Prescriptions of Strong Opioid Analgesics in Primary Care (Pharmacy Care)
Andrea Gazová1*, Mária Kolesárová2, Marek Orinák2, Dalibor Kolesár3, Jan Kyselovic4
1Institute of Pharmacology and Clinical Pharmacology, Faculty of Medicine Comenius University, Špitálska 24, 813 72 Bratislava, Slovakia
2Department of Human and Clinical Pharmacology, University of Veterinary Medicine and Pharmacology in Košice, Komenského 73, Košice, 041 81 Slovak Republic
3Department of Anatomy, Faculty of Medicine, Pavol Jozef Šafárik University in Košice, Trieda SNP 1, Košice, 040 11, Slovak Republic
4V.Department of Internal Medicine, Faculty of Medicine, Comenius University in Bratislava, Špitálska 24, 813 72 Bratislava, Slovakia
*Corresponding Author: Andrea Ga?ová, Institute of Pharmacology and Clinical Pharmacology, Faculty of Medicine Comenius University, Špitálska 24, 813 72 Bratislava, Slovakia
Received: 26 August 2018; Accepted: 10 October 2018; Published: 26 October 2018
Article Information
Citation:
Andrea Gazovz, Maria Kolesárova, Marek Orinak, Dalibor Kolesar, Jan Kyselovic. Prescriptions of Strong Opioid Analgesics in Primary Care (Pharmacy Care). J Pharm Pharmacol Res 2 (2018): 096-107.
View / Download Pdf Share at FacebookAbstract
Objective: Pain is a part of every human life and typically, example is a chronic pain in tumour diseases. In therapy of the chronic cancer pain, opioid analgesics have irreplaceable role.
Methods: This retrospective analysis was conducted from January 2015 to March 2015 using the prescriptions of strong opioid analgesics accepted by pharmacy VITAE. Prescriptions of strong analgesics were written in the Oncology Institute in Košice to adult patients. Opioid prescriptions for all patients in analyses had cancer medical diagnosis. Characterization of patients, diagnosis of cancer type, characterization of prescribed opioids analgesics – generic name and rug forms of prescribed medicament were analyzed in retrospective analyses of the prescriptions.
Results: In total, there were 332 prescriptions (100 % of cancer) of strong opioids for 151 (54% male; 46% female) patients treated in the East-Slovak Oncology Institute in Košice, Slovakia, during the study period. The youngest patient - woman was 27 and the oldest patient - woman was 88. The most frequent cancer diagnosis were the carcinoma of respiratory and thorax organs (male) and the carcinoma of breast (female). The number of package of strong opioids was 543 (fentanyl (44%), buprenorphine (26%), oxycodone (12%), tapentanol (10%), morphine (7%) and hydromorphone (1%)).
Conclusions: Our analysis demonstrated, that despite the fact that morphine is still considered as a gold standard in the oncologic pain therapy, other opioids are more frequently prescribed as fentanyl, buprenorphine, oxycodone or new molecule tapentadol. All substances were prescribed for intense pain emerged from different type of advanced tumour diseases.
Keywords
<p>Clinical praxis; Oncologic diseases; Pain; Prescription</p>
Article Details
1. Introduction
1.1 Pain and the cancer
The cancer pain is characterized as prolonged or re-entry pain, coupled with continual nociceptive stimuli and affected by psychological factors. It´s called as a total pain and has multiple dimensions: physical, psychological, social and spiritual [1,2].
The cancer pain is affected by patient and his age, then phase, type and therapy of the oncologic disease. Approximately 65 – 85 % of cancer pains are caused by oncologic disease and therapy. The cancer pain is directly activated by tumour and his metastasis, for example bone metastasis, the compression and infiltration of nerve structures, the infiltration and occlusion of blood vessels, and the obstruction of caval organs etc. Next 15-25 % of the cancer pain is generated by an outcome of the anticancer therapy, for example operations, invasive diagnostic and therapeutic methods, toxicity of chemotherapy, early and later outcomes of therapy. More or less 3-10% constitutes an associate pain within the context of disease or without the context of disease and its therapy, for example infections, muscle pains in immobile patients [3]. Approximately 80% of oncologic patients have more than one type of pain, 34% patients have more than four types of pain of different aetiology. Primary tumours of breast and prostate, which metastasis to bones and have 60 and 80% incidence of the pain [4].
Pain is the most feared symptoms of cancer disease. Cancer pain is often incapacitating and discouraging for patients; is demoralizing to family members and caretakers; and is taxing and difficult to subdue for the pain specialists [5]. About 30% of patients suffer from pain during assessment of diagnosis, and about 80 – 90 % patients suffer from pain during advanced phase of cancer disease [6]. Adequate and controlled therapy of cancer pain is inevitable, because uncontrolled pain paralyses immune system and leads to progression of oncologic diseases. Deficient therapy of pain leads to insomnia, exhaustion and depression. Patient with the pain often eats and drinks lesser, what contributes to worsening of nutritional state, immunity and function capacity. On one hand the immedicable pain decreases of patient motivation to therapy, aggravates his compliance and results of the anticancer therapy [2,8].
Findings and clinical experience need for adequate control of the cancer pain. Excessive attenuation of the pain in patient invades his physiological immune mechanism and produces other complications [1]. The consequence of both of these approaches is affliction, aggravation of quality of life and it is shortening. It ̍is required to diagnose pain, determine its pathological, psychological, and social characteristics and assess therapy. Conventional medical management of cancer pain includes prescription of opioids and coadjuvants at doses sufficient to control the symptoms without causing severe drug effects [9].
The type of the cancer pain can be acute and chronic. The acute cancer pain is the main symptom of the oncologic disease. The effective therapy of the acute cancer pain must be aggressive, which carries significant alleviation of the pain in patients and carries the promise of successful recovery. The chronic cancer pain is very complicated diagnostic and therapeutic problem, where the important role-plays psychologic factors and a degree of an affliction. The chronic prolonged pain is nameless, foreign and absurd pain. The patients suffer from depression, anxiety, and a disturbance of sleep, an aggravation of concentration, agitation and anger. These symptoms and continuing physical attenuation of the patient with oncologic diseases and its therapy contribute to exacerbating the pain and require more radical therapy than the therapy of the pain [8].
1.2 The therapy of the pain.
The successful treatment of the pain is a challenging task that begins with careful attempts to assess the source and magnitude of the pain. The amount of pain experienced by the patient is often measured by means of a pain Numeric Rating Scale or less frequently by marking a line on a Visual Analog Scale (VAS) with word descriptors ranging from no pain (0) to excruciating pain (10). In either case, the values indicate the magnitude of pain as mild (1-3), moderate (4-6), or severe (7-10). A similar scale can be used with children (Face, Legs, Activity, Cry, Consolability [FLACC] or Wong-Baker scales) and with patients who cannot speak, the Wong-Baker scale depicts five faces ranging from smiling (no pain) to crying (maximum pain).
In accordance to World Health Organisation (WHO), the basic strategy of the pain therapy is established by the three-step analgesic ladder [2]. The WHO Ladder was created in 1986 to promote awareness of the optimal treatment of pain for individuals with cancer and has helped improve pain care for cancer patients worldwide. Research in the hospice setting has also demonstrated that fixed-interval administration of opioid medication (i.e., a regular dose at a scheduled time) is more effective in achieving pain relief than dosing on demand [11].
The actual strategy of the pain therapy less differentiates between the causes of the pain, but accentuates to the intensity of the pain and its respond to the therapy [12]. The original WHO analgesic diagram consists of three steps representing the use of chemical analgesic agents of increasing potency, was revised to integrate advanced treatment options, and which includes the following: step one non-opioids (NSAIDs); step two weak opioids; step three strong opioids, methadone, oral, and transdermal formulations; and step four interventional techniques, pumps, and neuromodulation devices. Generally, the lower stepladder levels are indicated for acute and milder pain requirements and higher steps for chronic and more severe pain requirements [5].
In accordance with new recommendation there is possible to use the elevator system - in the therapy of the strong pain and jump over the 2nd degree of the WHO ladder. It means the direct advance from non-opioid analgesics to strong opioids. Pharmacotherapy of the chronic pain, there are used adjunctive analgesics – co-analgesics, which can attenuate some specific pain aspects, for example, neuropathic pain and implemental drugs assigned for the therapy of analgesics adverse effects [12].
1.3 The opioid analgesics
The pain associated with cancer must be treated aggressively and often requires a multidisciplinary approach for the effective management. Such conditions may require continuous use of potent opioid analgesics and are associated with some degree of tolerance and dependence. For a patient in severe pain, administration (oral, parenteral, neuralxial), duration of drug action, ceiling effect (maximal intrinsic activity), duration of therapy, the potential for adverse effects, and the patient´s past experience with opioids all should be addressed [13].
The opioids agonists produce analgesia by binding to specific G protein-coupled receptors that are located in the brain and spinal cord regions involved in the transmission and modulation of the pain. Three major classes of opioid receptors (μ, δ, κ) have been identified in various nervous system sites and in other tissues. At the molecular level, opioid receptors form a family of protein, that physically couple to G proteins and through this interaction affect ion channel gating, modulate intracellular Ca2+ disposition, and alter protein phosphorylation. The opioids have two well-established direct Gi/0 protein-coupled actions on neurons: 1. They close voltage-gated Ca2+ channels on presynaptic nerve terminals and thereby reduce transmitter release, and 2. They open K+ channels and hyperpolarize and thus inhibit postsynaptic neurons. The presynaptic action depressed transmitter release, including glutamate, acetylcholine, norepinephrine, serotonin and substance P [14].
New dosage forms of opioids that allow slower release of the drug are now available, for example, sustained-release forms of morphine (MS Contin) and oxycodone (OxyContin). Their purported advantage is a longer and more stable level of the analgesia. However, there is little evidence to support long-term (greater than 6 months) use of sustained release opioid to manage chronic pain in the non-cancer patient. If disturbances of gastrointestinal function, prevent the use of oral sustained-release morphine, then other forms of administration are used, for example, fentanyl transdermal system can be used over long periods, and next buccal transmucosal fentanyl can be used for short episodes of breakthrough pain. Administration of strong opioids by nasal insufflation is also efficacious, and nasal preparations are now available in some countries. In addition, stimulant drugs such as the amphetamines can enhance the analgesic actions of opioids and thus may be very useful adjuncts in the patient with chronic pain [15].
This publication presents results from retrospective analyses of pharmacotherapy of the pain caused by oncologic diseases in Slovak clinical praxis. This study aimed to described the characterization of patients (age and gender), the number of the most commonly prescribed strong opioids, the most frequently cancer diagnosis and the favourite and most commonly prescribed drug forms.
2. Materials and Methods
This retrospective analysis was conducted from January 2015 to March 2015 using the prescriptions of strong opioid analgesics accepted by pharmacy VITAE. Prescriptions of strong analgesics were written in the East-Slovak Oncology Institute in Košice to adult patients. Opioid prescriptions for all patients in analyses had cancer medical diagnosis.
Each prescription contains information about the patient, the gender, the age, the cancer diagnosis, the generic name of strong opioid, the drug form and the dose and who take up the drug from apothecary. In this retrospective analysis, we processed this all information. The strong opioid prescriptions were categorized after the cancer diagnosis, the patients´ demographic data (age) were stratified into seven groups (≤29, 30-39, 40–49, 50-59, 60-69, 70-79, 80-89 years old). Utilization measures for the six opioids included number of prescriptions, number of patients and the drug form.
3. Results
3.1 Characterization of patients
This work analyses opioid prescriptions of patients with oncology disease in apothecary shop VITAE, which is near the East-Slovak Oncology Institute in Košice, Slovakia from 1.1. 2015 to 31.1. 2015. In total, 332 prescriptions (100% for cancer pain) were prescribed for 151 users (number 82 - 54% male; number 69 - 46% female).
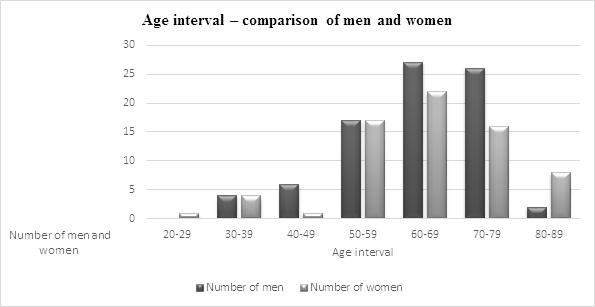
The mean age of men of strong opioids users was 64.3 ± 10.5 (T-test men vs women 0.2) and the mean age of women was 63.2 ± 14.8. There was a higher concentration of patients aged 60 – 69 years old (male 33%; female 32%), followed by aged 70-79 years old (male 32%; female 23%) and 50-59 years old (male 21%; female 25%) (see Graph 1). The youngest patient - was woman 27 years old and the oldest patient was a woman 88 years old.
3.2 Diagnoses of patients
Of all strong opioid users, 151 in our study had cancer diagnoses. The most frequently three diagnoses in male users were malignant tumours of respiratory and thorax organs (male 32% vs female 10%), malignant tumours of gastrointestinal organs (male 22% vs female 22%) and malignant tumours of lip, mouth cavity and pharynges (male 18% vs female 6%). On the other hand, the most common three diagnoses in female users were malignant tumours of breast (female 32% vs male 0%), malignant tumours of woman sex organs (female 22% vs male 0%) and malignant tumours of gastrointestinal organs (female 22% vs male 22%). The chronic excruciating pain without specific diagnosis was diagnosed in five patients (see Table 1). Ten patients had two diagnosis (6.6 %) and one patient had three oncologic diseases (0.6 %).
|
Diagnose |
Men |
Women |
|
Malignant tumours of lip, mouth cavity and pharynges |
15 |
4 |
|
Malignant tumours of gastrointestinal organs |
18 |
15 |
|
Malignant tumours of respiratory and thorax organs |
26 |
7 |
|
Melanoma and other malignant tumours of skin |
3 |
4 |
|
Malignant tumours of mesothelium and soft tissue |
2 |
1 |
|
Malignant tumours of breast |
0 |
22 |
|
Malignant tumours of woman sex organs |
0 |
15 |
|
Malignant tumours of man sex organs |
12 |
0 |
|
Malignant tumour of urinary system |
5 |
1 |
|
Non-specific malignant tumours |
5 |
1 |
|
Malignant tumours of lymphatic and hemopoetic tissue |
1 |
1 |
|
Chronic excruciating pain |
0 |
1 |
|
Another chronic pain |
2 |
2 |
|
Total |
163 |
|
Table 1: Diagnoses of patients
3.3 Characterization of prescribed opioids analgesics
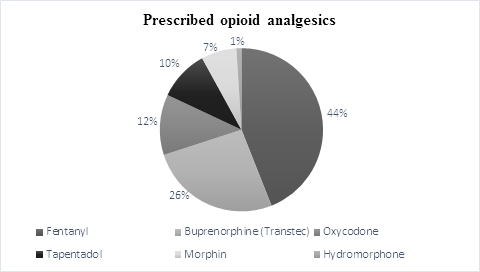
Strong opioids, total 543 in different package of analgesics, were prescribed by 332 prescriptions. The number of package of strong opioids was 543 (fentanyl (44%), buprenorphine (26%), oxycodone (12%), tapentanol (10%), morphine (7%) and hydromorphone (1%)) (see Graph 2). For all patients, the total number of strong opioids were 3.59 package during the study period.
3.4 Drug forms of prescribed medicament
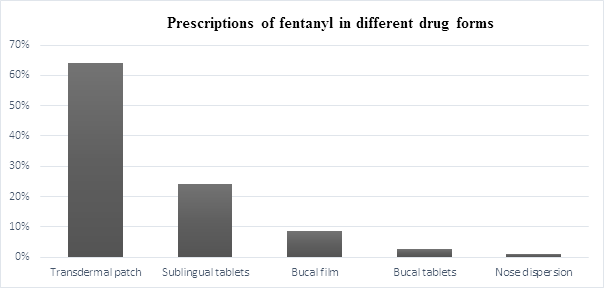
From mentioned drugs are available buprenorphine (48%) and fentanyl (52%) in transdermal patch. Fentanyl was prescribed in different drugs forms, the most often was used as transdermal patch (64%), then sublingual tablets (Lunaldin) (24%), buccal film (Breakyl) (8.5%), buccal tablets (Effentora) (2.5%) and nasal dispersion (Instanyl) (1%) (see Graph 3).
Within of the transdermal patch, fentanyl was prescribed in five different drug form. The most often Fentanyl transdermal patch was prescribed with force 25 µg/h and 100 µg/h. The least frequent was used Fentanyl transdermal patch with force 12 µg/h. The sublingual tablets of Fentanyl (Lunaldin) were prescribed in 5 different forces. The most often sublingual tablets with 100 μg Fentanyl were used. The least frequently was prescribed sublingual tablets with 300 and 800 μg of Fentanyl. Buccal film of Fentanyl constituted additional part of the pain pharmacotherapy. Buccal film of fentanyl (Breakyl) was prescribed in force 200 μg of Fentanyl (13 packages) and in force 400 μg of Fentanyl (7 packages). Besides buccal tablets of Fentanyl (Effentora) were prescribed in forces 100 μg Fentanyl (4 packages) and 200 μg of Fentanyl (2 packages) (Table 2).
|
Type of Fentanyl patch |
Number |
|
Fentanyl 12 µg/h EMP TDM 5x12 µg/h |
11 |
|
Fentanyl 25 µg/h EMP TDM 5x25 µg/h |
46 |
|
Fentanyl 50 µg/h EMP TDM 5x50 µg/h |
36 |
|
Fentanyl 75 µg/h EMP TDM 5x75 µg/h |
14 |
|
Fentanyl 100 µg/h EMP TDM 5x100 µg/h |
45 |
|
Total |
152 |
|
Sublingual tablets |
Number |
|
Lunaldin 100 µg TBL SLG 30 x 100 µg |
28 |
|
Lunaldin 200 µg TBL SLG 30 x 200 µg |
11 |
|
Lunaldin 300 µg TBL SLG 30 x 300 µg |
5 |
|
Lunaldin 400 µg TBL SLG 30 x 400 µg |
7 |
|
Lunaldin 800 µg TBL SLG 30 x 800 µg |
6 |
|
Total |
57 |
Table 2: Prescribed Fentanyl patches and Fentanyl sublingual tablets
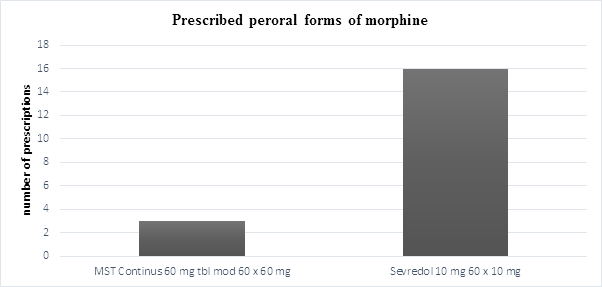
In comparison, morphine was prescribed in two drug forms as per oral (46%) and injection form (Morphin Biotika 1) (54%). Per oral morphine was prescribed in form of drug Sevredol 10 mg in 84% and preparation MST Continus 60 mg with continual liberation of morphine in 16% (Graph 4).
4. Discussion
The pain is the most often clinical symptoms, which motivates the patients to visit the doctor. The pain is always associated with oncologic disease and represents the commination and fear for patients [16]. Tumours of bones and pancreases are the most frequent oncologic diseases for the analgesics treatment [12]. Tumours of breast are mostly metastased to bones and have 60-80 % incidence of pain [17]. In our analyses there was not recorded diagnosis of malignant tumours of bones. Five men and five women suffered from tumour of pancreases, what is included under malignant tumours of gastrointestinal organs. The most men suffered from oncologic pain arised from malignant tumours of respiratory and thorax organs and the most women suffered from malignant tumours of breast.
Our analysis recorded only six opioid drugs, which were prescribed on 332 prescriptions for 151 patients in the whole amount of 543 packages in different forces and drug forms. Fentanyl, buprenorphine, oxycodone, tapentadol, morphine, hydromorphone were the most repeatedly prescribed mainly by oncologists and algesiologists.
Fentanyl (237 packages) and buprenorphine (139 packages) were the most regularly prescribed in the form of transdermal patches. On the other hand, fentanyl was prescribed behind transdermal patch in other various drug forms. Fentanyl was used in sublingual tablets (Lunaldin), buccal film (Breakyl), buccal tablets (Effentora) and nose dispersion (Instanyl) [18]. In our study the most prescribed was fentanyl in sublingual tablets about to 100 μg dose (28 packages) and the buccal tablets of fentanyl were used only in 6 packages in our analysis.
The benefit of buprenorphine is the independence on the excretion by kidney functions [16]. Additionally, partial agonist buprenorphine has an antidepressive potential, what may be a contribution in patients with depression. Buprenorphine exists in intranasal, sublingual, subcutaneous and intravenous form. In Slovakia, buprenorphine is only available in transdermal preparation Transtec.
Oxycodone has been the third most frequently prescribed opioid analgesics (66 package) in our study. This strong opioid analgesic is available for the therapy of the chronic non-oncologic pain, neuropathic pain, and sometimes for the therapy of an acute pain. Oxycodone is two-times stronger in equianalgesic doses than morphine. Beside drug forms with consistent liberation (Oxypro, Contiroxil), oxycodone was prescribed in combination with naloxone in the drug named Targin. This combined drug is available for patients, who suffer from the constipation. Per os administration of naloxon achieves to eliminate opioid induced constipation and retains the analgesic effect of oxycodone [16].
Tapentadol is fourth the most prescribed opioid analgesic in our analysis (53 packages). Tapentadol represent a new pharmacologic category of analgesics with dual mechanism of effect. Tapentadol in one molecule concurrently resolves somatic pains and attenuates psychic stress by mild antidepressive effect. Tapentadol is agonista of μ-opioid receptor and inhibitor of noradrenaline reuptake [19]. Our analysis presented that tapentadol with prescriptions of 53 packages exceeded prescriptions of 44 packages of morphine. Probably it was connected with less incidence of tapentadol adverse effects in the gastrointestinal tract and less risk of the development of the tolerance and physical dependence in comparison with morphine [20]. This analgesic effect was evidenced in the therapy of different types of pain: the acute nociceptive pain, the persistent fringe pain, the visceral and chronic neuropathic pain. Tapentadol is accessible in the retarded and fast-acting drug form, what allows for attenuation the basal and breakthrough pain [21].
Morphine represents the gold standard in the therapy of the oncologic pain. Its advance is accessibility in all pharmacologic drug forms and the low price [7]. Nevertheless, prescriptions of morphine declines and prescription of other opioids in the therapy of the oncologic pain heightens, what is indicated in our analysis. Morphine alone does not influence all components of pain, which is the most frequent causation of the failure in the analgesic therapy and has many adverse effects, mainly in gastrointestinal tract, developing tolerance and physical dependence. In our study, morphine was prescribed in two drug forms as per oral (46%) and injection form (Morphin Biotika 1) (54%). Injection form of morphine is advisable for the therapy of the strong acute pain. Per oral form of morphine was prescribed in the form of drug Sevredol 10 mg (84%) with immediate liberation, which is advisable for breakthrough pain. Per os forms with continual liberation of morphine (MST Continus 60 mg) were prescribed in 16 %. These drug forms are assigned for the therapy of chronic pain. The least prescribed opioid drug was hydromorphone (7 packages), which is accessible only in capsules with retarded liberation (Palladone) in Slovakia.
Our analysis also observed who picked up opioid analgesics on prescription. Patients themselves picked up 171 opioid analgesic on prescriptions, but in 161 cases, someone else picked up the strong opioids. We may assume, that advanced age (average was 64.6 year) and severe patients` diagnosis of did not allow to visit apothecary.
5. Conclusion
Our analysis demonstrated, that despite the fact that morphine is still considered as a gold standard in the oncologic pain therapy, other opioids are more frequently prescribed as fentanyl, buprenorphine, oxycodone or new molecule tapentadol. Probably it is connected with the fact that morphine does not achieve desired therapeutic profit in therapy of 10 – 30% patients with the oncologic pain because of adverse effects and insufficient analgesia.
Preferred are mainly drug forms as transdermal patches of fentanyl and buprenorphine with long-time control of the oncologic pain. Furthermore other drug forms of fentanyl are often prescribed, mainly sublingual tablets, buccal film and buccal tablets. These drug forms of fentanyl are more effective in the therapy of breakthrough pain in comparison with morphine. Additionally, new molecule Tapentadol is a great contribution in the therapy of the pain. This analgesic molecule is accessed in retarded and fast-acting drug form for attenuation basal and breakthrough pain with less incidence of adverse effects.
Sources of Funding
This publication is the result of the project implementation Biomakro ITMS 26240120027, APVV-14-0416
Acknowledgement
Special thanks to all authors.
References
- Kulichová M. Bolesť u onkologického pacienta - diferenciálno-diagnostické spracovanie, 2007. Onkológia (Bratisl.) roč. 2 (5): 287–291.
- Organization WH. Cancer pain relief : with a guide to opioid availability. Traitement de la douleur cancéreuse : complétée par une analyse des problèmes liés à la mise à disposition des opioïdes [Internet]. 1996 Available from: http://apps.who.int/iris/handle/10665/37896
- Bruera E, Neumann CM. Cancer pain. In: Max M, editor. Pain 1999: an updated review. Seattle: IASP Press 1999: 25–35.
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64 (2014): 252–271.
- Candido K, Kusper Teresa, Knezevic N. New Cancer Pain Treatment Options, Curr Pain Headache Rep 21 (2017): 12
- Nádorová bolest a možnosti její léčby | Urologické listy - proLékaře.cz
- Ripamonti C I, et al. Management of cancer pain: ESMO clinical practice guidelines. Annals of oncology 7 (2012): vii139-vii154.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 315 (2016): 1624–1645.
- Interventional Analgesic Management of Lung Cancer Pain [Internet]. [cited 2018 Jul 1]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5306685/
- Gul A, Ustundag H, Andsoy II, Kalkanli S. Anxiety and pain in surgically treated breast cancer patients. Asian Pac J Cancer Prev APJCP 16 (2015) :4261–4264.
- Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. J Neurosci 35 (2015): 13879–13888.
- Hakl M and Hříb R. Farmakoterapie léčby onkologické bolesti. In Interní medicína pro praxi 9 (2007): 299-300.
- Casy Alan F, and Robert T. Parfitt. Opioid analgesics: chemistry and receptors. Springer Science & Business Media, 2013.
- Gendron L, Cahill CM, von Zastrow M, Schiller PW, Pineyro G. Molecular Pharmacology of δ-Opioid Receptors. Pharmacol Rev 68 (2016): 631–700.
- Kučera P. Bolesť a neuropatia. In Via Practica 3 (2006): 562-566.
- Hakl M. Zásady podávání analgetik. Prakt Lékárenství 9 (2013): 173–176.
- Le Rhun E, Taillibert S, Chamberlain MC. Neoplastic Meningitis Due to Lung, Breast, and Melanoma Metastasis. Cancer Control J Moffitt Cancer Cent 24 (2017): 22–32.
- Fricová J. Léčba průlomovej bolesti u onkologických pacientu. Bolest 16 (2013): 111-114.
- Tzschentke TM, Christoph T, Kögel BY. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs 28 (2013): 319–29.
- Hoy SM. Tapentadol extended release: in adults with chronic pain. Drugs 72 (2012): 375–393.
- Imanaka K, Tominaga Y, Etropolski M, Ohashi H, Hirose K, Matsumura T. Ready conversion of patients with well-controlled, moderate to severe, chronic malignant tumor-related pain on other opioids to tapentadol extended release. Clin Drug Investig 34 (2014): 501–511.


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 