Anti-Mycobacterial and Synergistic Activity of Synthetic Acridine-9-Carboraldehyde and 9-Hydroxy-4-Methoxy Acridine Alkaloids against Clinical Isolates of Multidrug-Resistant Mycobacterium Tuberculosis
Lydia Bunalema1,*, Moses Ocan1, Godfrey S. Bbosa1, Aloysious Lubega 1, Hindum Lanyero1, Immaculate T. Nahereza2
1Department of Pharmacology and Therapeutics, Makerere University, College of Health Sciences, Kampala Uganda, P. O. Box 7072 Kampala Uganda
2Joint Clinical Research Center (JCRC), P.O Box 10005 Lubowa Kampala Uganda
*Corresponding author: Bunalema Lydia, Department of Pharmacology and Therapeutics, Makerere University, College of Health Sciences, Kampala Uganda, P. O. Box 7072 Kampala, Uganda.
Received: 04 April 2022; Accepted: 13 April 2022; Published: 06 May 2022
Article Information
Citation:
Lydia Bunalema, Moses Ocan, Godfrey S. Bbosa, Aloysious Lubega, Hindum Lanyero, Immaculate T. Nahereza. Anti-Mycobacterial and Synergistic Activity of Synthetic Acridine-9-Carboraldehyde and 9-Hydroxy-4-Methoxy Acridine Alkaloids against Clinical Isolates of Multidrug-Resistant Mycobacterium Tuberculosis. Journal of Pharmacy and Pharmacology Research 6 (2022): 25-34
View / Download Pdf Share at FacebookAbstract
Background: Tuberculosis treatment has become difficult due to development of strains that are resistant to both first and second-line anti-TB drugs. This calls for a continued development of new drugs with novel targets against multi and extensively drug-resistant strains of M. tuberculosis. The aims of the study were; i) to determine the antimycobacterial activity of two synthetic alkaloids i.e., acridine-9-carboraldehyde and 9-hydroxy-4-methoxy acridine on both susceptible and multidrug-resistant TB clinical isolates and ii) to determine interactions between the alkaloids and anti-TB drugs iii) to determine the toxicity profile of the most active alkaloid.
Methods: Resazurin reduction microplate assay (REMA) was used to determine the minimum inhibitory concentrations of acridine-9-carboral-dehyde and 9-hydroxy-4-methoxy acridine as well as their interactions with conventional anti-TB drugs ie isoniazid, rifampicin and ciprofloxacin.
Results: Acridine-9-carboraldehyde was active on both susceptible and multidrug-resistant strains of TB with minimum inhibitory concentrations of 0.238µg/mL and 0.108 µg/mL on the pan-sensitive and clinical susceptible strains and 0.157 µg/mL and 0.196 µg/mL on the rifampicin resistant and multidrug-resistant strains respectively. In addition, acridine-9-carboraldehyde was synergistic to both rifampicin and levofloxacin with a fractional inhibitory concentration index of ≤ 0.5.
Conclusion: Acridine-9-carboraldehyde is active against clinical isolates of multidrug-resistant M. tuberculosis. And it is synergistic with rifampicin and levofloxacin on susceptible and multidrug-resistant M. tuberculosis.
Keywords
<p>Mycobacterium tuberculosis, Synergistic Interactions, Drug resistance, acridine alkaloids</p>
Article Details
1. Introduction
Tuberculosis (TB) is a chronic, progressive and highly infectious mycobacterial infection that asymptomatically affects one quarter of the world’s population and is responsible for 2 million deaths annually worldwide [1, 2]. The increasing spread of TB is currently paralleled by a rapid increase in multidrug-resistant TB and extensively drug-resistant TB (MDR-TB and XDR-TB) making the disease incurable [3]. In 2018, about 484,000 cases of MDR TB were reported by WHO and these accounted for 20% of the patients with a history of previous TB treatment and 3.4% of the new TB infection [4]. The rise of drug-resistant M. tuberculosis and HIV /AIDS co-infections especially in low-and-middle-income countries (LMICs) has increased TB related mortality and thus threatens TB control and eradication efforts [5].
Successful implementation of the WHO strategy for tuberculosis control depends on early detection and prompt treatment of infectious TB cases [6]. However, the high level of drug resistance threatens to slow progress towards a TB free world. This is especially due to the challenges of managing drug-resistant M. tuberculosis which involves the use of second-line drugs which are costly with numerous adverse drug reactions and a prolonged duration of treatment [7].
Although discovery and development of new anti-TB drugs is key in addressing the challenge of high level of drug resistance in TB treatment, there is currently a limited number of novel agents in the TB drug development pipeline. For the first time in 50 years, very few new drugs including bedaquiline and delamanid have been developed and recommended by WHO as novel anti-TB agents [8, 9]. Bedaquiline and delamanid have novel specific targets in the M. tuberculosis organism as ATP synthase inhibitors and mycolic acid synthesis inhibitors respectively but they donot provide sufficient remedy against drug-resistant strains of M. tuberculosis especially XDR-TB [10, 11]. Despite the inclusion of bedaquiline and delamanid in MDR treatment regimens having shortened the treatment duration of MDR-TB from 20 months to less than 12 months and eliminating the necessity of using injectable anti-TB drugs [12], there have been recent reports of delamanid and bedaquilin drug resistance in patients with XDR-TB [13,14]. There is thus a need for continued search for novel agents in the treatment of Mycobacterium tuberculosis; acridine alkaloids being among such agents.
Acridine alkaloids and their derivatives have been investigated as possible therapeutic agents in the treatment of different ailments including cancer, Alzheimer’s disease, malaria, herpes simplex and bacterial infections [15, 16]. This is due to their varied structures and ability to affect several biochemical processes including DNA intercalation, protein and lipid metabolism [17]. Previous studies have reported that naturally occurring acridine alkaloids from Zanthoxyllum leprieurii have activity against preserved M. tuberculosis strains [18]. However, due to the challenges of mass production, the study sought to assess presence of anti-mycobacterial activity in synthetic acridine alkaloids. The activity of acridine alkaloids on clinical multidrug-resistant TB is unknown. This study aimed at; i) to determine the antimycobacterial activity of two synthetic alkaloids i.e., acridine-9-carboraldehyde and 9-hydroxy-4-methoxy acridine on both susceptible and multidrug-resistant TB clinical isolates ii) to determine the interactions between the synthetic alkaloids and anti-TB drugs.
2. Methods
2.1 Test Isolates: A total of four M. tuberculosis strains were included in this study including; a multidrug-resistant clinical isolate (ref #86074) not susceptible to all first-line drugs, rifampicin resistant clinical isolate (ref # 86333), a susceptible clinical isolate (ref # 86074) (that is sensitive to all first-line drugs) and a preserved pan-sensitive M. tuberculosis strain (H37Rv) that was used as a standard. All clinical isolates used in this study were donation samples obtained from Joint Clinical Research Centre (JCRC) at Lubowa in Kampala, Uganda, along Entebbe road (www.jcrc.org.ug).
2.2 Test Drugs: Rifampicin (Sigma, #R3501), isoniazid (Sigma, # 059k1640) and ciprofloxacin (Sigma, #098m4779v) were purchased from Sigma, Chemical Company (St Louis, MO). Two synthetic acridine alkaloids ie 9-hydroxy-4-methoxy acridine (fig 1) (101760-sv) and acridine-9-carboraldehyde (Fig 2) (#MKBP6466V) were bought from Sigma Aldrich co 3050 Spruce St Louis USA as pure compounds with a purity of 97%. Initial stock solutions of the anti-TB drugs were prepared according to the manufacturers’ instructions and stored at -70°C until use. The final concentrations ranged from 32- 0.065 µg/mL for the alkaloids and 16- 0.01 µg/mL for the anti-TB drugs.
2.3 Culture Medium: Middlebrook 7H10 (#8338821) and 7H9 (#6333553) broth supplemented with 10% oleic acid, albumin, dextrose, catalase (OADC) enrichment were used as the solid and liquid media, respectively, to grow the Mycobacterium sp. The culture media were prepared according to the manufacturer’s guidelines.
2.4 Inoculum Preparation: M. tuberculosis suspensions were inoculated on Middlebrook 7H10 and harvested in their growth log-phase after twenty-one days. The inoculum was scrapped and introduced into 7H9 broth (10 mL) supplemented with 0.2% (v/v) glycerol and OADC and incubated at 37°C for 24 hours. Using a nephrometer, the inoculum was adjusted to an optical density of 0.5 McFarland Standard (approximately 1.5x 108 CFU colony forming units) by adding normal saline until the required density.
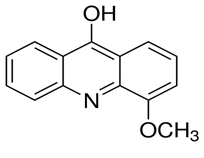
Figure 1: Structure of 9 hydroxy-4-methoxy acridine
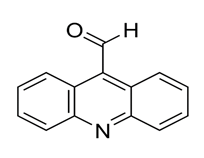
Figure 2: Structure of acridine-9-carboxaldehyde
2.5 Antimycobacterial Susceptibility Testing: Resazurin reduction microplate assay (REMA) was used as previously described [19]. In brief, 200 μl of sterile distilled water was added to all outer perimeter wells of 96 round bottomed plates. This was done to minimize dehydration. To the rest of the wells, 100 μl of Middle Brook 7H9 broth was added. One hundred micro liters (100 μl) of double concentration drug solutions were added to the wells in rows B to G in column 2 and serial dilutions were made through column 10 using a multi-channel pipette. To wells in rows 2–11, 100 μl of M. tuberculosis inoculum was added, bringing the final volume to 200 μl per well. The wells in column 11 were used as negative control wells containing only broth and inoculum. The plates were incubated at 37 °C for 24 hrs in a 5% CO2 incubator. The tests were prepared in triplicate for each of the strains used. Thirty micro liters of freshly prepared resazurin dye was added to one of the control wells and further incubated at 37 °C for 24 hrs. Observation of a color change from blue to pink indicated that there was growth and so the dye was added to all wells and further incubated at 37°C for 24 hours. The minimum inhibitory concentration (MIC) was defined as the lowest drug concentration which prevented a color change from blue to pink. The bioassays were performed in a level 3 bio-safety mycobacteriology laboratory at JCRC.
2.6 Combination Testing: Interactions between anti-TB drugs (rifampicin, Isoniazid and ciprofloxacin) and the test compounds (acridine 9 carboraldehyde and 9 hydroxy 4 methoxy acridine) were performed using the method described in 1.2.5 above. In total five combinations were tested including; (i) Rifampicin/acridine 9 carboradehyde, (ii) Isoniazid/acridine 9 carboradehyde on H37Rv and susceptible TB; (iii) Isoniazid/ acridine 9 carboradehyde and (iv) levofloxacin/ acridine 9 carboradehyde on the rifampicin resistant isolate; (v) acridine 9 carboradehyde/levofloxacin on MDR TB. The test results were subjected to a E-test where the fractional inhibitory concentration index (FICI) was calculated using the formula below;
FICI=(MICAcombi/MICAalone)+(MICBcombi/MICBalone).
where A and B are the two respective test agents. The interaction between two test agents in combination can either be synergistic, additive, no effect, or antagonistic. If FICI value is less than or equal to 0.5 then the MIC of the combination is lower than either of the drugs used alone and therefore there is synergism. If the FICI is greater than 0.5 but less than 1.0, then the interaction is indifferent and if the FICI is greater than 1.0, then the interaction is regarded as antagonist [20].
3. Ethical Considerations
The study sought ethical approval to carry out the study from the School of Biomedical Sciences Research and Ethics committee under approval number SBS-724. A waiver of consent was granted from the School of Biomedical sciences IRB to use the clinical isolates at JCRC.
4. Statistical Analyses
Data was entered in Microsoft Office Excel, 2010 and then transferred to Graph Pad prism version 9.2.0 INC, USA, for statistical analysis. The student’s t test was performed using a 95% confidence interval.
5. Results
The study investigated the antimycobacterial activity of two synthetic acridine alkaloids including acridine-9-carboraldehyde and 9-hydroxy-4-methoxy acridine against clinical strains of M. tuberculosis including, a susceptible isolate, rifampicin resistant isolate, a multidrug-resistant isolate, and a pan-sensitive strain using the resazurin reduction microtitre assay. In this study, rifampicin, isoniazid, and levofloxacin were used as positive controls.
5.1 Anti-mycobacterial activity of acridine-9-carboraldehyde and 9-hydroxy-4- methoxy acridine against susceptible and resistant clinical strains of M. tuberculosis
5.1.1 Susceptible clinical Strains: The MIC values of acridine-9-carboraldehyde on the preserved (H37Rv) and clinical susceptible strains were low i.e., 0.238 µg/mL and 0.108 µg/mL respectively (Table 1). When compared to the positive controls including isoniazid with MIC of 0.075 µg/mL on the H37Rv and 0.2 µg/mL on the susceptible strain, the activity of acridine-9-carboraldehyde was statistically not different (p=0.81 and 0.01 respectively).
Table 1: Minimum inhibitory concentrations of Rifampicin, Isoniazid and Levofloxacin in combination with acridine 9 carboraldehyde, FICI and interactions
|
Isolate |
|||||||||||||
|
Pan sensitive |
RIF Resistant |
Clinical wild strain |
MDR TB |
||||||||||
|
MIC (µg/ML) |
FIC |
MIC (µg/ML) |
FIC |
MIC (µg/ML) |
FIC |
MIC (µg/ML) |
FIC |
||||||
|
RIFACD |
0.5 |
0.5 |
0.03 |
0.4 |
|||||||||
|
INHACD |
0.1 |
1 |
0.1 |
1.2 |
0.08 |
1.3 |
|||||||
|
LVXACD |
0.42 |
0.5 |
|||||||||||
|
ACDRIF |
0.18 |
0.01 |
|||||||||||
|
ACDINH |
0.36 |
0.18 |
0.29 |
||||||||||
|
ACDLVX |
0.5 |
0.48 |
|||||||||||
RIF Rifampicin, INH isoniazid, LVX levofloxacin ACD Acridine 9 carboraldehyde, RIFACD Rifampicin in combination with ACD, INHACD Isoniazid in combination with ACD, LVXACD Levofloxacin in combination with ACD, MIC Minimum Inhibitory concentration, FIC Fraction inhibitory concentration
5.1.2 Resistant clinical strains:
Two clinical resistant strains of M. tuberculosis were tested in this study and these included; a rifampicin resistant strain and a multidrug-resistant strain. Acridine-9-carboraldehyde was active against both the rifampicin resistant and multidrug-resistant strains with low minimum inhibitory concentration values of 0.157 µg/mL (P=0.46) and 0.196 µg/mL (P=0.23) respectively. Isoniazid had MIC value of 0.2 µg/mL on the rifampicin strain while levofloxacin had an MIC value of 2 µg/mL on the MDR isolate. The difference between the MIC values of the controls and the test compound was statistically not significant.
5.2 Anti-mycobacterial activity of combinations of acridine-9-carboraldehyde and anti-TB drugs against M. tuberculosis clinical isolates
5.2.1 Rifampicin and acridine-9-carboraldehyde: When we combined rifampicin and acridine-9-carboraldehyde against the pan-sensitive strain, the MIC value of rifampicin was lowered fourfold from 2 µg/mL when singly tested to 0.5 µg/mL (Table 2). The calculated FIC was 0.5 meaning that there was synergism between the two.
Furthermore, against the susceptible clinical isolate, the MIC value of rifampicin in combination with acridine-9-carboraldehyde was lowered to 0.03 µg/mL and the calculated FICI value was 0.4. Acridine-9-carboraldehyde was synergistic to rifampicin against the clinical susceptible strain as well.
5.2.2 Isoniazid and acridine-9-carboraldehyde: A combination of acridine-9-carboraldehyde and isoniazid against the rifampicin resistant isolate (FICI= 1.2) and the pan-sensitive strain (FICI=1) did not show any synergistic interactions.
5.2.3 Levofloxacin and acridine-9-carboraldehyde: A combination of levofloxacin and acridine-9-carboraldehyde against the multidrug-resistant TB isolate decreased the MIC value of levofloxacin fivefold from 2 µg/mL to 0.42 µg/mL. From the calculated FICI value (0.48), there was synergy between levofloxacin and acridine-9-carboraldehyde against the multidrug-resistant clinical isolate.
Table 2: Minimum inhibitory concentration values, Fractional inhibitory concentration indices (FICI) of Rifampicin, Isoniazid and Levofloxacin in combination with acridine 9 carboraldehyde
|
H37Rv |
RIF Resistant |
Susceptible strain |
MDR TB |
|
|
Drug Minimum Inhibitory concentrations (μg/ml) |
||||
|
ACD |
0.238 |
0.157 |
0.108 |
0.196 |
|
HMA |
>25 |
>25 |
>25 |
- |
|
RIF |
2 |
>25 |
2 |
- |
|
INH |
0.075 |
0.2 |
0.2 |
- |
|
LVX |
0.5 |
2 |
0.5 |
2 |
Rifampicin, INH isoniazid, LVX levofloxacin ACD Acridine 9 carboraldehyde, RIFACD Rifampicin in combination with ACD, INHACD Isoniazid in combination with ACD, LVXACD Levofloxacin in combination with ACD, MIC Minimum Inhibitory concentration, FIC Fraction inhibitory concentration
No antagonistic effect was observed when compound 1 was combined with classical anti-tuberculosis drugs, but their anti-tuberculosis activities were increased instead.
6. Discussion
Treatment of tuberculosis faces a double challenge of limited new drugs in the development pipeline in addition to increased resistance to the existing therapies. This has limited the therapeutic options available for TB treatment especially in LMICs which bear the highest burden of the disease. Most local communities in LMICs resort to the use of traditional medicines in the management of TB and related symptoms. However, there is limited scientific literature to support the practice. A previous study (Bunalema et al., 2017) showed that naturally occuring acridine alkaloids present in some local medicinal plants have activity against M. tuberculosis. However, due to the challenges of mass production of the natural acridine alkaloids from plant sources, this study sought to determine the anti-mycobacterial activity of synthetic acridine alkaloids. In this study, acridine-9-carboraldehyde, a synthetic acridine alkaloid was active against the clinical susceptible TB isolates as well as the rifampicin resistant and multidrug-resistant strains of Mycobacterium tuberculosis. Presence of anti-TB activity in synthetic acridine alkaloids as found in this study is timely especially due to the challenges associated with mass production of medicines from natural sources like plants. The establishment of anti-TB activity of acridine alkaloids is welcome news for TB control efforts especially due to widespread drug resistance and the limited number of anti-TB compounds in the drug development pipeline. With the increasing resistance to the existing anti-TB drugs, the discovery and development of new compounds is urgently needed. This in addition to development of interventions to slow the development and spread of TB resistance are fundamental tenants in the fight against the disease.
Remarkably, in addition to in-vitro antimycobacterial activity, acridine-9-carboraldehyde showed synergistic activity when combined with first-line and second-line anti-TB drugs (rifampicin and levofloxacin) on sensitive and resistant clinical Mycobacterium tuberculosis respectively. The minimum inhibitory concentrations of both rifampicin and isoniazid were reduced to almost half (FICI=0.5) in combination with acridine-9-carboraldehyde. The exact mechanism by which the acridine decreased rifampicin and levofloxacin MIC is unknown. Nevertheless, Acridine alkaloids are known to be highly lipophilic and could have enhanced entry of rifampicn and levofloxacin into the cell increasing the intracellular concentration. However, rifampicin and levofloxacin are already known lipophilic drugs that rapidly penetrate the complex highly hydrophobic mycobacteria cell wall. Consequently, the increased cell wall permeability by the acridine may not be the only reason for the observed synergistic interaction. On the other hand, the synergy could be due to the acridine’s different mechanism of action on the M. tuberculosis. Several studies have demonstrated that acridine alkaloids are intercalating compounds that eventually inhibit DNA synthesis through inhibiting topoisomerase-I 21. In addition, previous studies have shown that rifampicin resistance is characterized by increased expression of the p-gp efflux pump which is modulated by acridine congeners 22, 23. The use of combination anti-TB drugs is core to the current modalities in anti-TB treatment and has been shown to reduce/slow resistance development in management of infectious diseases especially TB. Therefore, the discovery of new agents with synergistic activity with the current anti-TB drugs gives hope to the disease control efforts.
7. Conclusion
Our study has showed that acridine-9-carboraldehyde is active against both susceptible and multidrug-resistant strains of tuberculosis thus having potential to be developed into a novel anti-TB drug. Secondly, it interacts with both rifampicin and ciprofloxacin, two of the drugs that form a mainstay as first and second-line drugs thus showing potential of its use in combination with existing remedies.
Data availability
Data can be made available on special request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest
Acknowledgments
The authors would like to thank the international foundation of Science and Makerere University Research innovation fund for providing financial support. We acknowledge the support of Joint Clinical Research Center staff for the technical support they provided us.
References
- HoubenRMGJ & DoddPJ. The global burden of Latent Tuberculosis Infection: A re-estimation using mathematical modelling PLOS Medicine 13 (2016).
- World Health Organization.Global Tuberculosis Report. World Health Organization: Geneva, Switzerland (2020).
- Chisompola NK, Streicher EM, Muchemwa CMK, Warren RM and Sampson SL. Molecular epidemiology of drug-resistant Mycobacterium tuberculosis in Africa: a systematic review. BMC Infect Dis 20 (2020):
- Lacobino A, Fattorini L and Giannoni F. Drug-Resistant Tuberculosis 2020: Where We Stand. Applied Sciences 10 (2020).
- Sultana ZZ, Hoque FU, Beyene J, Akhlak Ul, Khan HR, Ahmed S, Hawlader DH & Hossain A HIV infection and multidrug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infectious Disease 21 (2021): 51.
- Gilpin C, Korobitsyn A, Migliori GB, Raviglione MC & Weyer K. The World Health Organization standards for tuberculosis care and management. European Respiratory Journal 51 (2018).
- Smith JP, Gandhi NR, Shah NS, Mlisana K, Moodley P, Johnson BA, Allana S, et al. The Impact of Concurrent Antiretroviral Therapy and MDR-TB Treatment on Adverse Events. Journal of acquired immune deficiency syndromes 83 (2020): 47–55.
- Pontali E, Raviglione MC & Migliori GB. Regimens to treat multidrug-resistant tuberculosis: past, present and future perspectives. European Respiratory Review 28 (2019).
- Cox E & Laessig K. FDA approval of bedaquiline- the Benefit–Risk Balance for Drug-resistant Tuberculosis. The New England journal of medicine 371 (2014): 689–691.
- Lewis JM & Sloan DJ. The role of delamanid in the treatment of drug-resistant tuberculosis. Therapeutic Clinical Risk Managemant 11 (2016): 779–791.
- Gualano G, Capone S, Matteelli A & Palmieri F. New antituberculosis drugs:from clinical trial to programmatic use. Infectious Disease Reports 8 (2016).
- Laniado-Laborín R. The WHO Shorter MDR-TB Regimen: Who Will Benefit with it? J Respiratory Research 4 (2016): 134-136.
- Polsfuss S, Hofmann-Thiel S, Merker M, Krieger D, Niemann S, Rüssmann H, et al. Emergence of Low-level Delamanid and Bedaquiline Resistance During Extremely Drug-resistant Tuberculosis Treatment, Clinical Infectious Diseases 69 (2019): 1229–1231.
- Yoshiyama T, Mitarai S, Takaki A, Aono A, Okumura Ohta K, Kato S. Multi-drug-resistant tuberculosis with simultaneously acquired-drug resistance to bedaquiline and delamanid, Clinical Infectious Diseases Advance online publication (2020).
- Zhang B, Li X, Li B, Gao C & Jiang Y. Acridine and its derivatives: a patent review (2009 - 2013). Expert opinion on therapeutic patents 24 (2014): 647–664.
- Rupar JS, Dobricic VD, Aleksic MM, Brboric J S, Cudina OA. A review of published data on acridine derivatives with different biological activities. Kragujevac J. Sci 40 (2018): 83-101.
- Gisela C. Muscia, Graciela Y. Buldain, Silvia E. Asís Design. Synthesis and evaluation of acridine and fused-quinolinederivatives as potentialanti-tuberculosis agents. European Journal of Medicinal Chemistry 73: 243-249
- Bunalema L, Fotso GW, Waako P, Tabuti JRS & Yeboah SO. Potential of Zanthoxylum leprieurii as a source of active compounds against drug-resistant Mycobacterium tuberculosis. BMC Complentary and Alternative Medicine. 17 (2017): 89.
- Wang G, Dong W, Lu H, Lu W, Feng J, Wang X, Chen H, Liu M & Tan C. Enniatin A1, A Natural Compound with Bactericidal Activity against Mycobacterium tuberculosis In Vitro. Molecules 25 (2020): 38.
- Orhan G, Bayram A, Zer Y & Balci I. Synergy Tests by E Test and checkerboard methods of antimicrobial combinations against Brucella melitensis. Journal of Clinical Microbiology (2005): 140–143.
- Prasher P and Sharma M. Medicinal chemistry of acridine and its analogues Medicinal Chemistry Communications 9 (2018): 1589-1618.
- Wu Q, Hossfeld A, Gerberick A, Saljoughian N, Tiwari C, Mehra S, Ganesan LP, Wozniak DJ & Rajaram M. Effect of Mycobacterium tuberculosis Enhancement of Macrophage P-Glycoprotein Expression and Activity on Intracellular Survival During Antituberculosis Drug Treatment. The Journal of infectious diseases 220 (2019): 1989–1998.
- Neves I, Costa M, Carvalho S, De Castro L. Expression of P-g P in patients with resistant tuberculosis European Respiratory Journal 40 (2012): 2643.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 