Entrapment of Ibuprofen by Multilamellar Liposomes for Aerosol Drug Delivery Towards Sustained Release Upon Pulmonary Administration
Ange Imanishimwe, Adam Taylor, Manal Almalki, and Edward P.C. Lai*
Ottawa-Carleton Chemistry Institute, Department of Chemistry, Carleton University, 1125 Colonel By Drive, Ottawa, ON K1S 5B6, Canada
*Corresponding author: Edward P.C. Lai, Ottawa-Carleton Chemistry Institute, Department of Chemistry, Carleton University, 1125 Colonel By Drive, Ottawa, ON K1S 5B6, Canada.
Received: 15 February 2024; Accepted: 04 March 2024; Published: 13 March 2024
Article Information
Citation: Ange Imanishimwe, Adam Taylor, Manal Almalki, and Edward P.C. Lai. Entrapment of Ibuprofen by Multilamellar Liposomes for Aerosol Drug Delivery Towards Sustained Release Upon Pulmonary Administration. Journal of Pharmacy and Pharmacology Research. 8 (2024): 1-11.
View / Download Pdf Share at FacebookAbstract
Purpose: Ibuprofen, a drug commonly used for treatment of inflammation and pain, has a short half-life which requires that the patient takes multiple doses a day to see a lasting effect in the cardiovascular system. Multilamellar liposomes (MLLs) have previously been shown to be effective nanocarriers in prolonging the administration cycle of similar drugs. The aim of this research is to entrap ibuprofen in MLLs and to evaluate these ibuprofen-loaded MLLs for sustained drug release during pulmonary delivery.
Methods: MLLs were prepared by the lipid hydration method wherein a thin film of ibuprofen and lecithin was heated past the critical temperature for efficient hydration. Stability test of the MLLs entrapping ibuprofen was confirmed by an incubation test at room temperature over 24 hours. To simulate physiological conditions expected in the lung, a surfactant (tween 20) was added to test the stability of the ibuprofen-entrapped MLLs. Then, any release of ibuprofen from the MLLs was determined by capillary electrophoretic analysis.
Results: Ibuprofen was successfully loaded into the MLLs with a diameter of 892-1713 nm at a high efficiency of 92%. There was no release of ibuprofen from the MLLs during the stability test under incubation at room temperature over 24 hours. However, sustained release of ibuprofen from the loaded MLLs at a rate of 0.09 mg/mL/hour was observed under physiological conditions expected in the lungs.
Conclusions: Our results suggest that MLLs could protect ibuprofen during administration in a pulmonary delivery system. Drying in a phosphate-buffered saline preserved the integrity of MLLs for long-haul transportation as well as long-term storage.
Keywords
<p>ibuprofen, entrapment, multilamellar liposomes, pulmonary delivery, sustained release, capillary electrophoresis</p>
Article Details
Introduction
Discovered in 1950, ibuprofen has proven to be one of the most powerful anti-inflammatory drugs for various types of fever, inflammation, and pain [1]. However, this drug has some disadvantages such as poor water solubility. Its short half-life of 2-4 hours, due to oxidative metabolism in the human body [2, 3] requires patients to take a high dosage of 1600 mg once (or two 800-mg tablets) daily to see a lasting therapeutic effect [4]. Accidental overdoses can damage the kidneys and may develop gastrointestinal ulcers in vulnerable patients. Among solutions to overcome these drawbacks, drug delivery system is proposed to be a viable candidate because of the awareness that nanocarriers can efficiently control the pharmacokinetic characteristics of drugs [5, 6] The system involves a formulation or device that enables therapeutic drugs to be delivered into the body and enhances its effectiveness and safety by controlling the rate, time, and place of drug release [7] Based on biological and physicochemical properties, there are several mechanisms such as dissolution, diffusion, osmosis, partitioning, swelling, erosion, and targeting available for consideration to modify and control drug delivery. These mechanisms involve capsules, liposomes, floating, micro-/nanoparticles, micro-/nanoemulsions, niosomes, transdermal, and implants [8] Their use depends on the particular application and may act individually or simultaneously at different stages of the drug release process. Nonionic micro-emulsions can be ideal nanocarriers and promising formulations for the transdermal administration of ibuprofen [9]. A redox-sensitive alginate–disulfide– ibuprofen derivative based anti-tumor target drug delivery system has been developed by attaching hydrophobic ibuprofen onto the hydrophilic alginate molecular skeleton through disulfide linkages [10]. Lidocaine-based ionic liquids have been explored for their utilization in topical and transdermal delivery of ibuprofen due to their high drug dissolution and expeditious drug delivery potential [11]. Metal–organic frameworks have been investigated as nanocarriers for delivery of ibuprofen owing to their remarkable impregnation of large quantities of drug with fast pharmacokinetics [12]. Density functional theory calculations have been performed to investigate the adsorption of ibuprofen by an iron-doped silicon carbide graphene monolayer as a drug delivery platform [13]. Microalgae possess an active surface, photosynthesis capabilities, and excellent biocompatibility to be highly promising as carriers for targeted drug delivery [14].
Considering the variety of nanocarriers, liposomes is a spherical-shaped vesicle that has one or more phospholipid bilayers [15, 16] and can be classified as either unilamellar liposomes (ULLs, one lipid bilayer) or multilamellar liposomes (MLLs, two or more lipid bilayers) [17]. Liposomes have been investigated to be effective and promising nanocarriers for drug delivery applications due to their capabilities of self- assembly, drug load controllability, variable release rates, rapid drug clearance prevention, flexible physiochemical properties for easy manipulation, high biocompatibility, modification of surface for target cells, and ability to entrap both hydrophobic and hydrophilic compounds [18, 19] Also, most liposome formulations are composed of glycerol and phospholipids with a lipid mass greater than 50%. Application of liposomes in medicine and drug delivery has been reported [20] in preparation and characterization of nano liposomes of Orthosiphon stamineus ethanolic extract in soybean phospholipids, [21] in liposomal liquid pharmaceutical composition comprising ibuprofen, [22] and in nanoliposome formulations developed for transdermal delivery of ibuprofen [23]. In addition, for liposomes to release the drug to the site of action, interactions between the liposomes and the cell surface must occur via adsorption, endocytosis/fusion, electrostatic attraction, hydrophobic interaction, and phospholipid exchange [24, 25] Interaction of liposomes with proteins from biological fluids and serum can form a corona layer that subsequently affects the uptake kinetics of liposomes by cells [26]. The USFDA approves non-targeted liposomes that largely reduce side effects and improve patient tolerability over conventional anti-cancer drugs, but improvement of therapeutic outcomes and patient survival rates cannot be expected.
Over the last decades, pulmonary administration has become a promising route for the systemic delivery of drugs due to the large surface area and abundant vasculature of the lungs [27] It prevents both the loss of drug in the gastrointestinal tract and metabolic destruction of the drug in the liver. Pulmonary administration is also a non-invasive drug delivery route that is convenient and safe if delivery devices are properly selected [28] Indeed, this route offers a way to administer ibuprofen, thereby providing a more rapid onset of action over the oral route. Forasmuch as the above reasons, the inhalation of ibuprofen via pulmonary drug delivery can reduce the adverse effects associated with oral administration by preventing overt gastrointestinal toxicity [29]. Hence, incorporation of liposome into the pulmonary drug delivery systems for achieving better therapeutic action at low dose seems most promising. Herein, the purpose of this study is to entrap ibuprofen in multilamellar liposomes (MLLs) and to evaluate the potential of these MLLs, prepared from commercial soybean lecithin, to retain ibuprofen for sustained release upon pulmonary administration. The MLLs were prepared by the lipid hydration method wherein a thin film of ibuprofen and lecithin was heated past the critical temperature for efficient hydration. They were evaluated for loading capacity, entrapment efficiency, stability under physiological condition using a surfactant Tween®20, transmission electron microscopy (TEM), and drug release test using capillary electrophoresis. The combination of pulmonary administration together with ibuprofen- loaded multilamellar liposomes (IMLLs) could ultimately help advance the field towards delivery of ibuprofen to its target destination with an extended therapeutic efficacy. Hydrocortisone, a hydrophobic pharmaceutical active, was recently encapsulated in MLLs composed of a mixture of phospholipids and Tween®80 to target skin penetration [30].
Materials and Method
Chemicals and reagents
Ibuprofen, chloroform, and sodium hydrogen phosphate (Na2HPO4) were purchased from Sigma- Aldrich (Oakville, Ontario, Canada). Soy lecithin in oil form was purchased from Vita Health Products (Winnipeg, Manitoba, Canada). Phosphate-buffered saline was obtained from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Tween 20 was purchased from BioShop (Burlington, Ontario, Canada). Distilled deionized water (DDW) was used throughout this work.
Preparation of IMLLs and MLLs
Ibuprofen-entrapped multilamellar liposomes (IMLLs) were prepared using a lipid hydration method adopted originally from Bangham et al. Briefly, soy lecithin (100 mg, molecular weight: 643.9 g/mol) was dissolved in chloroform (1 mL) together with ibuprofen (100 mg, molecular weight: 206.29 g/mol) to attain an experimental mole ratio of 3.1. The lipid-drug solution was deposited as a thin film on the bottom of a round flask and the solvent was removed under vacuum using a rotary evaporator (temperature: 40°C, rotation speed: 200 rpm, time: 60 min). The dry lipid-drug film was hydrated with a phosphate buffer saline (PBS, 4 mL) in water bath (above the transition temperature of 50°C) for 60 minutes to disperse the IMLLs for maximal entrapment efficiency. Finally, the resulting IMLLs suspension in PBS were homogenized for 5 minutes using an ultrasonic probe (300 W at 35%) to give a final ibuprofen concentration of 25 mg/mL. Similarly, multilamellar liposomes (MLLs) suspension in PBS was prepared using no ibuprofen in the chloroform.
Capillary electrophoresis analysis of ibuprofen
A lab-built CE-UV instrumentation system was used to analyze ibuprofen standard solutions. Ibuprofen (23.4 mg) were dissolved in methanol (1.5 mL) and PBS (13.5 mL) to obtain a stock concentration of 1.5 mg/ml. Then, the stock solution was diluted to 0.05, 0.1, 0.25, 0.50, 0.75, 1.0, 1.2 mg/mL concentrations. Prior to ibuprofen analysis, the capillary was conditioned with 10 M NaOH for 15 min, 1M NaOH for 15 min, and 10 mM BGE for 10 min, consecutively. The background electrolyte solution (BGE) was prepared with Na2HPO4 in DDW at pH 7.4 to be 10 mM. Capillary electrophoresis analysis of ibuprofen was next carried out at an applied voltage of 20 kV with UV detection at 190 nm. Standard solutions and experimental samples were introduced at the capillary inlet by electrokinetic injection at 17 kV for 12 s. Regularly, the capillary was flushed for 3 min with BGE between runs.
Characterization of IMLLs and MLLs by microscopy
The size distribution and morphology of MLLs and IMLLs were confirmed by TEM using FEI Tecnai G2 F20 microscope (Hillsboro, OR, USA) operating at 170 kV. A drop of sample solution, IMLLs or MLLs, was placed on the TEM carbon-copper grid for air drying over 24 hours before TEM images were taken. In addition, a stereo microscope was used to examine MLLs and IMLLs after a drop of MLLs or IMLLs suspension was placed on a glass slide for drying in air.
In vitro release test of ibuprofen from IMLLs
Centrifugal ultra-filtration was performed to test any release of ibuprofen from IMLLs in PBS suspension at 25oC for evaluating the stability of the liposomes when stored at room temperature. Specifically, 4 mL of IMLLs suspension was transferred into four microcentrifuge filter tubes (1 mL in each tube) and centrifuged at 5000 rpm (instead of 10000 rpm [31]) for 10 minutes using the Thermo Heraeus megafuge to remove any non-entrapped ibuprofen as the filtrate. Then, the four tubes were pooled together in one microcentrifuge tube for centrifugation to continue at 5000 rpm. After 5 minutes, 300 μL of supernatant was collected, followed by more collections every two hours until the end of 24 hours. Lastly, the supernatant samples were analyzed using the capillary CE-UV technique to determine if any changes in ibuprofen concentration occurred over time.
In vitro release test of ibuprofen from IMLLs with tween 20
To test the stability of IMLLs under conditions expected in the lungs, tween 20 was added to simulate the effect of pulmonary surfactant. The preparation of IMLLs was the same as described in section 2.2. Briefly, the standard liposome suspension was diluted into a buffer mimicking the lung surfactant and then the in vitro release procedure described in section 2.5 was conducted. In detail, after the ultrasonic homogenization process, tween 20 (0.44 mL) was added to the IMLLs in PBS suspension (4.00 mL) to attain a concentration of 120 mg/mL. This is the same concentration as that of pulmonary surfactant found in the lung [32].
Results and discussion
Capillary electrophoresis for ibuprofen determination
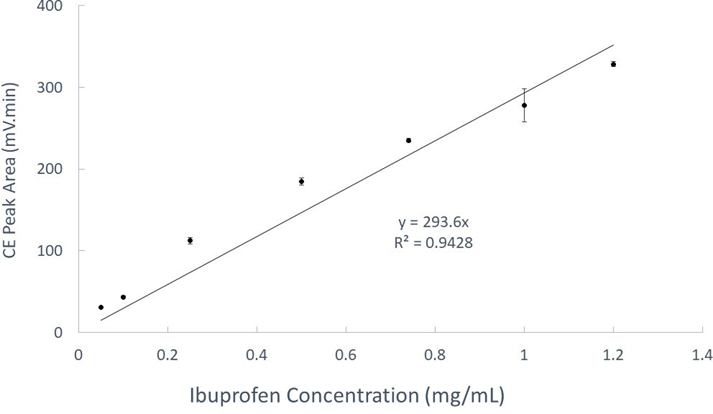
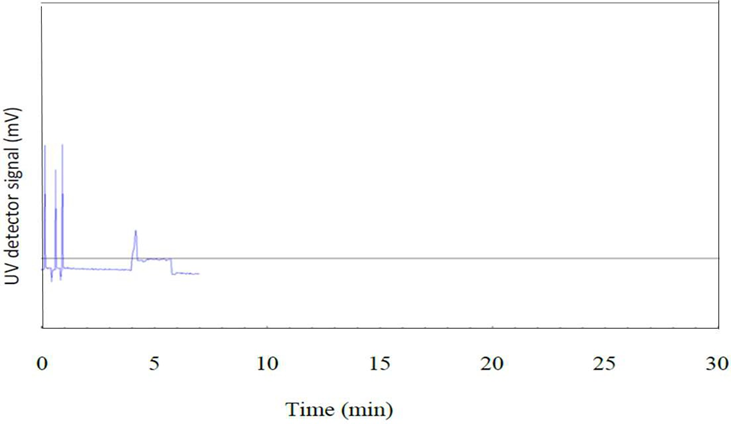
Previous researchers have used various instrumental methods such as high-performance liquid chromatography, size exclusion chromatography, and high-performance thin layer chromatography to analyze liposomes. However, capillary electrophoresis (CE) was recently demonstrated to be an efficient separation technique for rapid liposome analysis [33, 34] CE analysis requires less sample preparation and operates with more environmentally friendly aqueous background electrolyte solutions than the conventional methods. Electroosmosis is the fundamental process that drives the background electrolyte solution forward during CE analysis [35] Analytical separation by CE is based on differential migration of ions under the influence of a strong electrical field inside a fused silica capillary (50 µm diameter typically) [36]. It is also a promising technique for the separation of nanoparticles with ionic charge selectivity despite their size heterogeneity or chemical functionality. In the present work, a lab-constructed CE- UV system was used to analyze all the ibuprofen entrapped multilamellar liposomes (IMLLs) prepared from soy lecithin by the lipid hydration method. In analytical chemistry, a standard calibration curve validates the linear relationship between concentration (independent variable) and response (dependent variable) to predict the analyte concentration in an unknown sample [37]. Accordingly, standard ibuprofen solutions were first analyzed by CE with UV detection at a wavelength of 190 nm, as shown in Figure 1, to obtain peak areas for a calibration equation.
A standard calibration curve of increasing CE peak areas with higher concentrations of ibuprofen is illustrated in Figure 2. The trendline equation could be used to find the concentrations of free ibuprofen even in the presence of IMLLs. Since the square of the correlation coefficient (R2) is 0.9428, this means approximately 94% of the total variation in the collected data is explained by the least-squares regression line and approximately 6% is due to experimental errors. This value might not seem optimum compared to values typically obtained (R2 > 0.99) for HPLC methods. However, CE analysis operates with aqueous background electrolyte solutions that, unlike organic solvents, would not disrupt the structural integrity of IMLLs.
Microscopy of IMLLs and MLLs
Encapsulation of a drug has an impact on the size, elasticity, and zeta potential, the three key factors controlling MLLs. The prepared ibuprofen-entrapped multilamellar liposomes (IMLLs) were characterized by TEM to confirm their spherical morphology and measure their particle sizes for comparison with the original multilamellar liposomes (MLLs). According to Szoka et al., the normal size of unilamellar liposomes varies between 100 and 200 nm [38]. Our TEM micrographs revealed a size distribution from 892 to 1713 nm for IMLLs in Figure 3(a), and from 674 to 1106 nm for MLLs in Figure 3(b). Obviously, IMLLs show a slight increase of average size from the MLLs because IMLLs contain ibuprofen. Based on these size measurements, both MLLs and IMLLs were successfully prepared since they were significantly bigger than the normal size of unilamellar liposomes reported previously [39]. Chaves et al. had previously characterized multilamellar liposomes by small-angle X-ray scattering and small deformation rheological analyses to show spherical and near-spherical shapes with hydrodynamic diameters in the 680–1580 nm range. In addition, Moraes et al. obtained multilamellar liposomes with an average diameter of 1500 nm produced by hydration of proliposomes [40]. The size distribution of MLLs as nanomedical devices was also confirmed by Bozzuto et al. to be in the range of 1000-5000 nm. Furthermore, a minor observation was that the MLLs were not as round as the IMLLs likely because the former did not contain ibuprofen in the lipid bilayers to provide a rigid overall structure. Unfortunately, the TEM micrographs do not clearly show multilamellar layers because the instrument was not equipped for cooling the sample down to cryogenic temperatures.
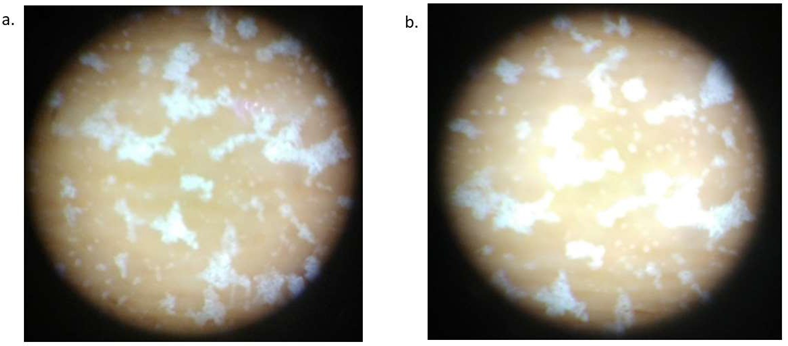
Additional evidence was gathered from a stereo microscope that showed a spherical morphology visibly for both IMLLs in Figure 4(a) and MLLs in Figure 4(b). The white stain was derived from the salts (KH2PO4 and NaCl in the phosphate-buffered saline) depositing on the clusters of circular liposomes. Apparently after drying in air, the inorganic salts cluttered as a residue around every liposome to pile up forming a 3-D structure like sand dunes. In comparison, an aliquot of PBS produced significantly smaller salt residues that were just barely visible as a uniform coating of tiny specks under the same magnification of stereo microscopy (micrograph not shown). Hence the aggregation/agglomeration of micron-sized MLLs or IMLLs is evidenced by the spatial organization of salt crystals across the microscope slide surface. It can be explained by water sipping up from the multilamellar structure of liposomes in a process similar to efflorescence [41]. These air-dried IMLLs could offer a cost-efficient way of long-term storage and long- haul delivery.
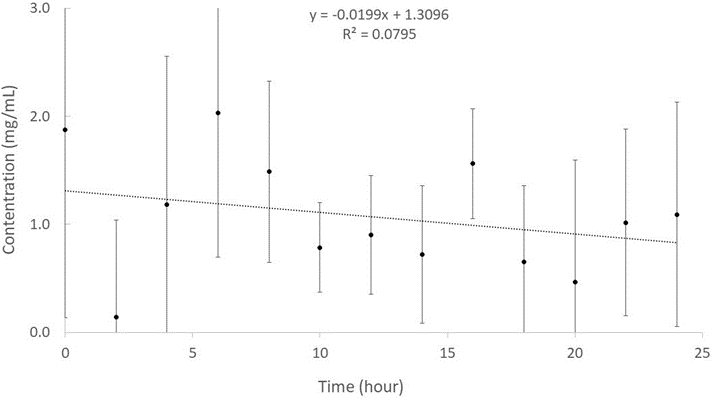
The delivery of liposome-encapsulated drugs depends on the structure and characteristics of the liposomes, the size and nature of the drug molecules, and their mutual interactions. In general terms, the release of drugs from multilamellar liposomes can be slower than that from unilamellar liposomes as it involves traversing only a single bilayer. An ibuprofen release test was performed to investigate the stability of the IMLLs during incubation at room temperature over a period of 24 hours. In order to obtain the time profile of ibuprofen release from the IMLLs, CE-UV was applied to measure the peak areas for ibuprofen in the PBS supernatants that were collected every 2 hours, as typified in Figure 5. Those peak areas were converted to concentrations of ibuprofen in the supernatant samples using the linear regression equation from the standard calibration curve presented above. Based on the release profile shown in Figure 6, there was no significant changes in ibuprofen concentration due to release of ibuprofen from the IMLLs for as long as 24 hours. The steady concentration of approximately 1.3 mg/mL ibuprofen represented just 8% of the initial concentration that was not entrapped by the liposomes during formation of IMLLs. This can be explained by several factors. The first one is based on the influence of lipid alkyl chain length wherein the longer the alkyl chain is, the longer the retention of entrapped hydrophobic drug in the MLLs becomes. Mohammed et al. have demonstrated how the ability of liposomes to retain poorly water-soluble drug correlates with the alkyl chain length of the lipid component, with longer retention in the order C24PC > DSPC > DMPC > PC [42]. The second could be the phase transition temperature (Tc) of the lipid excipients within the bilayer because as the alkyl hydrocarbon chain length increases, the van der Waals interactions between the lipid chains become stronger requiring more energy to disrupt the ordered packing, and therefore the phase transition temperature increases. Hence, the high Tc, the lesser the drug loss, and the longer the drug retention. Yadav et al. stated that lipids with a long acyl chain length are commonly used because a high phase transition temperature enables the drug to be retained due to strong van der Waals interactions. Anderson et al. also found that DSPC liposomes have the greatest encapsulation efficiency and drug retention over 48 hr at 37°C (85±10%) compared to DMPC liposomes which had (54±4%) at 37°C despite both systems being in the ordered gel phase [43]. The third could be the van der Waals interactions between the longer lipid chains where the increased lipid phase area within the liposomes enhancing the drug binding and bilayer stability [44]. These results are similar to previous reports that ibuprofen entrapped in liposomes containing soybean lecithin had shown good stability for the treatment of osteoarthritis [45]. In addition, Aisha et al. portrayed how liposomes prepared from soybean lecithin are highly stable at pH 5.5 and 7.4.
efflorescence [41]. These air-dried IMLLs could offer a cost-efficient way of long-term storage and long- haul delivery.Loading capacity and entrapment efficiency of IMLLs
Multiplying the volume of IMLLs suspension by the steady-state concentration obtained by CE-UV analysis above, the free mass of ibuprofen in the IMLLs suspension was calculated. The loading capacity and entrapment efficiency of IMLLs were next determined by converting the concentration into a free mass and using the this formula:
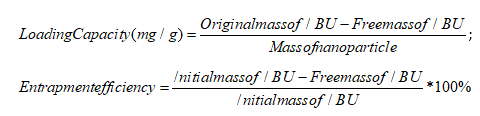
The IMLLs were found to have a loading capacity of 920 mg/g that represented a reasonable mole ratio of 3.1 between ibuprofen and lecithin, considering the molecular length of 1.03-1.30 nm for ibuprofen and thickness of 2.5-3.5 nm for the hydrophobic tails in a lipid bilayer [46]. Similarly, Eloy et al. reported that the encapsulation efficiency of hydrophobic drugs strongly depends on the length and packing density of the liposome acyl chains hosting them, whereas an increased loading capacity requires an increase in the bilayer lipophilic volume by choosing longer alkyl chain lipids [47]. The ibuprofen encapsulation or entrapment efficiency of 92% initially during preparation of IMLLs is similar to the 82% previously reported for paclitaxel in nanostructured lipid carriers [48] Chaves et al. had also reported a similar high entrapment efficiency of 93.3% for curcumin and 91.3% for cholecalciferol in multilamellar liposomes. These results suggest that a popular supplement dosage (1200 mg) of soy lecithin could entrap ibuprofen (1100 mg) just under the maximum daily dose (1200 mg) allowed for over-the-counter pain relievers [49, 50].
In vitro release studies of ibuprofen from IMLLs/tween 20
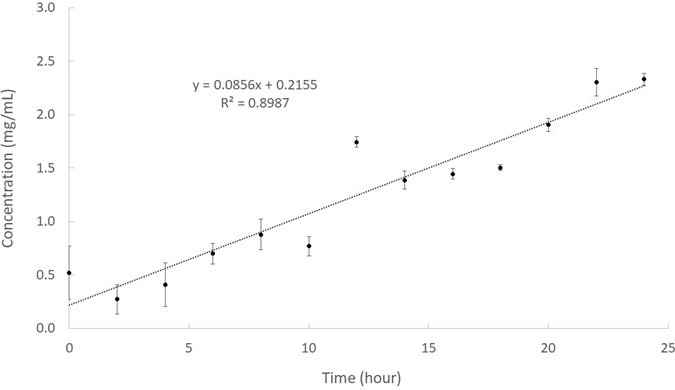
In another stability test of the IMLLs under conditions expected in the lungs, polyoxyethylene sorbitan monolaurate (Tween 20) was chosen from among other chemicals to simulate the effect of lung surfactant [51, 52]. Tween 20 is a polysorbate surfactant approved by the US FDA for use as an additive in pharmaceutical preparations, and polysorbate surfactants facilitate nanoparticulates to cross the blood- brain barrier [53]. The final concentration of Tween 20 was similar to the concentration of lung surfactant normally present. It was found that tween 20 allowed some ibuprofen leaking out from IMLLs to the PBS supernatant, as evidenced by the profile shown in Figure 7. The release rate was estimated to be 0.09±0.01 mg/mL/hour approximately, which is statistically significant in terms of 0.55% release per hour. Fortunately, this low release rate may be considered physiologically insufficient to cause non-cardiogenic pulmonary edema [54]. Preliminary biodistribution studies performed by Shah et al after the delivery of ibuprofen to mice by aerosol had demonstrated ibuprofen is rapidly transported from the lungs into the blood serum with minimum retention in lung tissue and bronchoalveolar lavage fluid [55].
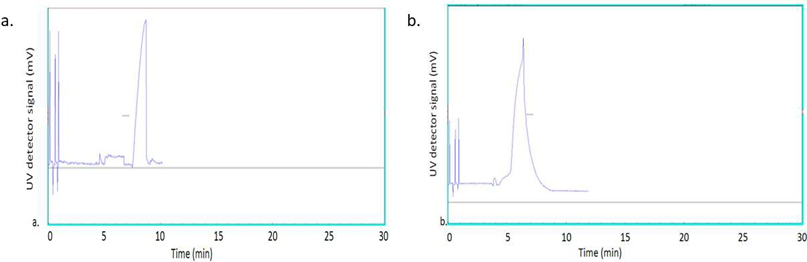
An investigation was last conducted to better understand the mechanism by which ibuprofen was released in the presence of tween 20 surfactant. Both IMLLs and IMLLs/tween20 were analyzed again by CE-UV. For IMLLs, a peak was observed at a longer migration time (8.7 min) as shown in Figure 8(a) which suggested that the liposomes were negatively charged. However, when IMLLs/tween 20 were analyzed, a peak was observed at a short migration time (6.4 min, closer to that for a neutral marker) in Figure 8(b). In addition to osmotic swelling of the IMLLs by the surfactant, [56] it can be hypothesized that the hydroxyl groups of tween 20 (a non-ionic surfactant) molecules covered the negatively charged phosphate head groups via hydrogen bonding. The surface lipid bilayer was probably disrupted by the fatty acid ester moiety of tween 20 to slowly release ibuprofen from the liposome [57]. This observation is similar to the previous report that in vitro polyphenols released from lipid vehicles demonstrated slower kinetics when compared to the free polyphenols in phosphate buffer solution at pH 7.4 [58]. From these results, it can be concluded that MLLs could protect ibuprofen during administration in a pulmonary delivery system. The drug release performance can be controlled by tuning the lamellarity and other physicochemical properties (membrane rigidity/fluidity, curvature, stress and permeability) of IMLLs [59]. In chemistry and chemical physics, a mesophase is a state of matter intermediate between liquid and solid. Biological structures such as the lipid bilayers of cell membranes are examples of mesophases.
Conclusion
In the present work, ibuprofen-entrapped multilamellar liposomes were successfully formed with a spherical shape that measured 892-1713 nm in diameter. By comparison, multilamellar lecithin liposomes without ibuprofen entrapment had a diameter in the range of 674-1106 nm. Sufficient entrapment of ibuprofen in MLL was attained, promising for new and novel pharmaceutical applications. The stability test of IMLLs showed there was no observable release of ibuprofen over 24 hours. However, repeating the stability test in the presence of tween 20 attained a small release of ibuprofen from the IMLLs at a rate of 0.09 mg/mL/hour. Pulmonary administration of IMLLs can deliver ibuprofen directly to epithelial cells in the lungs for local therapy, bypassing the gastrointestinal route altogether. In order for liquid formulations of IMLLs to be administered to the pulmonary region by intranasal drug delivery, [60, 61] they will have to be nebulized. We will test their stability during nebulization [62, 63] and verify that the nebulization process itself does not result in a burst of ibuprofen. It is expected that microencapsulation of ibuprofen by MLLs can offer bioavailability, control release, target delivery, mask bitter taste, provide stability, increase efficacy, and minimize side effects [64]. Our future work would focus on conducting more release experiments, in cell lines and animals, to investigate how the ibuprofen will be released from the multilamellar liposomes into the biological system. It would be interesting to study whether IMLLs interact with angiotensin conversion enzyme 2 receptors and look for evidence that ibuprofen can help alleviate some symptoms of chronic obstructive pulmonary disease.
Acknowledgment
Financial support from NSERC Canada (grant number RGPIN-2018-05320) is gratefully acknowledged.
Author Contributions:
Conceptualization: AI, AT, EPCL Writing Original Draft Preparation: AI Review & Editing: EPCL
Visualization: AI Supervision: EPCL
Co-supervision: AT, MA
Conflicts of Interest: The authors declare no conflict of interest.
References
- Ferrari WM, Nascimento AC, Moreira JV, Cremasco MA: Thermodynamic study of racemic ibuprofen separation by liquid chromatography using cellulose-based stationary phase. Chromatogr Res Int (2016).
- Priyanka BA, Hrushikesh JA. Phase solubility studies of ibuprofen with β-cyclodextrin and hydroxypropyl-β- cyclodextrin in different buffer media. Indo Am J Pharm Sci 4 (2017): 2386-2392.
- Evans AM. Comparative pharmacology of S (+)-ibuprofen and (RS)-ibuprofen. Clin Rheumatol 20 (2001): 9-14.
- Mazaleuskaya LL, Theken KN, Gong L, Thorn CF, FitzGerald GA, Altman RB, et al. PharmGKB summary: Ibuprofen pathways. Pharmacogenet Genom 25 (2015): 96.
- Cipolla D, Shekunov B, Blanchard J, Hickey T. Lipid-based carriers for pulmonary products: preclinical development and case studies in Hhumans. Adv Drug Delivery Rev 75 (2014): 53-80.
- Nguyen HD, Nguyen TD, Nguyen DH, Nguyen PT: Magnetic properties of Cr doped Fe3O4 porous nanoparticles prepared through a co-precipitation method using surfactant. Advances in Natural Sciences: Nanoscience and Nanotechnology 5 (2014): 035017.
- Bruschi ML: Strategies to modify the drug release from pharmaceutical systems. In: Bruschi ML, (eds). 4-Main mechanisms to control the drug release. W-Publishing, Cambridge (2015): 37-62.
- Bruschi ML: Strategies to modify the drug release from pharmaceutical systems, in: M.L. Bruschi (eds). 6-Drug delivery system. W-Publishing, Cambridge (2015): 87-194.
- Alkrad JA, Sayeh WN, Sijari A, Naser A, Neubert RHH, Dahmash EZ. In vivo and in vitro transdermal availability of ibuprofen using novel solubility enhancing fluid nanosized carrier systems. International Journal of Pharmaceutics 650 (2024): 123684.
- Bu Y, Wang H, Wu T, Chen X, Zhao S, Yan H, Lin Q. Development and evaluation of redox-responsive alginate– SS–ibuprofen derivative based anti-tumor target drug delivery system for the controlled release of doxorubicin. Journal of Drug Delivery Science and Technology 91 (2024): 105208.
- Mir B, Bakhtawar, Naz S, Uroos M, Ali B, Sharif F. Pharmaceutical applications of lidocaine-based ionic liquids – a remarkable innovation in drug delivery. Journal of Molecular Liquids 397 (2024): 124052.
- Pederneira N, Aina PO, Rownaghi AA, Rezaei F. Performance of MIL-101(Cr) and MIL-101(Cr)-pore expanded as drug carriers for ibuprofen and 5-fluorouracil delivery. Applied Biomaterials (2024).
- Esfahani S, Akbari J, Soleimani-Amiri S. Adsorption of ibuprofen by an iron-doped silicon carbide graphene monolayer: DFT exploration of drug delivery insights. International Journal of Nano Dimension 15 (2024): 63.
- Huang H, Lang Y, Wang S, Zhou M. Microalgae-based drug delivery systems in biomedical applications. Engineered Regeneration (2024).
- Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev 65 (2013): 36–48.
- Nkanga CL, Murhimalika AB, Okafor NI, Krause RW: General perception of liposomes: Formation, manufacturing, and applications. In Liposomes-Adv Perspect IntechOpen (2019).
- Bozzuto G, Molinari A: Liposomes as nanomedical devices. Int J Nanomed 10 (2015): 975-999.
- Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci 97 (2008): 4696-4740.
- Hidalgo A, Cruz A, Pérez-Gil J: Barrier or carrier? Pulmonary surfactant and drug delivery. Eur J Pharm Biopharm 95 (2015): 117-127.
- Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A: Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol 44 (2016): 381-391.
- Aisha AF, Majid AM, Ismail Z: Preparation and characterization of nano liposomes of Orthosiphon stamineusethanolic extract in soybean phospholipids. BMC Biotechnology 14 (2014): 23.
- Pinilla de Blas A, Vicente AO, Comin Aguirre J, Vaya Ibarra E. Topical ibuprofen formulation.
- Gaur PK, Bajpai M, Mishra S, Verma A, Development of ibuprofen nanoliposome for transdermal delivery: Physical characterization, in vitro/in vivo studies, and anti-inflammatory activity. Artif Cells Nanomed Biotechnol 44 (2016): 370-375.
- Szoka F, Jacobson K, Derzko Z, Papahadjopoulos D: Fluorescence studies on the mechanism of liposome-cell interactions in vitro. Biochim Biophys Acta - Biomembr 600 (1980): 1-8.
- Liu X, Huang G: Formation strategies, mechanism of intracellular delivery and potential clinical applications of pH-sensitive liposomes. Asian J Pharm Sci 8 (2013): 319-328.
- Yang K, Mesquita B, Horvatovich P, Salvati A: Tuning liposome composition to modulate corona formation in human serum and cellular uptake. Acta Biomaterialia 106 (2020): 314-327.
- Patton JS, Fishburn CS, Weers JG: The lungs as a portal of entry for systemic drug delivery. Proceedings of the American Thoracic Society 1 (2004): 338-344.
- Cazzola M, Cavalli F, Usmani OS, Rogliani P. Advances in pulmonary drug delivery devices for the treatment of chronic obstructive pulmonary disease. Expert Opinion on Drug Delivery 17 (2020): 635-646.
- Irvine J, Afrose A, Islam N: Formulation and delivery strategies of ibuprofen: challenges and opportunities. Drug Devel Ind Pharm 44 (2018): 173-183.
- Bernasqué A, Cario M, Krisa S, Lecomte S, Faure C: Transport of hydrocortisone in targeted layers of the skin by multi-lamellar liposomes. Journal of Liposome Research 33 (2023): 314-327.
- Vinod Ghodake, Jyoti Vishwakarma SL, Vavilala V. Patravale. Cefoperazone sodium liposomal formulation to mitigate P. aeruginosa biofilm in Cystic fibrosis infection: a QbD approach. International Journal of Pharmaceutics 587 (2020): 119696.
- Szoka F, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng 9 (1980): 467-508.
- Scholfield CR: Composition of soybean lecithin. J Am Oil Chem Soc 55 (1981): 889-892.
- Bilek G, Kremser L, Blaas D, Kenndler E: Analysis of liposomes by capillary electrophoresis and their use as carrier in electrokinetic chromatography. J Chromatogr B 841 (2006): 38-51.
- Popovska O, Simonovska J, Kavrakovski Z: An overview: Methods for preparation and characterization of liposomes as drug delivery systems. Int J Pharm Phytopharma Res 3 (2013): 182-189.
- Hamdan II: Capillary electrophoresis in the analysis of pharmaceuticals in environmental water: A critical review. J Liquid Chromatogr Relat Technol 40 (2017): 111-125.
- Heller M, Vitali L, Siqueira MA, Sako AV, Piovezan M, Micke GA: Capillary electrophoresis with UV detection to determine cocaine on circulated banknotes. ISRN Analytical Chem (2013).
- Azadeh M, Gorovits B, Kamerud J, MacMannis S, Safavi A, Sailstad J, Sondag P: Calibration curves in quantitative ligand binding assays: Recommendations and best practices for preparation, design, and editing of calibration curves. The AAPS J 20 (2018): 2.
- Szoka F, Olson F, Heath T, Vail W, Mayhew E, Papahadjopoulos D: Preparation of unilamellar liposomes of intermediate size (0.1–0.2 μm) by a combination of reverse phase evaporation and extrusion through polycarbonate membranes. Biochim Biophys Acta - Biomembr 601 (1980): 559-571.
- Chaves MA, Filho PLO, Jange CG, Sinigaglia-Coimbra R, Oliveira CL, Pinho SC: Structural characterization of multilamellar liposomes coencapsulating curcumin and vitamin D3, Coll. Surfaces A: Physicochem. Eng Aspects 549 (2018): 112-121.
- Moraes M, Carvalho JM, Silva CR, Cho S, Sola MR, Pinho SC: Liposomes encapsulating beta-carotene produced by the proliposomes method: Characterization and shelf life of powders and phospholipid vesicles. Int J Food Sci & Technology 48 (2013): 274-282.
- Veran-Tissoires S, Marcoux M, Prat M: Discrete salt crystallization at the surface of a porous medium. Phys Rev Letters 108 (2012): 054502.
- Mohammed AR, Weston N, Coombes AG, Fitzgerald M, Perrie Y: Liposome formulation of poorly water-soluble drugs: optimization of drug loading and ESEM analysis of stability. Int J Pharm 285 (2004): 23-34.
- Anderson M, Omri A: The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Delivery 11 (2004): 33-9.
- Lombardo D, Calandra P, Barreca D, Magazù S, Kiselev MA: Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 6 (2016): 125.
- Liang H: Medicinal composition containing ibuprofen liposome, chondroitin sulfate and curcumin for treating osteoarthritis, and preparation method thereof. Application: CN patent (2016).
- Gonzalez G, Sagarzazu A, Zoltan T: Infuence of microstructure in drug release behavior of silica nanocapsules. J Drug Deliv (2013).
- Eloy JO, Claro de Souza M, Petrilli R, Barcellos JP, Lee RJ, Marchetti JM: Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf B 123 (2014): 345–363.
- Kaur P, Garg T, Rath G, Murthy RR, Goyal AK: Development, optimization, and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box- Behnken design. Drug Deliv 23 (2016): 1912-1925.
- Swanson Health: Lecithin supplement dosage recommendations.
- Kuffler E. Over-the-counter (OTC) ibuprofen: cardiovascular safety & consumer use.
- Marques MRC, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolut Technol 18 (2011): 14-28.
- Kobayashi T, Shido A, Nitta K, Inui S, Ganzuka M, Robertson B: The critical concentration of surfactant in fetal lung liquid at birth. Respir Physiol 80 (1990): 181-92.
- Ravichandran V, Kesavan V, Cojean S, Loiseau PM, Jayakrishnan A: Polysorbate surfactants as drug carriers: tween 20-amphotericin B conjugates as anti-fungal and anti-leishmanial agents. Curr Drug Deliv 15 (2018): 1028-1037.
- Schreiber J, Schreiber C, Noncardiogenic pulmonary edema due to ibuprofen. Respir 74 (2007): 697.
- Shah PN, Marshall-Batty KR, Smolen JA, Tagaev JA, Chen Q, Rodesney CA, et al. Antimicrobial activity of ibuprofen against cystic fibrosis associated gram-negative pathogens. Antimicrob Agents Chemother 62 (2018): e01574-17.
- Cipolla D, Wu H, Gonda I, Eastman S, Redelmeier T, Chan HK: Modifying the release properties of liposomes toward personalized medicine. J Pharm Sci 103 (2014): 1851-1862.
- Johnson M: Detergents: Triton X-100, tween-20, and more. Mater Methods 3 (2013): 163.
- Pavaloiu RD, Sha’at F, Bubueanu C, Deaconu M, Neagu G, Sha’at M, Anastasescu M, Mihailescu M, Matei C, Nechifor G, Berger D: Polyphenolic extract from Sambucus ebulus L. leaves free and loaded into lipid vesicles. Nanomaterials 10 (2020): 56.
- Matsuura-Sawada Y, Maeki M, Uno S, Wada K, and Tokeshi M. Controlling lamellarity and physicochemical properties of liposomes prepared using a microfluidic device. Biomater Sci 11 (2023): 2419-2426.
- Xu D, Song XJ, Chen X, Wang JW, Cui YL, Advances and future perspectives of intranasal drug delivery: a scientometric review. Journal of Controlled Release 367 (2024): 366-384.
- Cipolla D, Gonda I, Chan HK: Liposomal formulations for inhalation. Ther Delivery 4 (2013): 1047-1072.
- Cipolla D, Wu H, Gonda I, Chan HK. Aerosol performance and stability of liposomes containing ciprofloxacin nanocrystals. J Aer Med Pulm Drug Del 28 (2015): 411-422.
- Yan C, Kim SR. Microencapsulation for pharmaceutical applications: a review. Journal of Controlled Release (2024).


 Impact Factor: * 3.3
Impact Factor: * 3.3 Acceptance Rate: 74.39%
Acceptance Rate: 74.39%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 