5-Aminolevulinic Acid Tumor Paint and Photodynamic Therapy for Chordoma: An In Vitro Study
Shachar Kenan*, 1, Haixiang Liang2, Ryan Nixon1, Howard J. Goodman1, Daniel A. Grande1, 2, Adam S. Levin3
1The Department of Orthopaedics, North Shore-Long Island Jewish Hospital, Northwell Health Medical Center, New Hyde Park, NY.
2Orthopaedic Research Laboratory, The Feinstein Institute for Medical Research, Manhasset, NY.
3The Department of Orthopaedic Surgery, The Johns Hopkins University School of Medicine, Baltimore.
*Corresponding author: Shachar Kenan. The Department of Orthopaedics, North Shore-Long Island Jewish Hospital, Northwell Health Medical Center, New Hyde Park, NY
Received: 20 November 2023; Accepted: 29 November 2023; Published: 13 December 2023
Article Information
Citation: Shachar Kenan, Haixiang Liang, Ryan Nixon, Howard J. Goodman, Daniel A. Grande, Adam S. Levin. 5-Aminolevulinic Acid Tumor Paint and Photodynamic Therapy for Chordoma: An In Vitro Study. Journal of Pharmacy and Pharmacology Research. 7 (2023): 243-250.
View / Download Pdf Share at FacebookAbstract
Wide resections of chordoma tumors are challenging for many reasons, primarily due to a lack of intraoperative visualization, leading to unacceptably high recurrence rates. Known techniques using five-aminolevulinic acid (5-ALA) for tumor paint and photodynamic therapy (PDT) may improve outcomes but have not been well described for chordomas. This study aimed to analyze chordoma cell response to 5-ALA tumor paint and PDT in vitro. Tumor paint: Human chordoma cells (MUG-Chor1) were cocultured with green fluorescence protein (GFP) rat adipose-derived stromal cells (ADS) with subsequent observation after 5-ALA exposure, visualized using filters to show GFP cells in green and 5-ALA positive cells in red color. PDT: MUG-Chor1 and ADS cells were exposed separately to 5-ALA followed by PDT treatment using 405-nm excitation and emission at 603-738-nm. Time-lapse images of live cells were captured every second for 15 minutes and then visualized sequentially at 50× speed. The tumor paint arm of the study successfully demonstrated selective red chordoma fluorescence, a finding which may facilitate visualization of a malignancy juxtaposed to surrounding benign healthy tissue. The second arm of the study, PDT, demonstrated selective chordoma cellular death, clearly evidenced by swelling and vesicle formation in contrast to the ADS control. The results of these experiments demonstrate the effective in vitro application of 5-ALA tumor paint and PDT on chordoma cells, documented visually using time-lapse photography. 5-ALA, with its double-edged applications of selective tumor identification and kill, may lead to transformative change in the management of soft tissue sarcomas.
Keywords
<p>5-ALA; 5-aminolevulinic acid; chordoma; primary bone sarcoma; tumor paint; photodynamic therapy; neoadjuvant therapy; orthopaedic oncology</p>
Article Details
1. Introduction
Chordomas are rare malignant primary bone tumors originating from embryonic notochord remnants [1]. Paralleling their vestigial distributions, they are most frequently found in the midline sacrococcygeal or sphenoocciptal regions. Wide en bloc resection with clear margins is the gold standard of care and is directly related to prognosis [2]. Multiple studies have confirmed significantly greater local recurrence rates with marginal (R1) and intralesional (R2) excision as opposed to wide resection (R0) [3-5]. Adjuvants such as postoperative radiation therapy, proton beam therapy, brachytherapy and recently developed targeted chemotherapy are commonly added in efforts to minimize the notoriously high local recurrence rate, which can be as high as 79% [6, 7]. The prognosis is poor, with a median survival of 6.29 years and 5- and 10-year relative survival rates of 67.6% and 39.9%, respectively, based on the SEER database from 1973-1995 [8]. Achieving negative margins, critical to improving prognosis, is often challenging due to the lack of visualization and infiltration into the surrounding complex pelvic retroperitoneal and intraperitoneal structures. Postoperative complication rates are therefore expectedly high and include wound dehiscence, infections, bladder and bowel dysfunction, sexual dysfunction, motor deficits, lymphatic complications, hematomas and cerebrospinal fluid fistulas [3, 9]. In the effort to battle such challenging tumors, advanced techniques such as tumor paint and photodynamic therapy (PDT) may be of benefit. Tumor paint refers to the method of selectively inducing fluorescence in tumor cells. PDT refers to the use of a photosensitizer activated by a light source to exert cytotoxic activity towards cells [10]. Although various compounds have been described for each of these purposes, one compound, five-aminolevulinic acid (5-ALA), has the capability of doing both.
In 1987, a breakthrough in the field of PDT was made when Malik and Lugaci [11] described 5-ALA, which became the first second-generation photosensitizer. Unlike earlier photosensitizers, 5-ALA is an endogenous inactive precursor in the heme biosynthesis pathway; therefore, the risk of adverse phototoxic effects that plagued its predecessors was reduced significantly. Tumor paint using 5-ALA has helped identify a variety of different tumor types in the fields of neurosurgery, urology and dermatology [12] but its use with chordoma tumors is not well described. One study by Cornelius et al., was the only study written so far in the literature on this topic, confirming in vitro cell fluorescence and destruction in response to 5-ALA [13]. The Cornelius study, however, involved fluorescence intensity measurement rather than direct image visualization, and moreover did not have a control group cell line for comparison. Eljamel et al. very briefly discussed a single case of 5-ALA guided resection of chordoma in vivo in their 2009 study but failed to mention the details of if and how it responded to 5-ALA. Eljamel included 114 patients who underwent 5-ALA guided resections of various brain tumors including gliobastoma multiforme (GBM), meningiomas, lung, colon and breast metastases, pituitary adenomas and myelomas, reporting a sensitivity of 89.5% and specificity of 100% for tumor identification [14]. The purpose of this study was to analyze the response of chordoma cells to 5-ALA exposure in vitro and to judge whether chordoma tumors would be eligible candidates for 5-ALA guided identification and resection. Our first hypothesis is that 5-ALA tumor paint will lead to selective fluorescence of chordoma cells. Our second hypothesis is that 5-ALA PDT will lead to chordoma cell destruction.
2. Materials & Methods
Tumor paint and PDT represented the 2 arms of this study. Rat adipose derived stromal cells carrying the green fluorescence protein gene (ADS-GFP), human adipose derived stromal cells (ADS, Lonza Inc., Allendale, NJ), and human chordoma cells (MUG-Chor1, Graz, Austria) were propagated according to established protocols, remaining free of contaminants [15, 16]. ADS-GFP and ADS cells were cultured using Dulbecco modified Eagle/F12 (50:50) medium (DMEM/F12) (Corning Life Sciences, Teterboro, NJ) containing 10% fetal bovine serum (FBS), 1% L-glutamine, 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 0.25 µg/mL of amphotericin B. MUG-Chor1 cells were suspended in a 4:1 mixture of Iscove’s modified Dulbecco’s medium (IMDM) to 1640 Roswell Park Memorial Institute (RPMI) medium and 1% Insulin Transferrin Selenium (ITS). Medium was changed every 2 days for all cell lines. Cells were maintained at 37° C in 5% CO2.
2.1 Tumor Paint
In the first experiment, 50,000 rat adipose derived stromal cells (ADS) carrying the GFP gene were co-cultured with 50,000 human chordoma cells (MUG-Chor1, Graz, Austria), with growth confined strictly to monolayer form [15]. After 4 weeks, 5-ALA was added to the medium at a concentration of 1000µg/mL. Cells were observed after 5 hours of 5-ALA exposure using phase microscopy at 10X magnification with an excitation wavelength of 395 to 440nm. A 509nm emission filter was used to detect GFP cells, which appeared green. A 635nm emission filter was used to detect 5-ALA fluorescence, which appeared red. In the second experiment, two three-dimensional micromass pellets consisting of 500,000 cells were separately formed using liquid drop techniques- one derived from the rat GFP-ADS cells and another derived from human chordoma cells. These two micromass pellets were then co-cultured for three weeks, at which point 5-ALA was added at a concentration of 1000µg/mL. Cell pellets were observed after 5 hours of 5-ALA exposure using phase microscopy at 10X magnification. Emission filter settings were unchanged from the prior experiment. In the third experiment, 50,000 human adipose derived stromal cells (ADS, Lonza, Allendale, NJ) and 50,000 human chordoma cells were co-cultured for two weeks, grown freely in suspension form (unlike the monolayer form in the first experiment), at which point 5-ALA was added at a concentration of 1000µg/mL. Cells were observed after 5 hours of 5-ALA exposure using phase microscopy at 10X magnification. Emission filter settings were unchanged from the prior experiment.
2.2 Photodynamic Therapy
MUG-Chor1 cells that had been exposed to 3 hours of 5-ALA were visualized using a confocal laser scanning microscope with a 10× objective at 405-nm excitation and emission at 603-738-nm wavelength. Time-lapse images of live cells were captured using bright field and 405-nm laser every second for 15 minutes. The captured frames were then visualized sequentially at 50× speed.
3. Results
3.1 Tumor Paint
Chordoma cells grown as a monolayer did not fluoresce much stronger than normal cells. However, chordoma cells grown as a micromass did fluoresce more than normal cells. In the first experiment, GFP positive rat adipose derived stromal cells can be seen as streaks of green fluorescence, while the chordoma cells appear to fluoresce very weakly in response to 5-ALA with minimal scattered red fluorescence (fig. 1). This contrasts with the second experiment, where chordoma cells, having been cultured in micromass form, fluoresced brightly and uniformly in response to 5-ALA. As a micromass, the distinction between chordoma cells (red color, center) and rat GFP adipose cells (green color, periphery) is clearly seen when both emission filters are combined (fig. 2). The third experiment was designed to mimic an in vivo growth pattern by allowing free mixture of both cell lines in a three-dimensional suspension, unlike the first experiment’s monolayer form and the second experiment’s micromass form. The results of the third experiment agreed with the earlier two. When grown as a free mixture, low concentrated regions of cells responded similarly to the monolayer form, with minimal fluorescence, whereas higher concentrated regions of the suspension behaved more like the second experiment, with high chordoma cell fluorescence (fig. 3).
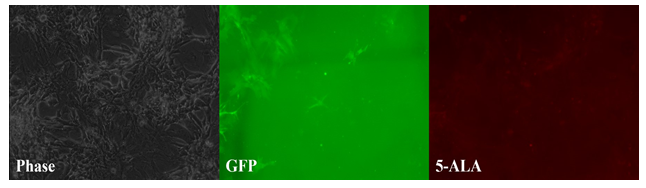
Figure 1: Tumor paint experiment #1 - cells grown in a monolayer. Phase microscopy under visible light (left), 509nm emission filter (middle), and 635nm emission filter (right). Streaks of green fluorescence in middle image correlates with rat GFP cells. Blank area in middle image correlates to chordoma cells. A paucity of fluorescence is observed in this same area on the red-light spectrum (right image), indicating lack of chordoma fluorescence in response to 5-ALA as a monolayer.
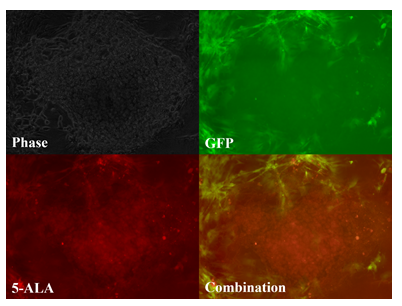
Figure 2: Tumor paint experiment #2 - cells grown in three-dimensional micromass form. Phase microscopy under visible light (upper left), 509nm (upper right), 635nm (lower left), merged 509nm and 635nm images (lower right). GFP-ADS cells can be seen fluorescing green, bordering the chordoma micromass, which fluoresces red after 5-ALA exposure.
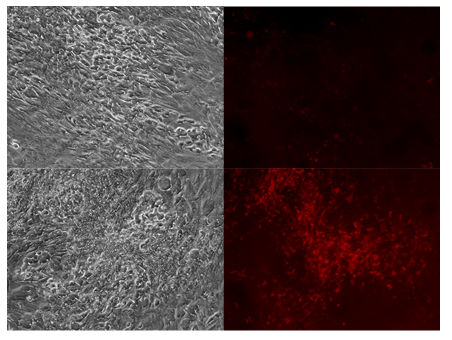
Figure 3: Tumor paint experiment #3 - cells grown in suspension form. Phase microscopy under visible light (upper and lower left), 635nm emission (upper and lower right). After addition of 5-ALA, chordoma cells do not appear to fluoresce in low density regions (upper right) but fluoresce heavily in high density, clustered regions (lower right).
3.2 Photodynamic Therapy
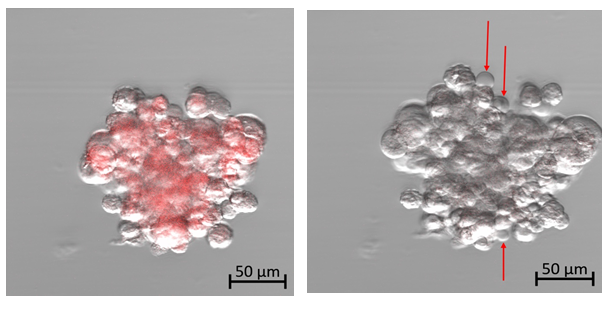
At the start of this experiment, the affected chordoma cells can be seen fluorescing red at an emission wavelength of 602-738 nm in response to the 405-nm excitation laser exposure (Fig. 4a). The cells appear densely packed with small round nuclei and abundant vacuolated cytoplasm, known as physaliferous cells, typical of a chordoma cell population. The captured frames were then visualized sequentially at 50× speed, resulting in a 5-second video (see supplementary material). As time progressed, there appeared to be increased intracellular swelling, with rapid formation of multiple vesicles exiting from the cellular membrane (Fig. 4b and supplemental video).
Figure 4a: Photodynamic therapy using confocal microscopy on human chordoma cells. Time: 0.
Figure 4b: Photodynamic therapy using confocal microscopy on human chordoma cells. Time: 15 minutes. Diffuse cellular swelling with multiple vesicle formation indicating cell death. (See time-lapse video for more visual clarity of vesicle formation)
4. Discussion
5-ALA-driven PDT was confirmed by the laser treatment under confocal microscopy. The bubble forming on the membrane of the cells indicated bubbling cell death, which is considered evidence of DNA damage caused by the accumulation of nitric oxide [17]. It has been reported that the PDT induced by 5-ALA exerts cytotoxic effects on malignant cells by activating the photosensitizer protoporphyrin IX. Type I and II reactions result, which release free radicals and singlet oxygen species, ultimately leading to cell death [10]. This process was first described by Malik and Lugaci in 1987 when they noted 95% of erythroleukemic cells were killed after 5-ALA exposure under ‘black-light’ (320-450nm) while cells which were not exposed to light had survived [11]. All the experiments carried out in the tumor paint arm showed increased and selective fluorescence of chordoma cells after exposure to 5-ALA when tumor cells were either heavily clustered or grown in micromass form. Micromass structures and freely suspended cell cultures were chosen for this study in order to better simulate in vivo conditions, allowing for more translatable clinical relevance. GFP positive rat adipose derived stromal cells were used to highlight the ability of 5-ALA to distinguish between malignant and benign cells (fig. 2). As chordoma tumors are faced with increased cellular crowding, a decreased ability to metabolize 5-ALA seems to ensue. This may be due to decreased exposed surface area to extracellular fluid, as well as possible loss of differentiation as the chordoma tumors increase in size, making 5-ALA expulsion more difficult. The fluorescent by-product, protoporphyrin IX, accumulates in these malignant cells but not in benign cells, leading to selective tumor fluorescence. This theory would also support Cornelius et al.’s findings of decreased cell viability in response to 5-ALA PDT in their high-density group as compared to their low-density group [13].
5-ALA guided tumor paint, pioneered by Stummer et al. for glioblastoma resections in 2000, has proven to be successful in a variety of fields such as neurosurgery, urology and gynecology [16, 18-21]. Additionally, the authors of the current study have shown selective and retained fluorescence in myxofibrosarcoma cells when compared to adipose derived stromal cells after 5-ALA exposure on a micromass scaled experiment [22]. Denzinger et al [20] found a significant drop in residual tumor rate in 88 patients treated with 5-ALA-assisted cystoscopy (4.5%), as opposed to the 103 patients without (25.2%) (p = 0.0003). A meta-analysis of 10 studies involving intraoperative 5-ALA fluorescence-guided resections of gliomas suggested that 5-ALA-guided surgery is more effective than conventional surgery, enhancing quality of life and prolonging survival [23]. A survival analysis of 646 patients across three studies was performed as part of this meta-analysis, with a 41% to 46% progression-free survival rate using 5-ALA-guided resections, as opposed to 21% to 28% for resections under plain visible light [23]. In 1999, 5-ALA was approved by the U.S. Food and Drug Administration (FDA) for topical application to treat basal cell carcinoma and actinic keratosis using photodynamic therapy and for cystoscopic detection of papillary bladder cancer in 2010 [12]. In Europe, the European Medicines Agency (EMA) has already approved 5-ALA for intravenous use in 2007, however, its approval for intravenous use by the FDA is still pending [24]. Side effects of 5-ALA are minimal, including non-clinically relevant mild alterations of blood cell counts and slight increases in liver enzymes [25]. Preclinical and safety literature confirmed these findings, with few reports of phototoxicity, largely limited to those receiving high doses (20 mg/kg). Contraindications include pregnancy, hypersensitivity to porphyrins and acute/chronic porphyria [26]. Overall, there is little evidence to support abnormal symptoms or laboratory results of clinical relevance [26].
The chordoma cell line was selected for this study due to its locally aggressive characteristics coupled with an unacceptably high recurrence rate. Chordomas account for 1% to 4% of all primary malignant bone tumors and have an average length of survival of 6.29 years when treated with resection with or without radiotherapy [8, 9]. Based on a recent review by George et. al. in 2015, the only three factors affecting prognosis are: completeness of resection, dedifferentiated forms, and recurrence [6]. Furthermore, George et. al. showed in their series with average follow-up of 6.5 years, that local recurrence and mortality rates correspond to the level of resection with a 75% recurrence and 63% mortality rate after partial resection, 47.5% recurrence and 52.5% mortality rate after subtotal resection and 28% recurrence and 20.5% mortality rate after total resection [27]. These results further justify the necessity of finding a means of achieving optimal resection at the time of initial surgical intervention. Lumbo-sacral chordomas tend to present as a slowly growing painless palpable mass. Since symptoms at onset are generally non-specific and not associated with rectal dysfunction or urinary incontinence, diagnosis is often delayed [1]. Plain lumbosacral spine radiographs and Computed Tomography (CT) scans may reveal a large destructive midline lesion extending into the intervertebral space and adjacent vertebral bodies. On Magnetic Resonance Imaging (MRI) chordomas will appear hypo-intense on T1-weighted images and hyperintense on T2-weighted images, with a possible associated soft tissue mass [1]. Management of chordomas is multifaceted. A multidisciplinary approach is necessary and should include a team of musculoskeletal surgical oncologists, medical oncologists, radiologists, pathologists, neurosurgeons, and plastic surgeons for soft tissue reconstruction. As previously mentioned, complete resection is the main factor affecting prognosis therefore as wide a resection as possible should be the initial step in management once the diagnosis is made. The main adjuvant therapy used is radiation therapy, although controversies exist over case-by-case use versus uniform use, as well as which type of radiation therapy to utilize. A meta-analysis performed by Di Maio in 2011 found that adjuvant proton beam therapy following resection proved beneficial for two chordoma subtypes, but not for the entire series [28]. Conventional chemotherapy has almost no effect on chordomas. However, targeted chemotherapy is a promising developing adjuvant for treatment given recent advances in the understanding of chordoma oncogenesis [1, 29] Signaling pathways such as mTOR and MAPK in addition to molecular receptors such as PDGFR, HER2, HGFR, and IGF-1R as well as the CDKN2A and Brachyury genes are currently being investigated as potential molecular targets [1].
5. Conclusion
The aim of this study was to perform an in vitro analysis of fluorescence and photodynamic therapy using chordoma cells after exposure to the photosensitizer 5-ALA. Based on the results of this study, chordoma cells selectively fluoresce in response to 5-ALA tumor paint and are sensitive to 5-ALA PDT. Our positive results justify a need for further testing in vivo. We believe visual detection using 5-ALA tumor paint, coupled with tumor bed sterilization using 5-ALA PDT can help the treating surgeon obtain clearer and wider margins. The prospect of improved margins, we hope, will lead to decreased recurrence rates, fewer complications, and improved overall survival.
Abbreviations
5-ALA: 5-Aminolevulinic acid; ADS: Adipose derived stromal cell line; MUG-Chor1: Human chordoma cell line; GFP: Green fluorescence protein; DMEM/F12: Dulbecco modified Eagle/F12; IMDM: Iscove’s modified Dulbecco’s medium; RPMI: Roswell Park Memorial Institute medium; ITS: Insulin transferrin selenium; FBS: Fetal bovine serum; FDA: Food and Drug Administration; PDT: Photodynamic therapy; GBM: Glioblastoma multiforme; CT: Computed tomography; MRI: Magnetic resonance imaging.
Declarations
Ethics approval and consent to participate: NA
Consent for publication: NA
Availability of data and materials: All datasets on which the conclusions of this report rely are available on request.
Competing interests: The authors declare that there are no conflicts of interest regarding the publication of this study.
Funding:
Funding was provided by the Department of Orthopaedic Surgery of North Shore/Long Island Jewish Medical Center and the H. Craig and Lora Treiber Orthopaedic Research Foundation.
Authors’ Contributions:
All authors have read and approved this manuscript. Research design, or the acquisition, analysis, or interpretation of data, and drafting the paper or revising it critically were performed by Kenan, the first author. Research design, or the acquisition, analysis, or interpretation of data were performed by Liang, the co-first author. Research design, or the acquisition, analysis, or interpretation of data, and drafting the paper or revising it critically were performed by Nixon. Research design, or the acquisition, analysis, or interpretation of data, and drafting the paper or revising it critically were performed by Grande. Research design, or the acquisition, analysis, or interpretation of data, and drafting the paper or revising it critically were performed by Goodman. Research design, or the acquisition, analysis, or interpretation of data, and drafting the paper or revising it critically were performed by Levin.
Acknowledgements
:Rachel Box, MS, ELS and Jenni Weems, MS, ELS - medical editors at The John Hopkins University.
References
- Garofalo F, di Summa PG, Christoforidis D, Pracht M, Laudato P, Cherix S, et al. Multidisciplinary approach of lumbo-sacral chordoma: From oncological treatment to reconstructive surgery. Journal of surgical oncology 112 (2015): 544-554.
- Angelini A, Pala E, Calabro T, Maraldi M, Ruggieri P. Prognostic factors in surgical resection of sacral chordoma. Journal of surgical oncology 112 (2015): 344-351.
- Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. The Journal of bone and joint surgery American volume 87 (2005): 2211-2216.
- Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M. Surgical margins and local control in resection of sacral chordomas. Clinical orthopaedics and related research 468 (2010): 2939-2947.
- Radaelli S, Stacchiotti S, Ruggieri P, Donati D, Casali PG, Palmerini E, et al. Sacral Chordoma: Long-term Outcome of a Large Series of Patients Surgically Treated at Two Reference Centers. Spine 41 (2016): 1049-1057.
- George B, Bresson D, Herman P, Froelich S. Chordomas: A Review. Neurosurgery clinics of North America 26 (2015): 437-452.
- Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC, Jr. Lumbosacral chordoma. Prognostic factors and treatment. Spine 24 (1999): 1639-1645.
- McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer causes & control: CCC 12 (2001): 1-11.
- Sciubba DM, Cheng JJ, Petteys RJ, Weber KL, Frassica DA, Gokaslan ZL. Chordoma of the sacrum and vertebral bodies. The Journal of the American Academy of Orthopaedic Surgeons 17 (2009): 708-717.
- Wachowska M, Muchowicz A, Firczuk M, Gabrysiak M, Winiarska M, Wanczyk M, et al. Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of cancer. Molecules 16 (2011): 4140-4164.
- Malik Z, Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer 56 (1987): 589-595.
- Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci 7 (2008): 283-289.
- Cornelius JF, Eismann L, Ebbert L, Senger B, Petridis AK, Kamp MA, et al. 5-Aminolevulinic acid-based photodynamic therapy of chordoma: In vitro experiments on a human tumor cell line. Photodiagnosis and photodynamic therapy 20 (2017): 111-115.
- Eljamel MS. Which intracranial lesions would be suitable for 5-aminolevulenic acid-induced fluorescence-guided identification, localization, or resection? A prospective study of 114 consecutive intracranial lesions. Clinical neurosurgery 56 (2009): 93-97.
- Rinner B, Froehlich EV, Buerger K, Knausz H, Lohberger B, Scheipl S, et al. Establishment and detailed functional and molecular genetic characterisation of a novel sacral chordoma cell line, MUG-Chor1. International journal of oncology 40 (2012): 443-451.
- Millon SR, Ostrander JH, Yazdanfar S, Brown JQ, Bender JE, Rajeha A, et al. Preferential accumulation of 5-aminolevulinic acid-induced protoporphyrin IX in breast cancer: a comprehensive study on six breast cell lines with varying phenotypes. J Biomed Opt 15 (2010): 018002.
- Chang NS. Bubbling cell death: A hot air balloon released from the nucleus in the cold. Exp Biol Med (Maywood) 241 (2016): 1306-1315.
- Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93 (2000): 1003-1013.
- Denzinger S, Rossler W, Otto W. [Photodynamic diagnostic of superficial bladder carcinoma]. Dtsch Med Wochenschr 132 (2007): 2332-2335.
- Denzinger S, Burger M, Walter B, Knuechel R, Roessler W, Wieland WF, et al. Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study. Urology 69 (2007): 675-679.
- Regula J, MacRobert AJ, Gorchein A, Buonaccorsi GA, Thorpe SM, Spencer GM, et al. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX--a pilot study. Gut 36 (1995): 67-75.
- Kenan S, Liang H, Goodman H, Grande D, Levin A. Five-Aminolevulinic Acid Photodynamic Therapy for Myxofibrosarcoma. Orthopaedic Research Society (ORS) Annual Meeting, San Diego, Ca (2017).
- Zhao S, Wu J, Wang C, Liu H, Dong X, Shi C, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PloS one 8 (2013): e63682.
- Teixidor P, Arraez MA, Villalba G, Garcia R, Tardaguila M, Gonzalez JJ, et al. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PloS one 11 (2016): e0149244.
- Agency EM. Gliolan: EPAR - Scientific Discussion (2015).
- Perez MH, Rodriguez BL, Shintani TT, Watanabe K, Miyanari S, Harrigan RC. 5-aminolevulinic acid (5-ALA): analysis of preclinical and safety literature. Food and Nutrition Sciences 4 (2013): 1009-1013.
- George B, Bresson D, Bouazza S, Froelich S, Mandonnet E, Hamdi S, et al. [Chordoma]. Neurochirurgie 60 (2014): 63-140.
- Di Maio S, Temkin N, Ramanathan D, Sekhar LN. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg 115 (2011): 1094-1105.
- Presneau N, Shalaby A, Idowu B, Gikas P, Cannon SR, Gout I, et al. Potential therapeutic targets for chordoma: PI3K/AKT/TSC1/TSC2/mTOR pathway. Br J Cancer 100 (2009): 1406-1414.
Supplemental files
Name: Supp1- Chordoma Lysis after 5-ALA.mp4
Title: Time-lapse video of MUG-Myx1 cells after exposure to 5-ALA PDT
Description: Time-lapse video showing photodynamic therapy using a 405-nm laser with confocal microscopy to target human chordoma cells. Video speed is 50x real time, accounting for a 15-minute period of time. Multiple intracellular vesicles can be seen rapidly enlarging and exiting from the membrane (red arrows), indicating initial stages of cell death.
Name: Supp2- ADS control after 5-ALA.mp4
Title: Time-lapse video of ADS cells after exposure to 5-ALA PDT
Description: No fluorescence, swelling, or vesicle formation is observed after treatment.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 