Antimicrobial Evaluation of Novel Metals Complexes of n- Isonicotinamido-2-hydroxy-5-methoxybenzalaldimine
Husain I. Alarabi1, Sofian S. Mohamed2, Wahiba A. Suayed1, Inass A. Al-Sadawe3, Salah M. Bensaber3,Fathi M. Sherif4*, Anton Hermann5, Abdul Gbaj2,3
1Department of Chemistry, Faculty of Science, University of Zawia, Zawia, Libya
2Unit of Medicinal Chemistry, National Medical Research Center, Zawia, Libya
3Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Tripoli M16, Libya
4Department of Pharmacology, Faculty of Pharmacy, University of Tripoli, Tripoli M16, Libya
5Department of Cell Biology and Physiology, University of Salzburg, 5020 Salzburg, Austria
*Corresponding Author: Fathi M.Sherif, Department of Pharmacology, Faculty of Pharmacy, University of Tripoli, Libya
Received: 21 March 2018; Accepted: 05 April 2018; Published: 09 May 2018
Article Information
Citation: Husain I. Alarabi, Sofian S. Mohamed, Wahiba A. Suayed, Inass A. Al-Sadawe, Salah M. Bensaber, Fathi M. Sherif, Anton Hermann, Abdul Gbaj. Antimicrobial Evaluation of Novel Metals Complexes of n-Isonicotinamido- 2-hydroxy-5-methoxybenzalaldimine. J Pharm Pharmacol Res 2 (2018): 039-055.
View / Download Pdf Share at FacebookAbstract
Objective: The appearance of resistant bacteria reduces the efficiency of antimicrobial therapies, thereby increasing the need for more efficient drugs for infections treatment. Many studies have shown an enhance in antimicrobial activity after the interaction of many agents with metal ions. Complexes of the metal ions with ligands which are polydentate have been the theme of demanding research as they have interesting spectral, magnetic properties and a miscellaneous spectrum of biological activities.
Methods: New isoniazid based compounds and their transition metal complexes (cobalt (II), copper (II), nickel (II) and zinc (II)) were produced using microwave synthesis technique. The All compounds which were synthesized (free ligand and their metal complexes) were fully characterized by many spectroscopic techniques (FT-IR spectra, UV/visible electronic spectra, mass spectra and 13C NMR and 1H NMR spectra). In addition, CHN, XRFA, AAS merged with other spectroscopic data were utilized to allocate the precise ligand to metal ratio and geometry. The synthesized ligands and their complexes were tested for in vitro antimicrobial activity against Candida albicans (ATCC 10231), Aspergillus niger (ATCC 16404), Escherichia coli (ATCC 25922), and Staphylococcus aureus (ATCC 29213) by using agar-well diffusion.
Results: Based on analytical and spectroscopic findings, the ligands proceed as a coordinate and monoanionic tridentate throughout phenolic oxygen, azomethine nitrogen, and carbonyl oxygen. New complexes of nisonicotinamido- 2-hydroxy-5-methoxy benzalaldimine with Cu(II), Co(II) and Zn(II), having a formula of the type [M (L) 2].nH2O, (M = Co(II), n = 1.5; Zn(II), n = 0; Cu(II), n = 6) and with Ni(II), featuring a formula of the type [M (L) (H2O)] (ac).nH <p>Antimicrobial activity; Metal complexes and Schiff base</p>
Management of infectious diseases remains significant and challenging crisis due to several factors as increasing multi-drug resistant microbial pathogens and emerging infectious diseases. Despite of several chemotherapeutics and antibiotics available for clinical use, still there are requirements for developing new antimicrobial agents. There is also an urgent requirements for novel compounds have antimicrobial activity, probably through new mechanism of actions, which is different from those of recognized classes of antimicrobial medicines to which numerous pathogens are resistant to. Metal ions play a fundamental role in many biological processes such as: catalytic activity of metalloenzymes; regulation of nucleic acids replication and hemoglobin that contains iron-porphyrin complexes. Its function as oxygen carrier which is being connected to the capability of the iron atoms to manage molecules of oxygen reversibly [1]. Imine (also known as Schiff base) compounds participate a function in inorganic chemistry as they can simply form stable complexes with the majority of transition metal ions [2;3]. These are described by existence of azomethine group (πN=CHR) which was formed by condensation of carbonyl compound and primary amine (aldehydes or ketones) [4]. Due to existence of lone pair of electrons at the nitrogen atom, the electron donating nature of unsaturated double bond and low electronegativity of nitrogen of azomethine group (>C=N) proceeds as a excellent donor and Schiff base constituting active ligands. The bonding capability of the ligands depends on the character of atoms that act at site of the coordination, their steric and electronegativity factors. The structure of chelates offers additional stability to the complexes particularly when the ring is five or six membered. Consequently, presence of functional group with replaceable hydrogen atom next to > C=N will be an extra factor for affording stability. To prevent them from rapidly decomposing or polymerizing, aryl group ought to be bonded to the nitrogen or to the carbon of the C=N double bond [5]. Schiff bases also showed wide range of biological activities, such as anti-inflammatory [6;7], antimalarial [8;9], antitumor [10-15] and antimicrobial abilities [16-19]. Improvement of chemical process to decrease environmental contamination arises as urgent mission for chemical researchers [31]. Microwave assisted organic and inorganic chemical synthesis start to be a new and rapid developing area in the synthetic organic chemistry field. This new technique is depended on the experimental study that some organic reactions progress very quickly with superior products yields using microwave irradiation when compared to the classical heating procedures. In a number of circumstances, reactions that generally consume several hours at reflux temperature under traditional conditions can be finished within few minutes or even some times seconds by using microwave chemical synthesis [32-34]. Thus, the aim of this study was to search for novel anti-microbiotics, effective in treatment of infections caused by multi-resistant bacteria. We synthesized isoniazid based compounds and their transition metal complexes (cobalt, copper, nickel and zinc) using microwave assisted chemical synthesis and explored the antimicrobial activity of both the complex and the free ligand. All the chemicals and solvents used in the synthesis of Schiff base and
their complexes of highest purity and they were purchased from Sigma Aldrich
(UK) and Fluka (UK) and used without any further purification. 2.2 Instruments 2.3 General procedure for synthesis of N-isonicotinamido-2-hydroxy-5-methoxy benzalaldimine (ligand): 2.4 General procedure for synthesis of metal complexes The Schiff base ligands and their metal complexes were evaluated for their in vitro antibacterial activity against several strains of microorganisms: Staphylococcus Aureus (ATCC 29213), Escherichia Coli (ATCC 25922), Candida Albicans (ATCC 10231) and Aspergillus Niger (ATCC 16404). Strains were obtained from the American Type Culture Collection (ATCC) and were recognized based on the American type of cell culture collection (ATCC) by agar-well diffusion method. Bacteria were inoculated into nutrient broth (Difco), incubated for twenty four hours and fungi kept inoculated in malt extract broth (Difco) for forty eight hours. In the agar-well diffusion method, Malt Extract Broth (Difco) and Mueller Hinton Agar (Oxoid) were sterilized in a flask and cooled to 45-50°C, and were distributed into twenty millilitter aliquots to sterilized petri dishes after injecting ten microlitter cultures of bacteria which was prepared as stated earlier and were keep to solidify. The dilution plate method was utilized to quantify the microorganisms (105 bacteria ml-1 and fungi 103-104 ml-1) for twenty four hours [35]. Wells were dug in the culture plates by using a sterilized cork borer (7 mm diameter). The synthesized compounds were dissolved in dimethyl sulfoxide (0.2 ml) and then added to the wells aseptically. The petri dishes were left at 4° C for two hours and then the plates were incubated at 30° C for bacteria (18-24 hours) and at 25° C for fungi (72 hours). At the end of the incubation period the inhibition zones produced on the medium were assessed as millimeters (mm). The control samples were only loaded with only DMSO. Blank tests showed that the concentration of DMSO used has not any affect and the antimicrobial activity obtained will be correlated to the synthesized compounds. Tetracycline and Amphotericin B as antimicrobial agents were used as references. 4.1 Chemistry part *Melting points more than 300 0C 1H and 13C NMR spectra of the Schiff base: 4.1.2 IR spectra Table 2: IR stretching frequencies of various functional groups of ligands and its metal complexes 4.1.3 Electronic spectral analysis Table 3: Electronic spectral data and geometries for the ligand and their complexes 4.1.4 Elemental analysis, X-ray fluorescence analysis and atomic absorption spectroscopy. Table 4: The results of elemental analysis, AAS and XRF of the ligands and their complexes: 4.1.5 Mass spectra Table 5: Mass fragmentation of the ligand and its metal complexes 4.1.6 Thermal analysis (TGA and DTG) Table 6: Thermal analysis of the ligand and its metal complexes 4.2 Biology part Table7: Antimicrobial activity data of the ligand and its metal complexes 5.1 IR spectra The infrared spectra spectrum of the ligandexhibits a strong band at 1652 cm-1 due to γ(C=O) of the amide group. The band of this ligand has shifted and was powerfully displaced in all complexes confirming coordination throughout the carbonyl oxygen. A band at 1578 cm-1 is due to γ(C=N) azomethine group which has shifted to the lower frequencies for the entire complexes. This suggests the involvement of the azomethine nitrogen in coordination. Another important ligand band, which allocated at about 3380 cm-1 owing to the phenolicπhydroxyl group, was missing in the complexes. This proofs the phenolicπOH is deprotonated and it is on coordination with metal. The band due to phenolic CπO stretching vibration is observed at 1261 cm-1 in the free ligand. In the entire complexes this band appears at higher or lower frequencies in 1268-1219 cm-1 proving the participation of the phenolic oxygen in the coordination with the metal ions. As indicated in the literature the Ni complex shows absorption bands, one in the 1572 cm-1 and the other in 1425 cm-1 regions for symmetric γ(COOπ )and asymmetric γ(COOπ)stretching [42;43]. 5.2 Electronic spectral analysis 5.3 Elemental analysis, X-ray fluorescence analysis and atomic absorption spectroscopy 5.4 Mass spectra 5.5 Thermal analysis (TGA and DTG) MCl2.nH2O + 2 L → [M (L) 2].nH2O+2HCl+nH2O The ligand acts as a monoanionic tridentate (O, N and O) throughout the carbonyl oxygen, phenolic oxygen and azomethine nitrogen. Composition was construed from FT-IR, elemental analyses, UV-VIS,XRF, AAS, MS and TGA. The analytical data of the complexes specify that the ligand forms a 1:2 (M : L) complex with Zn (II), Co (II) and Cu (II) and 1:1 (M: L) with Ni (II) ions. The atomic absorption and mass spectral data substantiate the monomeric structure of the metal complexes whereas the TGA studies substantiate the existence of water molecules in some complexes and XRF data demonstrate that chloride anions are absent outside the coordination sphere in Zn (II), Co (II) and Cu complexes. According to the indicated analytical and spectral data, the common structure formula and stoichiometry of synthetized metal complexes are illustrated in Figure 2. Figure 2: structural formula proposed for; (A)[M (L) 2].nH2O ,(M = Co (II), n=1.5; Zn (II), n=0 ; Cu (II), n=6) and (B)[M (L) (H2O)] (ac).nH2O,(M=Ni (II), n=0,ac=CH3COO¯) 5.6 Biology part It has been reported that, there are 5 fundamental mechanisms that suggest an increased level of cellular resistance to metals: (1) intra- or extracellular sequestration (2) efflux of the toxic metal from the cell, (3) enzymatic conversion, (4) prohibiting by a permeability barrier, and (5) diminution in sensitivity of cellular targets [54-56]. Comparative studies of ligands and their complexes signify that zinc complexes display higher antimicrobial activity than the free ligand and others complexes. It has been reported that most the metal complexes are more active than their respective Schiff bases [57]. In several cases, Schiff bases and their complexes have comparable activity against bacteria and fungi, i.e. chelation may improve or restrain the biochemical prospective of bioactive organic species [57]. Such generation or improvement in activity of the metal complexes can be explained based on Overtone’s concept and chelation theory. Per Overtone’s notion of cell permeability, the lipid membrane that envelops the cell helps the passage of lipid soluble substances owing to their liposolubility which is a vital factor that manages antimicrobial activity[58]. On chelation, the polarity of the metal ion is abridged due to the overlap of the ligand orbital and fractional sharing of the (+) charge of the metal ion with donor groups. Additionally, it enhances the delocalization of pi-electrons over the entire chelate ring and enhances the lipophilicity of the complex. This increased lipophilicity in turn enhances and this could help the penetration of the complexes into lipid membranes and ultimately blocks metal active binding sites on the enzymes of the microorganisms [59;60]. Moreover, coordination might escort to considerable reduction of drug π resistance [61]. In addition another factors, like conductivity, dipole moment and solubility effected by the existence of metal ions and might enhance the bactericidal activity of the metal complexes in comparison to the uncomplexed compounds [62]. Low activity of the some metal complexes, as shown in this study, may be related to their low lipophilicity which reduces penetration of the complex through the lipid membrane and therefore these complexes cannot achieve their target to block or inhibit the growth of microorganisms [63;64]. In conclusion, we synthesized and characterized four new complexes of n-isonicotinamido-2-hydroxy-5-methoxy benzalaldimine with Cu(II), Co(II), Ni(II) and Zn(II). The spectroscopic data demonstrate that Schiff base acts as monoanionic tridentate ligand. Schiff base and some of the metal complexes were active against some of representative bacterial and fungal strains and complexation enhances their activity. The activity may be due to increase in cell permeability caused by increase of lipophilicity of metal conjugates, which allows intracellular drug accumulation and target accessibility. It is possible that intracellular reduction of metal compounds leads to higher cytoplasmic concentration of metal species, which proves lethal for bacteria and fungi. This study also shows superior antimicrobial activity of metal complexes relative to their ligands. MIC values indicate their potential for pharmacological use.Keywords
Article Details
1. Introduction
To ascertain novel compounds with an improved pharmacological profile, researchers oriented their work towards the complexation of Schiff bases as some of them showed increased activity upon coordination/chelation with some metal ions. Some of these complexes have broad application spectrum in inorganic, organic, pharmaceutical, industrial and analytical use, in addition to their important roles in orgometallic synthesis and catalysis [20-24]. Isonicotinic acid hydrazide (INH) is first-line medication used to treat Mycobacterium tuberculosis and as prophylactic for human immunodeficiency virus patients who are at threat to be infected with tuberculosis [25;26]. Isonicotinic acid hydrazide can form metal chelates with numerous divalent ions [27]. Fungicide and bactericide characteristics of different mixed ligand complexes of metal ions with hydrazone and isoniazid derivatives were attained and characterized [28-30].2. Methods
2.1 Chemistry
Synthesis of compounds (ligand and metal complexes) was performed in a microwave closed system (Milestone start E 2450 MHz, Italy). For thin layer chromatographic (TLC) analysis pre-coated aluminum plates (silica gel 60778, Fluka analytical, UK) were used. TLC spots were detected with both short wave (254 nm) and long wave (366 nm) UV light. Melting points were determined in open capillary tubes using a melting point apparatus (Electrothermal SMP30, Stuart, UK). The Infra-red spectra of the synthesized compounds were recorded in the region of 4,000-400 cm-1
by Varian FT-IR spectrophotometer (Cary-Varian 660, Australia). UV-visible
spectra were recorded by Cary spectrophotometer (5000, UV\VIS\NIR, Varian,
Australia) using one cm Starna quartz cuvettes. Proton NMR (1H-NMR) and carbon
NMR (13C-NMR) spectra were recorded in dimethyl sulfoxide (DMSO-d6) by using
tetramethylsilane as an internal standard with a Bruker Avance 400 MHz NMR
Spectrometer (400 MHz, Bruker, France). Mass spectra were recorded by EI
technique at 70eV using Shimadzu QP-2010 plus. The thermogravimetric analysis
(TGA and DTG) was determined in a dynamic nitrogen atmosphere (30 ml/min) with a
heating rate of 10πC per min using Shimadzu TGA-50H thermal analyzers. All
analyses were performed at the National Medical Research Center, Zawia, Libya
and the Microanalytical Center, Cairo University, Giza, Egypt.
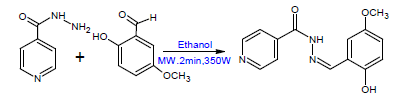
As shown in Scheme 1, equimolar amounts of both starting materials (0.5 g isoniazid and 0.55 g 2-hydroxy-5-methoxy benzaldehyde) were weighed and triturated to form a homogeneous mixture using clean and dry Teflon vessels with the addition of 3-4 drops of ethanol. The reaction mixture was subjected to microwave irradiation at 350 - 600 Watt power for about 2 minutes with maximum heating of 60o C. The optimum reaction time was determined based on the reaction completion using TLC and the appropriate solvent system. The reaction mixture was allowed to cool and the crude solid product was collected through vacuum filtration and washed with three volumes of acetone, dried over anhydrous magnesium sulphate, evaporated and finally recrystallized from the appropriate solvent (ethanol). The achieved crystals were dried and their melting points were determined. The chemical purity was investigated by TLC using chloroform/ethanol (90:10) as mobile phases.
Metal complexes were prepared by weighing and triturating equimolar amounts of Schiff base ligand and metal chloride or acetate metal salts using clean and dry Teflon vessels. The reaction mixture was subjected to microwave irradiation at 600 - 800 Watt power for about 3 - 5 minutes using 0.5 ml of dry ethanol as a solvent after milling. The optimum reaction time was determined based on the reaction completion using TLC and the appropriate solvent system. The crude product achieved after filtration under vacuum was washed several times with hot ethanol and finally dried and their melting points were determined. Metal salts used were ZnCl2, CoCl2.6H2O, CuCl2.2H2O and Ni (CH3COO)2.4H2O.3. Biology
3.1 Evaluation of antimicrobial activities
4. Results
4.1.1 Physical properties
Some physical properties of the ligandand their complexes are shown in Table1. 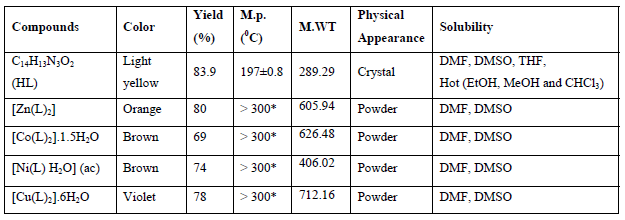
Table 1: Physical properties of the ligand and its complexes
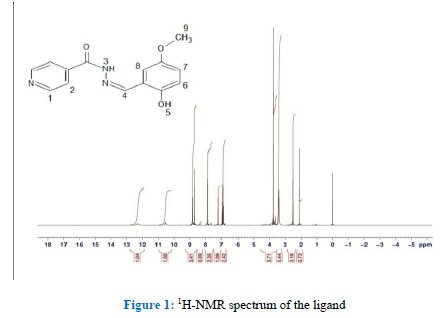
The 1H-NMR (400 MHz, DMSO-d6): [ppm] 12.3 (s, 1H, πNH), 10.5 ppm (s, 1H, πOH), 8.68 ppm (s, 1H, N=CH), 8.80 ppm for H1 (d, JH1-H2 = 5Hz), 7.85 H2 (d, JH2-H1 = 5Hz), 7.20-6.85 (m, 3H, ArH), 3.75 ppm (s, 3H, OCH3). The 13C NMR (400 MHz, DMSO-d6): π [ppm] 161.34 (C4, *C =O), 148.10 (C15, *C=N), 55.44(C12, *CH3O), 152.12, 151.49, 150.34, 140.04, 121.50, 118.95, 118.64, 117.31,111.55 for C7, C10, C1, C3, C2, C6, C9, C8 and C11, respectively. Figure 1 shows a 1H-NMR spectrum of the ligand.
The main stretching frequencies of the IR spectra of the ligandand its complexes are shown in Table 2
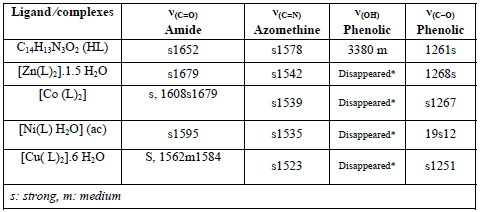
*The peak in the Ligand spectra at 3380 cm-1 due to the deformation of OH group and disappeared in the complexes. This indicates deprotonation of phenolic OH, on coordination with metal ion.
The electronic spectra of the free ligand showed two strong absorption bands in the Ultraviolet-Visible region (294-361 nm), allocated to the transitions π→π* and n →π *. These transitions are found only in the spectra of the complexes. But they are shifted and strongly displaced in all complexes, verifying coordination of metal ions to the ligand. The spectra of the complexes also showed new bands that were attributed to the formed ligand complexes (Table 3). 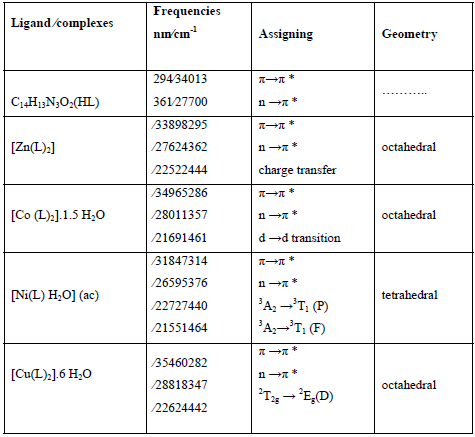
Elemental analysis of the free ligand and its metal complexes along with X-ray fluorescence analysis and atomic absorption spectroscopy are listed in Table 4. 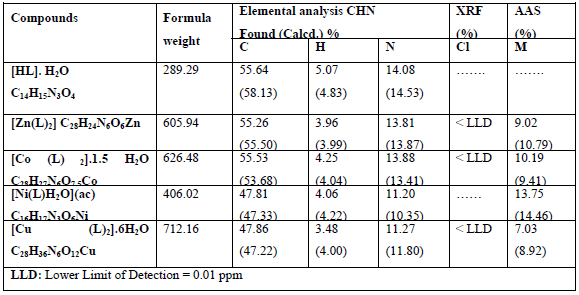
The most important peaks in the EI mass spectral data of all complexes with ligandare listed in Table 5. 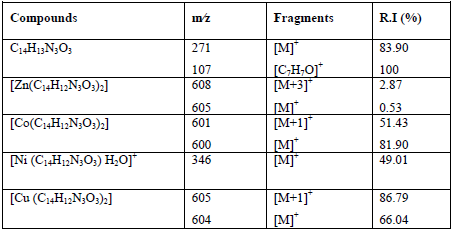
Thermogravimetric analysis of the ligand and its complexes were used to obtain information about the thermal stability of these new complexes. In addition, to make a decision whether the water molecules (if it is available) are outside or inside the inner sphere coordination of the central metal ion. The results of the thermal analysis of the ligand and its metal complexes are given in Table 6.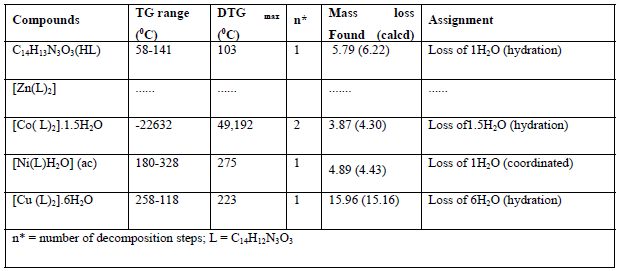
4.2.1 Evaluation of antimicrobial activity
The antimicrobial activity studies of the ligand and its metal complexes were performed by using different fungi and bacteria and the results of the inhibition are summarized in Table 7.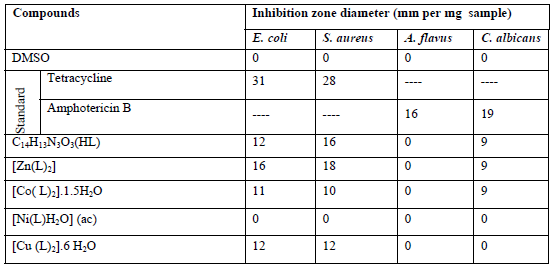
5. Discussion
In general, the entire synthesized amides demonstrate two absorption bands: one is the the carbonyl absorption band near 1640 cm-1 which is known as amide-I band and two is the strong band in the 1500 - 1600 cm-1 region which is known as amide-II band. The origin of these two bands can be seen in hydrazones, in which the carbonyl absorption accountable for the amide-I band, which is probable to be lowered [36;37] occasionally by the NH group as in standard amides. The amide-I band in isoniazide derivative, however, can be seen at 1700 and 1655 cm-1 [38]. In the entire hydrazones, the absorptions such as 1540, 1520 cm-1 have been allocated to absorption of amide-II. The NH stretching absorption in free ligands occurs at π3300 and 3220 cm-1 as described in the literature [39]. The other significant band occurs at π1585-1600 cm-1 allocated to γ(C=N) (azomethine) mode [35;39]. The strong bands allocated at 1000π1080 cm-1 and 1520π1575 cm-1 are possibly allocated to symmetric and asymmetric γ(C=C) + γ(C=N) of the pyridine ring and pyridine ring deformations and breathings, respectively [40;41].
Theelectronic spectrum of the zinc (II) complex shows a broad absorption band at 444 nm which may be assigned to a charge transfer transition, due to the coordination of the ligand with metal ion [44]. The electronic spectrum of the cobalt (II) complex is expected to show three absorption bands due to the electronic transitions, namely 4T1g→4T1g(P), 4T1g(F)→4A2g and 4T1g→4T2g, but bands due to these transitions usually overlap to give a broad absorption band. The broad band allocated in the complex approximately 461 nm is in agreement with octahedral arrangements for Co (II) ion. The electronic spectrum of the nickel (II) complex shows two d-d absorption bands at 440 and 464 nm, while the third d-d band is not observed. These bands are assigned to 3A2→3T1 (P) and 3A2→ 3T1 (F) transitions, respectively. In fact, the 3A2 (F) →3T2 is missing. The transitions correspond to the tetrahedral geometry of the complex. The spectrum of electronic of the copper (II) complex reveals one d-d absorption band at 442 nm. This band is assigned to 2T2g →2Eg (D) transition. Which designates the existence of Cu (II) complex in octahedral structure [45].
Elemental analysis of the free ligand and the complexes of the metal in addition to the atomic absorption spectroscopy and the X-ray fluorescence analysis and demonstrated in the results section (Table 4) and are in a good conformity with the expected values.
The mass spectrum of ligand showed the molecular ion peak at m/z = 271 that corresponds to its molecular formula [C14H13N3O3]+, with a virtual intensity of 83.90%. The fragment at m/z = 107 (R.I. = 100%, base peak) is allocated to the [C7H7O]+ ion. The other peaks signify fragments of the molecular ion. The EI mass spectral data of Zn(II), Co(II), Ni(II) and Cu(II) complexes show an strong molecular ion peak m/z [M]+. The mass spectra of several compounds also exhibit a important peak matchingto m/z [M+1]+, [M+2]+ or [M-1]+ and the enduring peaks signify the successive degradation of the complexes [46]. The peak intensity provides an thought of the stability of the fragments.
Thermogravimetric analysis of the ligand and its complexes demonstrated good conformity with the theoretical formula as suggested from the elemental analysis. TGA of ligand, shows the initial weight loss 103°C is allocated to the loss of lattice water molecule which is allocated to one H2O corresponding to (found 5.79%; calculated 6.22%). The remaining steps, that occur inside the temperature range 204-799°C attributed to decomposition of the ligand, involve mass losses of 96.55%. TGA for Co(II) complex, demonstrates 2- stages of decomposition within the range of 32-226 °C, which is attributable to the loss of 1.5 uncoordinated water molecules (weight loss; found /calculated . 3.87π4.30 % ( , while TG for Cu(II) complex shows one stage of decomposition within the range of 32-226 πC, which is attributable to the loss of 6- uncoordinated water molecules (weight loss; found π calculated. 15.96π15.16% ( . The molecular formulae of the complexes Co(II) and Cu(II) construed from elemental analysis points out the existence of lattice water. In case Ni(II) complex, TG shows one stage of decomposition within the range of 180-328 πC, which is due to the loss of one coordinated water molecule (weight loss; found π calcd . 4.89 π 4.43 ( . But in the zinc (II) complex, there is no considerable weight loss below 180°C proposing the nonappearance of lattice water molecules. The succeeding steps (280-800πC) in all complexes match to the exclusion of the organic part of the ligand [47;48]. The complexes have been synthesized by microwave methods from reaction of CoCl2.6H2O, CuCl2.2H2O, ZnCl2 and Ni (CH3COO) 2. 4H2O with Schiff base (ligand) in presence of ethanol as solvent. Formation of the complexes may have proceeded per the following equations.
M = Co (II), n=1.5; Zn (II), n=0 and Cu (II), n=6
M (ac) 2 .4H2O+L→ [M (L) H2O] (ac).nH2O+ CH3COOH+ nH2O
M=Ni (II), n=0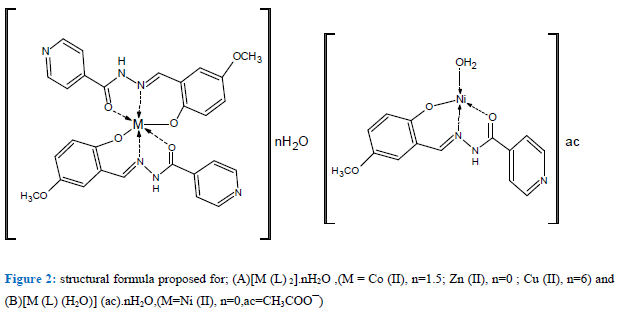
5.6.1 Evaluation of antimicrobial activity
Microorganisms require the presence of a number of metals that play essential biochemical roles as catalysts, enzyme cofactors, activity in redox processes and stabilizing protein structures [49-51]. Metals might build up exceeding normal physiological concentrations and effecting the transport systems, and might become toxic. The intracellular metals could cause toxic effects through forming coordinate bonds with some anions and this could block the efficient groups of enzymes. This formed coordinate bonds with the enzymes will be inhibiting the transport systems and disturbing cellular membrane integrity [52;53].References
Article Views: 2178
Journal Statistics




Discover More: Recent Articles