mueller et al.[16] .The higher percentage (33.33%) of CTCA changes in hypertensive patients having venous grafts establishes a correlation between hypertension and neointimal hyperplasia responsible for venous graft occlusion on being subjected to high aortic pressures.
The uniqueness of the study lies in its role in highlighting a specific high-risk cardiac profile, which must be given more significance when ECG changes appear in the post op period. This study also emphasizes the fact that even though the grafts fail on ECG and CTCA, they cause imperceptible immediate clinical consequences to the patients and this finding supports that by Loeb et al[17]; however, the changes might pose a threat to the patient in the long run. The authors claim the following cardiac profile to be at absolute high risk for a positive correlation between ECG changes and graft blocks:
- Normal Ejection fraction 50-60%.
- Native right coronary artery involvement.
- Grafts to posterior left ventricular artery.
The relative high-risk profile as per our study for the same being:
- Age group 61-70 years.
- Left main involvement with triple vessel disease.
- Ejection fraction 30-40%.
- Hypertensive patient.
- Grafts to the posterior descending artery.
Endpoints:
High risk profile patients are identified and counselled regarding potential threat to bypass grafts. These patients are requested to maintain follow up with absolute compliance. Similarly, the ECG changes that can be attributed to reasons other than graft occlusion have been identified and these patients need not be subjected to CT scan
Limitations:
It is important to put light on the type of grafts used and their various combinations, the type of anastomoses performed, the size of the native coronary vessel, the way how grafts lie, flow studies of grafts, and long-term study of patency rates which the authors could not explain in the present study for purpose of simplicity.
Ethics approval and consent:
Institutional ethics approval and written informed consent of human participants taken.
5. Conclusion
In postoperative patients, electrocardiographic changes include broad differentials like pH changes, electrolyte shifts, temperature alterations, sympathetic effects, complications of cardioplegia or aortic cross-clamping, pericarditis, as well as coronary-graft anastomotic trouble. The risk for misdiagnosis of an MI exists for all postoperative patients and may occur more frequently than recognized at present due to these pseudo-infarction patterns[18] .The study highlights the correlation between immediate and late postoperative ECG changes to CTCA changes. Overall, early and late ECG changes in patients having EF 50-60% and native right coronary lesions with posterior descending and posterior left ventricular artery grafts warrant vigilance and close follow-up even in the absence of hemodynamic compromise, and such patients should be subjected to CTCA. Thus, both ECG and CTCA should always be correlated as they are valuable in determining graft patency and also help detect clinically occult and potentially life-threatening complications.
6. Acknowledgements
The conception and study design of the project was given by Dr. D V Kulkarni and the authors cannot thank him enough for the constant support.
References
- Bruss J, Meyerowitz C, Greenspan A, et al. The significance of the electrocardiogram after open heart surgery. In: Kotler M, Alfieri A, editors. Cardiac and noncardiac complications of open heart surgery 10(1992):39–65.
- Klein MS, Coleman RE, Weldon CS, et al. concordance of electrocardiographic and scintigraphic criteria of myocardial injury after cardiac surgery. J Thorac CardiovascSurg 71(1976):934–7.
- Taussig AS, Schlant RC. Misleading ECG's: patterns of pseudoinfarction. J Cardiovasc Med 29(1983): 1147-1151.
- Gardner MJ, Johnstone DE, Lalonde L, et al. Perioperative myocardial infarction with coronary artery surgery: diagnosis, incidence and consequences.Can J Cardiol3(1987): 336-341.
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: Angiographic follow up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J Am Coll Cardiol 28(1996):616-26.
- Achenbach S, Moshage W, Ropers D, et al. Noninvasive three dimensional visualizationvof coronary artery bypass grafts by electron beam tomography. The American Journal of Cardiology79(1997): 856-861.
- Lytle BW, Loop FD, Cosgrove DM, et al. Long term (5-12years) serial studies of internal mammary artery and saphenous vein coronary bypass grafts. The Journal of Thoracic and Cardiovascular Surgery89(1985):248-258.
- Dewey M. Cardiac CT. 2nd ed. Berlin Heidelberg: Springer 34 (2014).
- Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: Clinical implications. Journal of Vascular Surgery51(2010):736-746.
- Barbero U, Iannacone M, d’ Ascenzo F, et al. 64 slice coronary computed tomography sensitivity and specificity in the evaluation of coronary artery bypass graft stenosis: A meta analysis. International Journal of Cardiology216(2016):52.
- Donkol RH, Mahmoud ZS, Elrawy M. evaluation of coronary artery bypass by CT coronary angiography. Surgery 10 (2014).
- Hendel RC, Patel MR, Kramer CM, Poon M, Car JC, et al. ACCF/ ACR/ SCCT/ ASNC/ NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. Journal of the American College Cardiology48(2006):1475-1497.
- Rupprecht et al.Impact of coronary angiography early after CABG for suspected postoperative myocardial ischemia. Journal of Cardiothoracic Surgery 54(2019).
- Puskas JD, Williams WH, Mahoney EM, et al. Off-pump versus conventional coronary artery bypass grafting: Early and 1-year graft patency, cost and quality of life outcomes: a randomized trial. JAMA 291(2004):1841-9.
- Glineur D, Hanet C, D’hoore W, et al. Causes of non-functioning right internal mammary used in a Y graft configuration: insight from a 6month systematic angiographic trial. Eur J Cardiothorac Surg36(2009):129-35.
- Mueller J, Jeudy J, Poston R, et al. Cardiac CT angiography after coronary bypass surgery: Prevalence of incidental findings. AJR Am J Roentgenol189(2007): 414–419.
- Loeb HS, Gunnar WP, Thomas DD. Is new ST-segment elevation after coronary bypass of clinical importance in the absence of perioperative myocardial infarction? J Electrocardiol40(2007): 276-81
- Bilazarian SD. Pseudoinfarction pattern on electrocardiogram after coronary artery bypass. Chest J98(1990): 1271-4.


 Impact Factor: * 5.6
Impact Factor: * 5.6 Acceptance Rate: 74.36%
Acceptance Rate: 74.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks