A Pre-clinical Standard Operating Procedure for Evaluating Orthobiologics in an In Vivo Rat Spinal Fusion Model
Andrew L Alejo1,4, Scott McDermott2, Yusuf Khalil1,4, Hope C Ball4, Gabrielle T Robinson3,4, Ernesto Solorzano3,4, Amanda M Alejo1,4, Jacob Douglas1,4, Trinity K Samson1,3,4, Jesse W Young1,3,4, Fayez F Safadi1,3,4,5,6
1College of Medicine, Northeast Ohio Medical University (NEOMED), Rootstown, OH, USA
2Roper St. Francis Physician Partners Orthopaedics, Summerville, SC, USA
3College of Graduate Studies, NEOMED, Rootstown, OH, USA
4Musculoskeletal Research Group, NEOMED, Rootstown, OH, USA
5Rebecca D. Considine Research Institute, Akron Children’s Hospital, Akron, OH, USA
6GPN Therapeutics Inc., Redi Zone NEOMED, Rootstown, OH, USA
*Corresponding Author: Fayez F Safadi, College of Medicine, Northeast Ohio Medical University (NEOMED), Rootstown, OH, USA.
Received: 18 August 2022; Accepted: 29 August 2022; Published: 05 September 2022
Article Information
Citation:
Alejo AL, McDermott S, Khalil Y, Ball HC, Robinson GT, Solorzano E, Alejo AM, Douglas J, Samson TK, Young JW, Safadi FF. A Preclinical Standard Operating Procedure for Evaluating Orthobiologics in an In Vivo Rat Spinal Fusion Model. Journal of Orthopedics and Sports Medicine 4 (2022): 224-240.
View / Download Pdf Share at FacebookAbstract
The rat animal model is a cost effective and reliable model used in spinal pre-clinical research. Complications from various surgical procedures in humans often arise that were based on these pre-clinical animal models. Therefore safe and efficacious pre-clinical animal models are needed to establish continuity into clinical trials. A Standard Operating Procedure (SOP) is a validated method that allows researchers to safely and carefully replicate previously successful surgical techniques. Thus, the aim of this study is to describe in detail the procedures involved in a common rat bilateral posterolateral intertransverse spinal fusion SOP used to test the efficacy and safety different orthobiologics using a collagen-soaked sponge as an orthobiologic carrier. Only two orthobiologics are currently FDA approved for spinal fusion surgery which include recombinant bone morphogenetic protein 2 (rhBMP-2), and I-FACTOR. While there are many additional orthobiologics currently being tested, one way to show their safety profile and gain FDA approval, is to use well established preclinical animal models. A preoperative, intraoperative, and postoperative surgical setup including specific anesthesia and euthanasia protocols are outlined. Furthermore, we describe different postoperative methods used to validate the spinal fusion SOP, which include μCT analysis, histopathology, biomechanical testing, and blood analysis. This SOP can help increase validity, transparency, efficacy, and reproducibly in future rat spinal fusion surgery procedures.
Keywords
<p>Standard Operating Procedure (SOP); Protocols; Spinal fusion; Rat model; Posterolateral intertransverse fusion model; Orthobiologics; rhBMP-2</p>
Article Details
Abbreviations:
AMQP: The Animal Model Qualification Program; BMAC: Bone Marrow Aspirate Concentrate; CRO: Contract Research Organizations; ELISA: Enzyme-Linked Immunosorbent Assay; H and E: Hematoxylin and Eosin; IP: Intraperitoneal; OVX: Ovariectomy; PBS: Phosphate Buffered Saline; PRP: Platelet-Rich Plasma; MSCs: Mesenchymal Stem Cells; NBF: Neutral Buffered Formalin; NELL-1: Neural Epidermal Growth Factor-Like 1 Protein; rhBMP-2: Recombinant Human Bone Morphogenetic Protein 2; SOPs: Standard Operating Procedures; µCT: Micro-Computed Tomography
1. Introduction
Spinal fusion surgery is one of the most common surgical procedures performed to correct multiple pathologies of the spine, including degenerative disorders of the intervertebral discs, spinal weakness or instability, herniated disk stabilization, spinal stenosis, tumors, trauma, chronic back pain and deformities [1]. In the United States alone, approximately 500,000 spinal fusion procedures are performed annually costing approximately 32 billion dollars [2,3]. These costs include spinal fusion surgery, inpatient follow up in the intensive care unit to monitor for inflammation and swelling ($3000/day), and the potential need to harvest bone graft material from the iliac crest if necessary ($550/procedure) [4]. The population of Americans over the age of 65 is projected to double over the next 40 years [5]. With such a surge in the aging population, an increase in the number of spinal fusion procedures will likely occur. The rate of spinal fusion surgeries has already increased 32.1% from 2004 to 2015, rising from 60 to 80 surgeries per every 100,00 U.S. adults [6]. Although spinal fusion is considered a generally safe procedure, with any surgery potential complications can arise which include infection, poor wound healing, bleeding, blood clots, injury to nerves or blood vessels adjacent to the spine, pain at the bone graft site, lingering pain, instability, and pseudoarthrosis, which occurs in up to 15-25% of spinal fusion surgeries [7,8]. In these patients, multiple revision surgeries are often required, increasing financial burden, prolonging recovery time, and increasing the risk of additional serious complications such as occult infection, devascularization of adjacent tissue, epidural fibrosis and scarring, and chronic pain [9]. Since these complications are fairly common, advancement in research is needed to continue searching for better alternatives, such as the use of orthobiologics in these procedures.
Orthobiologics are biologically-derived materials intended to augment both bone and soft tissue healing [10]. They are used in various orthopaedic procedures as biological supplements to screws and cages that are used in spinal surgery. Orthobiologics can be used in minimally invasive spinal fusion surgeries which help decrease operative time and avoid complications of a larger, open spinal surgery [11]. A patient can undergo a minimally invasive spinal fusion to temporarily decrease symptoms prior to a more definitive spinal fusion surgery using screws, rods, and cages. Only two orthobiologics are currently FDA approved for spinal fusion surgery which include recombinant bone morphogenetic protein 2 (rhBMP-2), and I-FACTOR. Several other orthobiologics that are available to use include Platelet-Rich Plasma (PRP), Bone Marrow Aspirate Concentrate (BMAC), Mesenchymal Stem Cells (MSCs), autograft, Neural Epidermal Growth Factor-Like 1 protein (NELL-1), and bone void fillers (tricalcium phosphate, calcium phosphate, calcium sulfate) (Table 1) [10,12-15]. Currently, the most common orthobiologic agent used from the ones previously listed is rhBMP-2 [16]. It is used “off-label” for various orthopaedic procedures including fractures and long bone nonunion when used in combination with an autograft [17]. Side effects include male sterility, severe postoperative radiculitis, vertebral osteolysis and/or edema, ectopic bone formation, bone cyst formation, inflammatory complications, nerve root injury, and carcinogenesis, many of which have been shown to be dose dependent [18-20]. Autografts are usually harvested from the patient’s anterior or posterior iliac crest, which poses its own risks and complications that include early post-operative pain, fracture, chronic pain, scarring, bleeding, and infection [21]. However, it is considered the “gold standard” orthobiologic due to its optimal osteoconduction, osteoinduction, and osteogenesis [22]. Although this procedure is quite successful, pseudoarthrosis is reported in up to 27% of cases [23,24]. The prevalence and severity of these side effects highlight the need, for safer and more effective orthobiologics.
To further enhance clinical practice, the need for adequate and safe animal models is essential. There are multiple spinal fusion models reported in the literature that include rabbit, dog, pig, and sheep [25-28]. The rat model is a common in vivo animal model for spinal fusion due to its reliability and cost effectiveness prior to pursuing surgical experimentation in a larger, more costly model [29,30]. Additionally, it has been reported that the mechanical performance of rat and human discs are very similar, which may suggest that disc tissue material properties are largely conserved across animal species [31-33]. Therefore, the rat model is a great pre-clinical model to evaluate new spinal fusion methods and/or supplementary drug applications targeted for use in humans. Although there has been a plethora of spinal fusion in vivo rat models previously described in the literature, none have been described in detail that evaluate orthobiologic application using collagen sponges as a carrier.
Standard Operating Procedures (SOPs) are validated methods that ensure production of accurate and reliable results in pre-clinical trials [34]. SOPs help ensure safety, consistency, quality, and minimize complications in animal experimentation which help advance pre-clinical research. They can be used as models by other laboratories to harbor similar results within their own studies. Therefore, this paper is aimed at providing a descriptive preoperative, intraoperative, and postoperative surgical SOP for spinal surgery in a pre-clinical in vivo rat model.
|
Orthobiologic |
FDA approval |
Indication |
Animal model |
Clinical Trial |
References |
|
rhBMP-2 |
Yes |
Cervical and Lumbar spine and long bone non-union |
Yes |
Yes |
[35-38] |
|
I-FACTOR |
Yes |
Cervical spine |
Yes |
Yes |
[14, 39-42] |
|
PRP |
N/A |
Osteoarthritis, Ligament, tendon, muscle, joint and skin injury |
Yes |
Yes |
[43-47] |
|
MSCs |
N/A |
Regenerative medicine: neurological, cardiac, endocrine and bone/cartilage diseases |
Yes |
Yes |
[48-53] |
|
BMAC |
N/A |
Arthritis, tendinitis, lower back pain, and meniscus tears |
Yes |
Yes |
[54-58] |
|
Autograft |
N/A |
Spine |
Yes |
Yes |
[59-64] |
|
NELL-1 |
No |
Osteoporosis, spinal fusion and long bone non-union |
Yes |
Yes |
[65-69] |
|
Bone Void Fillers |
Yes |
Any bony void or gap of the skeletal system |
Yes |
Yes |
[70-76] |
Table 1: Current orthobiologics being tested in various systems. Various orthobiologics that are currently being tested via animal models and clinical trials. Most have an indication for spinal fusion surgery, with some having indications for multiple systems. Currently, there are only two FDA approved orthobiologics for spinal fusion surgery which include rhBMP-2 and I-FACTOR. Some orthobiologics are not applicable for FDA approval due to the material being used coming from the patients’ blood and is therefore not considered a drug.
2. Materials
2.1 Experimental design
Here, we describe in detail a SOP for rat spinal fusion surgery to test differences in orthobiologics. The collagen-soaked sponge acts as a carrier of the orthobiologic or rhBMP-2 (as an example presented throughout the paper) that allows it to slowly release into the surrounding tissues. A high dose of orthobiologics is necessary when using rhBMP-2, as there is a 30% loss the orthobiologic when it comes in contact with the collagen sponge [77]. Different orthobiologics can be evaluated and compared to FDA approved rhBMP-2 or an autograft, both of which are currently used in spinal fusion [78-80]. In our experiments, we describe the steps performed in our spinal fusion model, outlined in Figure 1. This protocol will be important to assist future researchers who plan to replicate our procedure using different proteins/therapies of interest. The surgical procedure was carried out under sterile conditions with all instruments autoclaved prior to surgery. The study was conducted according to the approval of Northeast Ohio Medical University’s Institutional Animal Care and Use Committee (protocol #19-09-236 and #19-16- 019). The study design follows the ARRIVE guidelines (https://arriveguidelines.org/, assessed 27 April 2020) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals [81].
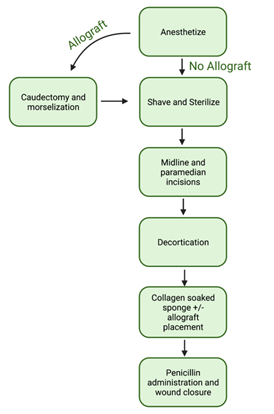
Figure 1: Overview of the spinal fusion surgery steps. Outlined are the critical and major steps of the standard operating procedure developed from start to finish. The flow chart also includes the additional step of the caudectomy and morselization if the allograft is going to be used as a comparison.
2.2 Materials
- Treatment/Control
- rhBMP-2 (0.75-1.50mg/cc, 20 L) (R & D Systems, MN)
- 10× Phosphate Buffered Solution (20 L) (Thermo Fischer Scientific, MA)
- Orthobiologic of interest (0.75-1.50mg/cc, 20 L)
- Drugs
- Ketamine hydrochloride (75mg/kg) (Covertus, OH)
- Xylazine (3mg/kg) (Covertus, OH)
- 1-4% isoflurane vaporizer (EZSystems, PA)
- Penicillin (20,000 U/kg) (Covertus, OH)
- Buprenorphine (slow release) (ZooPharm, WY)
- Surgical equipment
- 100% CO2 (AirGas, OH)
- 27 G Precisionglide needles (BD, NJ)
- Surgical lubricant/Ophthalmic ointment (Surgilube, PA)
- Cotton tipped applicator (Henry Schein, NY)
- Electric shaver (Wahl, IL)
- Betadine (povidone-iodine, 10%) (Betadine, CT)
- #10 Surgical blades (Henry Schein, NY)
- #3 Scalpel (Henry Schein, NY)
- Sterile collagen sponge (2 × 2 × 16 mm) (Medline, OH)
- 1mL sterile syringe with plunger (Worldwide Medical Products, PA)
- 4-0 Coated vicryl plus antibacterial absorbable sutures (Ethicon, NJ)
- Neutral Buffered Formalin (4%) (LabChem, PA)
- Ethanol (70%) (Pharmaco-Aaper, KY)
- Sponge gauze (Jorvet, CO)
- Webcol Alcohol prep, 2 ply, medium (COVIDEN, MA)
- Sterile compress (Fischer Scientific, NY)
- Non-sterile linen surgical drape (Aspen Veterinary Resources, MO)
- 1 mL cryovials (CELLTREAT Scientific Products, MA)
- 18 mL syringe (Worldwide Medical Products, PA)
- 8mL Heparin coated tubes (Medline, OH)
- Curved forceps (Fischer Scientific, NY)
- Needle holder (Alimed, MA)
- Surgical forceps (Fischer Scientific, NY)
- Retractors (Alimed, MA)
- Suture scissors (Fischer Scientific, NY)
- Curette (WPI, FL)
- Kerrison rongeur (Integra, OH)
- Dental bone mill (Salvin Dental Specialties, NC)
- High-speed oscillating burr/ideal micro drill (CellPoint Scientific, MD)
- Jewelers saw (Megacast, DE)
- Veterinary surgical lamp (Avante, KY)
- Cauterizer (Fischer Scientific, NY)
- Small animal heat lamp (Morganville Scientific, NJ)
- Centrifuge 5425R (Eppendorf, CT)
- Viva CT 80 (ScanCo Medical, PA)
- Microtome (Leica, IL)
- Scanner (Epson, CA)
- Electropuls E3000 linear-torsion all-electric dynamic test instrument (Instron, MA)
- Personal protective equipment
- Sterile surgical gloves (Cardinal Health, OH)
- Barrier medical face mask (Molnlycke, ME)
- Bouffant caps (Medline, OH)
- Isolation gown (Labsource Co, IL)
- Surgical shoe covers (Labsource Co, IL)
2.3 Equipment
- AAALAC accredited animal facility with rodent operation room (Northeast Ohio Medical University; Rootstown, OH)
- Anesthesia station (equipped with a gas scavenging system)
- Rodent surgical monitor with heating pad, thermometer, pulse oximeter, respiratory rate and heart rate (Vetcorder, WI)
- Clear euthanasia chamber connected to CO2 tank (Conduct Science, MA)
- Standard animal cages (water, food, and bedding) (Ancare, NY & TR Last, PA)
2.4 Animals
Sprague-Dawley rats were used in the experiments (Charles River Laboratories, NY). Experimental designs address the key scientific and commercial barriers with adequate numbers based on power calculations, include positive and negative controls for the recombinant protein, and include negative controls for the animal studies. In the first experiment, 40 sixteen-week old male Sprague-Dawley rats were used (10 for control, 10 for orthobiologic, 10 for orthobiologic plus autograft, and 10 for autograft alone). In the second experiment, 136 female Sprague-Dawley that were ovariectomized at twelve weeks-old were used (34 for control, 34 for orthobiologic, 34 for orthobiologic + autograft, and 34 for autograft alone; 34 was the number of animals needed to have significant power for the experiment that were split between µCT, histology, and biomechanical analysis). Bilateral posterolateral intertransverse fusion procedure occurred twelve weeks after ovariectomy (24 weeks old). A 24-week-old rat corresponds to an approximate 12.2- year-old human [82,83]. For this SOP, any rat model can be used. All rats were housed in the comparative medicine unit at Northeast Ohio Medical University (NEOMED; Rootstown, OH).
3. Methods
3.1 Ovariectomy (optional)
Charles River performed the Ovariectomy (OVX) procedure in accordance with Charles River SOP at 12-weeks of age [84]. Following surgery, animal well-being and survival were monitored for one to two weeks by the vendor according to Charles River SOP [85]. They were shipped to our facility for the spinal fusion procedure. Spinal fusion was performed 12 weeks post OVX. The OVX procedure is a well-established animal model used to induce loss of bone mass (osteoporosis) in female rodents and are a good model to evaluate therapeutics for spinal fusion procedures in osteoporotic patients [86].
3.2 Anesthesia
Anesthesia was prepared by collecting ketamine hydrochloride (75mg/kg), Xylazine (3mg/kg), and two 27G needles. The first 27G needle was used to withdraw the ketamine hydrochloride to perform an Intraperitoneal (IP) injection. For IP injections, the animal is held in a head-down position and a 27G needle is inserted into the lower left abdominal quadrant just off the midline. The second 27G needle was used to withdraw the Xylazine for the IP injection as described above. These two anesthetics plus maintenance with isoflurane (see below) will maintain animal anesthesia with appropriate monitoring [87]. If needed during the procedure, 1/3 dose of ketamine can be supplemented to prolong anesthesia. Xylazine can be reversed with 1-2 mg/kg Yohimbine IP [88] if needed.
Following anesthesia induction, the animals were observed for 5-10 minutes to ensure full surgical anesthesia identified by the lack of a withdrawal reflex following forelimb pinch with fingers or forceps. Isoflurane (1-4%) plus O2 was then used to maintain anesthesia during the procedure.
3.3 Surgical setup
Anesthetized animals were placed prone on a heating pad with their snout in the nose cone of the anesthetic system (Figure 2a) to decrease pain sensitivity and assist with maintenance of body temperature [89,90]. Sterile non-medicated ophthalmic ointment was applied to both eyes using cotton tipped applicator to prevent desiccation during the procedure. Oxygen saturation and heart rate were monitored with a pulse oximeter on the paw, using the Rodent Surgical Monitor (Figure 2b). Isoflurane/Oxygen flow and temperature (<40°C) of the heating pad were monitored and adjusted throughout the procedure as needed. The orthopedic/neurosurgeon will be on the caudal side of the rat with their assistant/second surgeon overlooking the procedure on the right. The surgical instruments are placed next to the heating pad on a sterile cover (Figure 2c). The assistant/second surgeon can prepare the next animal’s injection with Ketamine and Xylazine as the main surgeon is suturing the animal during the last step to minimize time spent waiting for full anesthesia. The main surgeon will disinfect the rat’s surgical site by using alcohol-based skin disinfectant. They will then disinfect their hands and put on sterile gloves to begin the procedure.
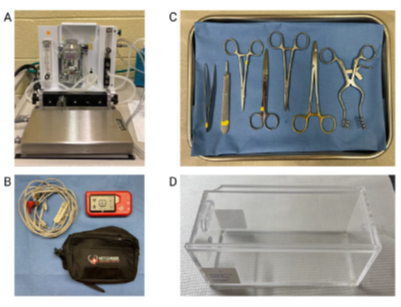
Figure 2: Surgical instruments setup. Image A depicts the 1-4% isoflurane vaporizer setup with the nose cone lying in front of the metal tray where the animal will be placed. Image B depicts the Vetcorder animal vitals monitoring device. Image C depicts the surgical tray including forceps, scalpel, needle driver, suture scissors, curved forceps, and a retractor. Image D depicts the clear euthanasia chamber which is connected to 100% CO2.
3.4 Preparing the surgical site
To prepare the site for surgery, hair is removed from the midline using an ethanol-sterilized electric shaver. The skin was then sterilized with betadine (povidone-iodine, 10%). To prepare animals for autografts, the tail was also sterilized with betadine (povidone-iodine, 10%) prior to surgery.
3.5 Surgical procedures
Rat autografts were obtained prior to beginning the spinal fusion procedure. Rat tails were amputated via caudectomy, using a cauterizer to control bleeding while the incision was closed using 4-0 absorbable suture. From the amputated tail, seven tail vertebrae were extracted, and the soft tissues were removed. Six vertebrae were taken and morselized using the rongeur and the one remaining vertebra was ground using the dental bone mill. The morselized and milled bone were mixed together and inserted into a 1 mL sterile syringe and compacted. Rat’s not undergoing autograft also had their tails amputated and disposed to maintain identical surgical procedures for all animals.
The spinal fusion procedure began with a midline incision down to the spine to expose the dorsal lumbar fascia. Next, two paramedian fascial incisions were made 4mm from the spinal processes on each side. This exposed the spinal vertebrae and the area where the collagen sponges will lie on top of the bed for the spinal fusion. Next, the transverse processes of L4 and L5 were located and bilaterally decorticated with the high-speed oscillating burr in addition to the facets and lamina which helped create the bed for the spinal fusion.
The rats then received sterile collagen sponges (2 ×2 × 16 mm) pre-soaked in either 20 µL of PBS (control), 20 µL of rhBMP-2, 20 µL of the desired orthobiologic, 20 µL of the desired orthobiologic + autograft, or 600 µL of the untreated autograft alone. This sponge or autograft was bilaterally placed in the newly formed bed (Figure 3 and 4a). To avoid bias, the surgeons are blinded in regard to treatment groups with the exception of the bone autografts. Following the placement of the sponges, 20,000 U/kg of penicillin was administered onto the wound. The wounds were closed with 4-0 absorbable suture and the surgical site was cleaned with ethanol wipes.
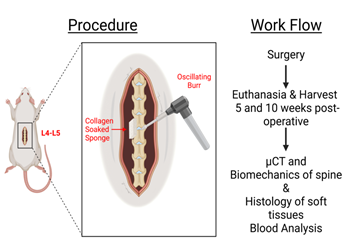
Figure 3: Overview of procedure and workflow. Presented is a visual representation of the decortication of the spine and placement of the collagen sponge located at spinal levels L4-L5 in the rat. Additionally, the workflow of the post-operative procedure with the evaluation of µCT imaging, biomechanics, histology and blood analysis is shown.
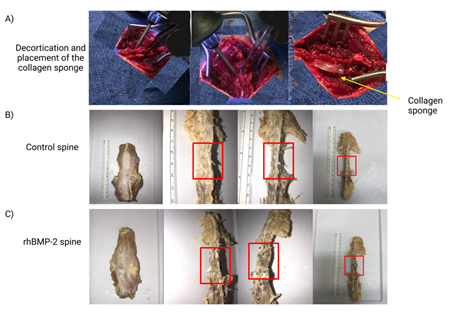
Figure 4: Gross images of the spinal fusion during surgery and after dissection. Images in A depict different views of the decortication process and placement of the collagen sponge at the spinal fusion site. Images B and C, control (PBS) and rhBMP-2 respectively, are the dissected spines after animal sacrifice. The red boxes are approximately the L4-L5 area where the collagen sponge was placed.
3.6 Post-op monitoring
To reduce pain and distress to the rats following surgery, Buprenorphine (slow release) was administered at a dose of 1mg/kg subcutaneously. Rats were allowed to recover in a clean cage under a heat lamp alone, to prevent suture/wound disruption by another animal. Fluid replacement is critical to the survival of the rats so ensure that clean water is being constantly supplied. A minimum of 48 hours of post-operative analgesia must be provided for this procedure. All animals were monitored twice daily (morning and afternoon) for 96 hours (4 days) following surgery to evaluate the animals for pain and distress such as difficulty walking, lethargy, rubbing of the surgical site and/or alterations in food or water intake. Lastly, the rats were weighed one week postop to confirm body weight is maintained. If any signs of distress are observed, consultation with the institution’s veterinarian as soon as possible is required.
3.7 Euthanasia
For our experiments, animals were sacrificed at 5 and 10 weeks postop to compare the progress of the spinal fusion. These time points were chosen as previously publications have noted spinal fusion occurring as early as week 4 [91-93]. Animals were individually placed into a clear and clean euthanasia chamber (Figure 2d). Sudden exposure of conscious animals to CO2 concentrations of 70% or greater has been shown to be distressful [94], yet death should be induced as quickly as possible. Without pre-charging the chamber, 100% CO2 (with a gas displacement rate of 10-30% of the chamber volume/min) was introduced slowly to minimize animal distress. Gas immersion and displacement were controlled with a pressure-reducing regulator and flow meter following IACUC guidelines. The euthanasia chamber was thoroughly cleaned between animals.
Following exposure to CO2, animals’ death was confirmed with cervical dislocation. Following euthanasia, tissues/organs, such as the heart, liver, lung, spleen, kidney and skeletal muscle next to the spinal fusion, were harvested and preserved via 24-hour exposure to 4% Neutral Buffered Formalin (NBF). Blood was collected using an 18G syringe rinsed with heparin (to prevent clotting) and spun down for 5 min at 3000 RPM. The serum was collected and aliquoted into 1 mL cryovials and stored at -80°C for further analysis. Finally, the spines were removed and fixed (Figure 4b-c) for future assessments of spinal fusion differences between treatments.
4. Results
To determine if the spinal fusion surgery was successful, µCT, histology, blood analysis, and biomechanical testing was done. Each of these methods has been shown to be accurate ways to exhibit results from each of the orthobiologics tested. µCT can be used as a visual construction to see new bone growth; histology can be used to visualize and calculate inflammation within tissues; blood analysis can be used to assess inflammatory cytokines; and biomechanical testing can be used to assess mechanical strength and bone quality. The following analyses are typical of assessing the effects of orthobiologics used in spinal fusion.
4.1 µCT analysis
Successful spinal fusion was achieved in both experiments with no cases of post-surgical infection. To evaluate the spinal fusion variations between treatments, µCT imaging was performed on week 5 and week 10 rat spines using a Viva µCT 80 (ScanCo Medical, PA) with a 20-micron resolution. µCT is a pronounced imaging modality that can be used to compare and contrast the protein/therapeutic of interest to the control due to its ability to conjure a 3D image of the spine [95]. The µCT scans were then analyzed using the BoneJ plug-in ImageJ to determine bone volume, bone volume/trabecular volume, trabecular thickness, trabecular spacing, bone mineral density, and connectivity [96]. Results showed rhBMP-2 treatment increased bone mass at the L4-L5 site compared to the control (Figure 5).
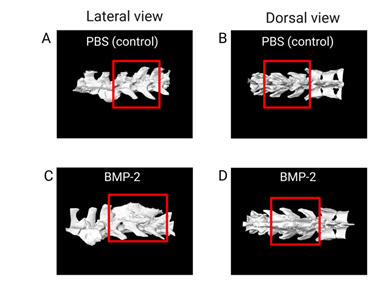
Figure 5: µCT analysis of the spinal fusion site. Images A-D depicts the µCT images of the control in PBS and the treatment rhBMP-2. The red box outlines the L4-L5 area in which the spinal fusion bed was created, and collagen-soaked sponge was placed. Images A and C are the lateral views of the spine, with images B and D being the dorsal views of the spine. As shown in C and D in comparison to the control, rhBMP-2 increases bone regeneration at the level of the spinal fusion.
4.2 Histology
Histopathology is an accurate and efficacious way to evaluate the gross inflammatory profile of the therapeutic agents in soft tissues. Systemic inflammation is an important aspect to review for the safety profile of any new therapeutic. Local inflammation is commonly assessed in muscles surrounding the spinal fusion [97]. Soft tissues were processed and embedded in paraffin. Then, 6-μm sections were cut using a microtome (Leica, IL) and sections were stained with hematoxylin and eosin (H and E) [98]. Photomicrographs were acquired using an Olympus BX61VS microscope. Analysis of photomicrographs was performed using NIS-Elements software (Nikon). Results of systemic inflammation assessment in our spinal fusion animals showed increased systemic inflammation in the rhBMP-2 treatment compared to PBS treated controls. Here we presented an example of two soft tissues (Figure 6).
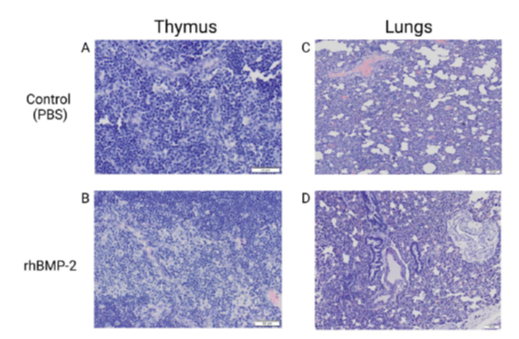
Figure 6: Thymus and lung samples of control and rhBMP-2 treatment. Images A-D depicts the histology staining and imaging post spinal fusion at 5 weeks. Images A and C show the control (PBS) while images B and D exhibit the rats treated with rhBMP-2. As presented in images B and D, rhBMP-2 induced a greater summation of inflammation (dark purple color) in samples as evidenced by increased quantity and morphed cell structure.
4.3 Blood analysis
Blood analysis can be implemented to assess how the orthobiologic affects various systemic protein secretions. Rat spinal fusion plasma was subjected to Enzyme-Linked Immunosorbent Assay (ELISA) assays to assess alterations in proinflammatory cytokines (IL-1 b , IL-6, MMP-1, MMP-3, TNF-α) [99], anti-inflammatory markers (IL-4, IL-10, IL-17, TGF-b ) [100], and markers of organ damage (FOXO1, SIRT-5, VEGF-C, SMAD-1) following the various treatments [101-104].
4.4 Biomechanical testing
With the production of new orthobiologics, it is important to assess how therapy affects mechanical strength and bone quality relative to existing approved therapeutic options. Common biomechanical assessment techniques include 3-point bending, tensile, and compression testing [105-107]. Prior to biomechanical assessment, soft tissues were removed from the spine (Figure 4b-c) and the area of spinal fusion (L4-L5) was isolated using a jeweler saw, resulting in a 10mm section (Figure 7b-e). Total area of each individual section was determined by scanning (Epson, CA). Then, we performed compression testing of the spine sections using an Electropuls E3000 linear-torsion all-electric dynamic test instrument (Instron, MA) (Figure 7a). Using the generated force displacement curves, we calculated yield strength (MPa) and determined compressive stiffness (Young’s modulus). Results suggested rhBMP-2 treatment not only increases ectopic bone formation but also generates a lower quality bone.
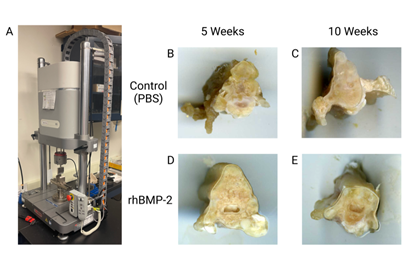
Figure 7: Biomechanical testing with 10mm sections of spinal fusion L4-L5. Image A is the Electropuls E3000 linear-torsion all-electric dynamic test instrument (Instron) used for biomechanical analysis of the spine sections. Image B and C are the controls (PBS) at 5 and 10 weeks respectively with image D and E being rhBMP-2 at 5 and 10 weeks, respectively.
5. Discussion
Orthobiologics are biologically-derived materials intended to augment both bone and soft tissue healing [10]. They can come from an individual’s own tissues (autograft), where less side effects are encountered, or from other sources (allograft) [108]. Autografts also have been shown to reduce the debilitating effects of osteoarthritis and accelerate healing of tendon or ligamentous injuries [109]. Orthobiologics can serve as biological supplements to traditional orthopaedic hardware such as rods, screws, and cages, and have recently played a role in advancing the field of regenerative medicine [110]. Most commonly, orthobiologics are used to treat bone defects, bone fractures, spinal fusion, cranial defects, tendon/ligamentous injuries, arthritis, inflammation, knee pain, muscles strains and tears, and overuse injuries [111-113].
It has been reported that anywhere from 9% - 45% of primary adult spinal fusion surgeries have been revised [114]. This number can vary widely based on the location of the spinal fusion surgery, patient demographics, other concurrent injuries or comorbidities, number of levels fused, and type of spinal fusion performed (screws/cages, orthobiologics, bone grafts) [115]. Pre-clinical animal models demonstrated the efficacy of spinal fusion procedures and were used establish the safest protocols prior to use in humans [116]. Furthermore, pre-clinical animal models were vital to determining the best approaches for spinal fusion as various locations along the spinal column as well as the most appropriate location-specific hardware [117].
The use of injectable orthobiologics is an alternative treatment option for individuals who want to prevent or delay surgery. Orthobiologics are also a suitable option when conventional procedures require revision and a lack of self-healing is present, often seen in aging, metabolic diseases, genetic diseases, and rare diseases. Here, we described a SOP that can be used to enable researchers to be able to study the effects of various orthobiologics in a pre-clinical spinal fusion rat model. Pre-clinical animal models are good conduits to clinical trials in humans and are necessary to advance an orthobiologics safety and efficacy profile that can be later used to get approval from the FDA for utilization in humans. Orthobiologics shown in Table 1 have all undergone pre-clinical trials using different animal models, especially rhBMP-2 and I-FACTOR, which have both been approved by the FDA [14,41].
I-FACTOR and rhBMP-2 are currently the only two orthobiologics approved for posterolateral spinal fusions. Although these proteins are effective orthobiologics, they still pose many undesirable clinical side effects. In pre-clinical animal models, major side effects of I- FACTOR include pain, neural impingement, physical impairment, loss of function, allergic reaction, abnormal bone formation, excessive or incomplete bone formation, and adjacent level degeneration [14]. Major side effects experienced from rhBMP-2 are inflammation induction that leads to abnormal bone formation [24], and increased osteoclast induced activity through the increase of RANK-Ligand production [118]. There have also been findings of cyst-like bone cavities filled with fatty marrow in lieu of the typical trabecular structure [24]. In humans, major side effects have ranged from ectopic bone [119,120], inflammatory complications, and osteolysis (through the over-activation of osteoclasts) [121,122]. Bladder retention and retrograde ejaculation are a few urogenital events that are also noted as side effects [123,124]. The other major side effects that occur are wound complications, including but not limited to hematoma, infection, and wound dehiscence [125-127].
Common orthobiologics used during spinal fusions (such as rhBMP-2) are known to cause inflammation following spinal fusions [128]. This side-effect is reflected through systemic inflammatory markers in the serum as well as tissue [129]. Serum and histological analyses are some of the most common methods to assess inflammation during spinal fusions [130,131]. Histological assays may also be used on the fusion masses to determine the quality and quantity of new bone formed by the orthobiologics at hand [132]. When subjected with immunohistochemical analyses, various markers of interests can be stained and quantified to determine their response to the orthobiologic [68]. In addition to quantification of pro- inflammatory markers, blood assays can also reveal fluctuations in bone related markers such as vitamin-D, calcium, osteocalcin, and N-terminal of telopeptide [133-135].
The use of a spinal fusion model to assess the effectiveness of orthobiologics has gained a great popularity in the recent years due to its clinical relevance and various methods to determine successful spinal fusion. While we have presented various parameters to be assessed during spinal fusions, this list is not exhaustive. Micro-computed tomography (µCT) is one of the best methods to describe the changes in bone as previously presented [136]. In addition, µCT may also be used to assess spinal segment stability during 3-point bending, predicting effectiveness of varying sets of rods and screws, and aggregation of bone cements [137-139].
While µCT provides invaluable data capable of describing the newly formed bone during spinal fusions, biomechanical testing is still necessary to prove fusion effectiveness under physiological conditions. Young’s Modulus is a reliable method for measuring tensile strength, which is one of the main forces driving spinal movement [140]. Spinal compressive stress, flexion, and lateral bending may also be measured through a variety of methods as described [141,142]. Each of these methods may further describe the effectiveness of an orthobiologic during spinal fusions and how it may translate its effectiveness to humans. In addition to biomechanical testing, other modalities can be used for supplementary analysis including DEXA scanning, X-ray imaging, RNA isolation and expression, and bio-material property testing. All these modalities are commonly used to assess different variables shown in each pre-clinical animal model before moving to clinical trials.
The FDA approves animal models based on criteria in the “The Animal Model Qualification Program (AMQP).” Once an animal model is qualified through this program, the FDA focuses on two main components. One is the reliability of the proposed animal model to produce a disease process or pathological condition that in multiple important aspects correlates to primary elements of the human disease or condition of interest. The second is the appropriate use and application of the qualified animal model in drug development and regulatory review. In addition, these details provide measures of quality control and quality assurance when the models are replicated [143].
The anatomy and overall methodology of the pre-clinical animal model should be akin to that seen clinically. Because of inherent advantages and disadvantages, specific models are chosen over others. Table 2 is a recapitulation of current animal models organized by approach. In addition, it is also worth considering an organism's size, cost, and handling. A broad spectrum of species, from rats to non-human primates has been used to study orthobiologics in spinal fusion. While smaller model organisms have their advantages, non-human primates are the most closely related to humans and are thus considered the most valid pre-clinical model [144]. The progression from smaller to larger animal models demonstrates an evolving cascade of evidence known putatively as the “Burden of Proof” [144]. The burden of proof is defined by a series of steps: proof of concept studies, feasibility studies, and efficacy studies [145]. The bone graft substitute must have osteoinductive properties that can be confirmed, i.e., induce the formation of de novo bone heterotopically in rat muscle pouches, to establish a proof of concept [145]. However, heterotopic bone induction in rat muscles pouches does not suggest that it will produce sufficient surgical immobilization of adjacent bones, i.e., vertebra [145]. Moreover, success in feasibility studies does not foreshadow success in efficacy studies. Orthobiologics may produce one effect in a lower organism but fail to produce any clinically significant effect in humans [145]. Taken together, these steps serve to underscore the importance of progressing to larger, more evolutionarily similar model organisms.
|
Model |
Considerations |
|
Mice (mouse model) |
- Cost effective and easily housed |
|
- Reproducible and quick to breed |
|
|
- Low complication rates |
|
|
Rat (rodent model) |
- Smallest–Sprague-Dawley being most common |
|
- Size limited to only dorsal, or dorsal lateral fusions |
|
|
- Cost effective and easily housed |
|
|
- Resistant to infection |
|
|
- Resilient to anesthesia |
|
|
Rabbit (lapine model) |
- Used in lumbar fusions |
|
- The New Zealand white rabbit is used in dorsal and dorsolateral lumbar spinal fusions |
|
|
- Ventral cervical, thoracic, and lumbar fusion studies have been reported infrequently |
|
|
Cat (feline model) |
- Repudiated model in society |
|
Dog (canine model) |
- Suitable for all approaches (i.e., Ventral & Dorsal) |
|
- Suitable for biomechanical analyses |
|
|
- Easy to handle |
|
|
- Repudiated model in society |
|
|
Goat (caprine model) |
- Suitable model for cervical fusions |
|
Sheep (ovine model) |
- Considerable background data |
|
- Due to comparable size to humans, surgical techniques are readily preformed |
|
|
- The lumbar spine is frequently studied, whereas the thoracic and cervical segments are less profound |
|
|
- Considerable housing is required |
|
|
- Biomechanical testing is common and baseline data is well established |
|
|
Swine (porcine model) |
- Minimally invasive techniques are supported using live pigs; however, most other techniques are reported in cadaveric models |
|
- Similar to human spine lumbar segment is most studied |
|
|
- Challenging to handle due to size and veterinary issues |
|
|
Cattle (bovine model) |
- Cadaveric specimens of the skeletally immature cow spine are used |
|
- Instrumentation can easily be placed |
|
|
- High availability and relatively low cost |
|
|
Primate (primate model) |
- Ideally the last step in establishing burden of proof |
|
- Offers greatest genetic homology (i.e. approximating human upright posture) |
|
|
- Most difficult model when considering financial, housing, and ethical implications |
Table 2: Current animal models used in spinal fusion surgery [144]. Various animal models that are being studied to be used during spinal fusion surgery. Most importantly, each model has considerations whether it is or is not a good fit for the proposed experiment.
Animal models of osteoporosis are suitable tools for studying new prevention and treatment modalities. The first choice, and the most employed for such studies, is the ovariectomized rat model [146]. To better understand the utility of the OVX model, it is crucial to define menopause. Menopause is when the menstrual cycle ceases due to a reduced production of the ovarian hormones estrogen and progesterone [147]. Removal of the ovaries mimics the menopausal phase, which is an essential approach to better comprehend the biological process involved in menarche [148]. The ultimate action of estrogen on the skeleton is to decrease bone resorption and remodeling, while maintaining bone formation [149] (Figure 8). Therefore a deficiency in estrogen results in accelerated trabecular and cortical bone loss over life, resembling patterns of osteoporosis in humans [150].
Posterolateral spinal fusions have used specific animal models from the aforementioned species, which all range in varying advantages. The four major types of spinal fusions in humans are posterolateral gutter fusions, posterior lumbar interbody fusions, anterior lumbar interbody fusions and transforaminal lumbar interbody fusions [151]. The variation in these different techniques aid in the accessibility of the effected vertebrae. Posterolateral gutter fusions are performed through the back and include a bone graft isolated from the iliac crest, being placed at the posterolateral region of the spine [152]. Whereas posterior lumbar interbody fusions are also performed through the back, while including the removal of a disc between two vertebrae [153]. Bone graft is then inserted into the space created between the two vertebral bodies, all of which can be obtained by the use of a single incision. An advantage of this method is the allowance of access to nerve roots, which is beneficial in neural decompression [153,154]. Anterior lumbar interbody fusions are similar to posterior lumbar interbody fusions, being that a disc is removed from between two vertebrae and the bone graft is then inserted into the space created between the two vertebral bodies. The primary difference is this procedure is performed from the front of the patient [155,156]. In doing so posterior and iliopsoas muscles are avoided and promote better stability for patients in their recovery process [156]. A Transforaminal lumbar interbody fusion allows for access to the foraminal spaces via a unilateral manner. This is achieved through using the posterior spine which avoids ligaments and muscle tissues, which allows for an effective healing process and increased biomechanical stability [157,158].
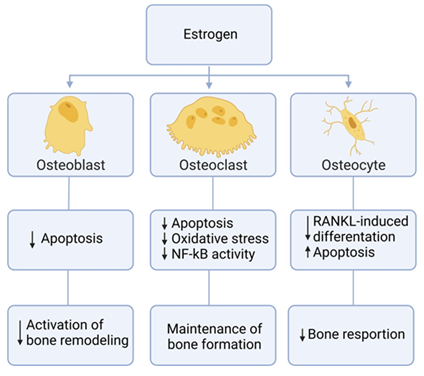
Figure 8: Effects of estrogen on bone. Summarized chart of how estrogen affects different components of bone cells.
The murine models are easily genetically manipulated [159]; athymic rats display a decrease in inflammatory responses to xenogeneic proteins [160]. Additionally, mice models are very easy to inbreed [161]. Rabbit models are the most favorable animal models used for posterolateral spinal fusions [162], whereas canine models are deemed as easy working models but they are less societally accepted [163]. Both swine and primate models are similar to human beings in that swine share a similar spine [164], and primates share an anatomical homology with humans [163]. Murine models are easily inbred, but these models possess a decrease in genetic variability when compared to humans and are not ideal for inflammatory studies [165]. Sheep are beneficial animal models that share similar anatomical and physiological structure of humans, which makes them a better animal model for assessing disease states [166, 167]. Primates are the best animal models as they share genetic, biochemical, and psychological characteristics similar to that of humans [168].
In conclusion, the use of orthobiologics has been gaining traction within the United States and can often be a preferred method of treatment prior to or during surgical correction. Without the proper studies enacted by researchers that are experts in the field in animal surgical models, orthobiologics would have not come to fruition in today’s day and age. Often time Contract Research Organizations (CRO) have be used to help expedite the process of collecting valuable data on orthobiologic testing, including in vivo and in vitro experiments. These previous experiments have provided evidence to determine the doses of orthobiologics that are safe [169]. Although these CRO’s may come at a higher cost of operation, the data they produce is necessary to gain FDA approval and validate prior laboratory data from academic research. Possible ways to establish a consistent process in orthobiologic testing can be establishing a center for training or using online or in-person training that ensures that the researchers are up to date on the most current methods and procedures for pre-clinical animal models. Therefore, the SOP we describe is one of many ways’ researchers are able to test orthobiologics in a pre-clinical rat spinal fusion model. We thank all the athletes who have agreed to participate in this study.
Acknowledgements
This study is supported by the NIH, National Institute of Aging, SBIR Phase-1 grant # R43AG063627.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Schnake KJ, Rappert D, Storzer B, et al. Lumbar fusion-Indications and techniques. Orthopade 48 (2019): 50-58.
- Beckerman D, Esparza M, Lee SI, et al. Cost Analysis of Single-Level Lumbar Fusions. Global Spine J 10 (2020): 39-46.
- Weiss AJ, Elixhauser A. Trends in Operating Room Procedures in U.S. Hospitals, 2001–2011: Statistical Brief #171, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US): Rockville (MD) (2006).
- Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med 33 (2005): 1266-12 71.
- White MS, Burns C, Conlon HA. The Impact of an Aging Population in the Workplace. Workplace Health Saf 66 (2018): 493-498.
- Martin BI, Mirza SK, Spina N, et al. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976) 44 (2019): 369- 376.
- Peters MP, Willems R, Weijers R, et al. Pseudarthrosis after lumbar spinal fusion: the role of 18F-fluoride PET/CT. Eur J Nucl Med Mol Imaging 42 (2015): 1891-1898.
- Leven D, Cho SK. Pseudarthrosis of the Cervical Spine: Risk Factors, Diagnosis and Management. Asian Spine J 10 (2016): 776-786.
- Eichholz KM, Ryken TC. Complications of revision spinal surgery. Neurosurg Focus 15 (2003): E1.
- Bravo DL, Jazrawi DA, Cardone M, et al. Orthobiologics A Comprehensive Review of the Current Evidence and Use in Orthopedic Subspecialties. Bull Hosp Jt Dis (2013) 76 (2018): 223-231.
- Greene AC, Hsu WK. Orthobiologics in minimally invasive lumbar fusion. J Spine Surg 5 (2019): S11-S18.
- Sezgin EA, Atik O. Are orthobiologics the next chapter in clinical orthopedics? A literature review. Eklem Hastalik Cerrahisi 29 (2018): 110-116.
- Obana KK, Schallmo MS, Hong IS, et al. Current Trends in Orthobiologics: An 11-Year Review of the Orthopaedic Literature. Am J Sports Med (2021): 3635465211037343.
- Arnold PM, Sasso RC, Janssen ME, et al. i-Factor™ Bone Graft vs. Autograft in Anterior Cervical Discectomy and Fusion: 2- Year Follow-up of the Randomized Single-Blinded Food and Drug Administration Investigational Device Exemption Study. Neurosurgery 83 (2018): 377-384.
- Yuan W, James AW, Asatrian G, et al. NELL-1 based demineralized bone graft promotes rat spine fusion as compared to commercially available BMP-2 product. J Orthop Sci 18 (2013): 646-657.
- Duarte RM, Varanda P, Reis RL, et al. Biomaterials and Bioactive Agents in Spinal Fusion. Tissue Eng Part B Rev 23 (2017): 540-551.
- Morimoto T, Kaito T, Matsuo Y, et al. The bone morphogenetic protein-2/7 heterodimer is a stronger inducer of bone regeneration than the individual homodimers in a rat spinal fusion model. Spine J 15 (2015): 1379-1390.
- James AW, LaChaud G, Shen J, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev 22 (2016): 284-297.
- Glassman SD, Howard J, Dimar J, et al. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine (Phila Pa 1976) 36 (2011): 1849-1854.
- Mesfin A, Buchowski JM, Zebala LP, et al. High-dose rhBMP-2 for adults: major and minor complications: a study of 502 spine cases. J Bone Joint Surg Am 95 (2013): 1546-1553.
- Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am 93 (2011): 2227-2236.
- Greer N, Yoon P, Majeski B, et al. Evidence-based Synthesis Program Reports, in Orthobiologics in Foot and Ankle Arthrodesis Sites: A Systematic Review. Department of Veterans Affairs (US): Washington (DC) (2020).
- Watkins R 4th, Watkins R 3rd, et al. Non-Union Rate with Stand-Alone Lateral Lumbar Interbody Fusion. Medicine 93 (2014).
- Zara JN, Siu RK, Zhang X, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17 (2011): 1389-1399.
- Conway JC, Oliver RA, Wang T, et al. The efficacy of a nanosynthetic bone graft substitute as a bone graft extender in rabbit posterolateral fusion. Spine J 21 (2021): 1925-1937.
- Sato K, Kumagai H, Funayama T, et al. Posterolateral lumbar spine fusion with unidirectional porous beta-tricalcium phosphate in a canine model. J Artif Organs 23 (2020): 365-370.
- Güngör A, Berikol G, Berke Göztepe M, et al. Thoracic vertebra interbody fusion surgery with robotic assisted system in a swine model. J Clin Neurosci 92 (2021): 85-88.
- Van Horn MR, Beard R, Wang W, et al. Comparison of 3D-printed titanium-alloy, standard titanium-alloy, and PEEK interbody spacers in an ovine model. Spine J 21 (2021): 2097-2103.
- Dietrich MR, Ankeny RA, Chen PM. Publication trends in model organism research. Genetics 198 (2014): 787-94.
- Meek S, Mashimo T, Burdon T, From engineering to editing the rat genome. Mammalian Genome 28 (2017): 302-314.
- Elliott DM, Sarver JJ. Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine (Phila Pa 1976) 29 (2004): 713-722.
- Showalter BL, Beckstein JC, Martin JT, et al. Comparison of animal discs used in disc research to human lumbar disc: torsion mechanics and collagen content. Spine (Phila Pa 1976) 37 (2012): E900-E907.
- Beckstein JC, Sen S, Schaer TP, et al. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine (Phila Pa 1976) 33 (2008): E166-E173.
- Freeman KP, Cook JR, Hooijberg EH. Standard operating procedures. Journal of the American Veterinary Medical Association 258 (2021): 477-481.
- Okada R, Kaito T, Ishiguro H, et al. Assessment of effects of rhBMP-2 on interbody fusion with a novel rat model. Spine J 20 (2020): 821-829.
- Golubovsky JL, Ejikeme T, Winkelman R, et al. Osteobiologics. Oper Neurosurg (Hagerstown) 21 (2021): S2-S9.
- Pan HC, Lee S, Ting K, et al. Cyst-Like Osteolytic Formations in Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) Augmented Sheep Spinal Fusion. Am J Pathol 187 (2017): 1485-1495.
- Liau ZQG, Lam RWM, Hu T, et al. Dose-dependent Nerve Inflammatory Response to rhBMP-2 in a Rodent Spinal Nerve Model. Spine (Phila Pa 1976) 42 (2017): E933-E938.
- Thaci B, Yee R, Kim K, et al. Cost-Effectiveness of Peptide Enhanced Bone Graft i-Factor versus Use of Local Autologous Bone in Anterior Cervical Discectomy and Fusion Surgery. Clinicoecon Outcomes Res 13 (2021): 681- 691.
- Arnold PM, Sasso RC, Janssen ME, et al. Efficacy of i-Factor Bone Graft versus Autograft in Anterior Cervical Discectomy and Fusion: Results of the Prospective, Randomized, Single-blinded Food and Drug Administration Investigational Device Exemption Study. Spine (Phila Pa 1976) 41 (2016): 1075-1083.
- Arnold PM, Vaccaro AR, Sasso RC, et al. Two-Year Clinical and Radiological Outcomes in Patients With Diabetes Undergoing Single-Level Anterior Cervical Discectomy and Fusion. Global Spine J 11 (2021): 458-464.
- Axelsen MG, Overgaard S, Jespersen SM, et al. Comparison of synthetic bone graft ABM/P-15 and allograft on uninstrumented posterior lumbar spine fusion in sheep. J Orthop Surg Res 14 (2019): 2.
- Lazarescu AE, Vaduva AO, Hogea GB, et al. Comparing PRP and bone marrow aspirate effects on cartilage defects associated with partial meniscectomy: a confocal microscopy study on animal model. Rom J Morphol Embryol 62 (2021): 263-268.
- Liu X, Wang L, Ma C, e t al. Exosomes derived from platelet- rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg Res 14 (2019): 470.
- Boru CE, Manolescu N, Ulmeanu DI, et al. Platelet-rich plasma PRP vs. absorbable mesh as cruroplasty reinforcement: a study on an animal model. Minim Invasive Ther Allied Technol 31 (2022): 252-261.
- Barman A, Prakash S, Sahoo J, et al. Single intra- articular injection with or without intra-osseous injections of platelet-rich plasma in the treatment of osteoarthritis knee: A single-blind, randomized clinical trial. Injury 53 (2022): 1247-1253.
- Randelli PS, Stoppani CA, Santarsiero G, et al. Platelet-Rich Plasma in Arthroscopic Rotator Cuff Repair: Clinical and Radiological Results of a Prospective Randomized Controlled Trial Study at 10-Year Follow-Up. Arthroscopy 38 (2022): 51-61.
- Yu L, Shi Q, Zhang B, et al. Genetically modified mesenchymal stem cells promote spinal fusion through polarized macrophages. Laboratory Investigation 102 (2022): 312-319.
- Stephan SR, Kanim LE, Bae HW. Stem Cells and Spinal Fusion. Int J Spine Surg 15 (2021): 94-103.
- Hu T, Liu L, Lam RMW, et al. Bone marrow mesenchymal stem cells with low dose bone morphogenetic protein 2 enhances scaffold-based spinal fusion in a porcine model. J Tissue Eng Regen Med 16 (2022): 63-75.
- Lina IA, Ishida W, Liauw JA, et al. A mouse model for the study of transplanted bone marrow mesenchymal stem cell survival and proliferation in lumbar spinal fusion. Eur Spine J 28 (2019): 710-718.
- Blanco JF, Villarón EM, Pescador D, et al. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: results of a prospective phase I/II clinical trial with long-term follow-up. Stem Cell Res Ther 10 (2019): 63.
- Fomekong E, Dufrane D, Berg BV, et al. Application of a three-dimensional graft of autologous osteodifferentiated adipose stem cells in patients undergoing minimally invasive transforaminal lumbar interbody fusion: clinical proof of concept. Acta Neurochir (Wien) 159 (2017): 527-536.
- Liu XN, Yang CJ, Kim JE, et al. Enhanced Tendon-to-Bone Healing of Chronic Rotator Cuff Tears by Bone Marrow Aspirate Concentrate in a Rabbit Model. Clin Orthop Surg 10 (2018): 99-110.
- Cavinatto L, Hinckel BB, Tomlinson RE, et al. The Role of Bone Marrow Aspirate Concentrate for the Treatment of Focal Chondral Lesions of the Knee: A Systematic Review and Critical Analysis of Animal and Clinical Studies. Arthroscopy 35 (2019): 1860-1877.
- Harford JS, Dekker TJ, Adams SB. Bone Marrow Aspirate Concentrate for Bone Healing in Foot and Ankle Surgery. Foot Ankle Clin 21 (2016): 839-845.
- Olifirenko O, Savosko S, Movchan O. Knee Joint Structural Changes In Osteoarthritis And Injections Of Platelet Rich Plasma And Bone Marrow Aspirate Concentrate. Georgian Med News 303 (2020): 184-188.
- Viganò M, Ragni E, Marmotti A, et al. The effects of orthobiologics in the treatment of tendon pathologies: a systematic review of preclinical evidence. J Exp Orthop 9 (2022): 31.
- Crowley JD, Oliver RA, Dan MJ, et al. Single level posterolateral lumbar fusion in a New Zealand White rabbit (Oryctolagus cuniculus) model: Surgical anatomy, operative technique, autograft fusion rates, and perioperative care. JOR Spine 4 (2021): e1135.
- Laratta JL, Vivace BJ, López-Peña M, et al. 3D-printed titanium cages without bone graft outperform PEEK cages with autograft in an animal model. Spine J 22 (2022): 1016-1027.
- Lehr AM, Oner FC, Delawi D, et al. Efficacy of a Standalone Microporous Ceramic Versus Autograft in Instrumented Posterolateral Spinal Fusion: A Multicenter, Randomized, Intrapatient Controlled, Noninferiority Trial. Spine (Phila Pa 1976) 45 (2020): 944-951.
- Lehr AM, Delawi D, van Susante JLC, et al. Long-term (> 10 years) clinical outcomes of instrumented posterolateral fusion for spondylolisthesis. Eur Spine J 30 (2021): 1380-1386.
- García de Frutos A, González-Tartière P, Coll Bonet R, et al. Randomized clinical trial: expanded autologous bone marrow mesenchymal cells combined with allogeneic bone tissue, compared with autologous iliac crest graft in lumbar fusion surgery. Spine J 20 (2020): 1899-1910.
- Lehr AM, Oner FC, Delawi D, et al. Increasing Fusion Rate Between 1 and 2 Years After Instrumented Posterolateral Spinal Fusion and the Role of Bone Grafting. Spine (Phila Pa 1976) 45 (2020): 1403-1410.
- Li C, Zhang X, Zheng Z, et al. Nell-1 Is a Key Functional Modulator in Osteochondrogenesis and Beyond. J Dent Res 98 (2019): 1458-1468.
- James AW, Shen J, Zhang X, et al. NELL- 1 in the treatment of osteoporotic bone loss. Nat Commun 6 (2015): 7362.
- Siu RK, Lu SS, Li W, et al. Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A 17 (2011): 1123- 1135.
- Li W, Lee M, Whang J, et al. Delivery of lyophilized Nell-1 in a rat spinal fusion model. Tissue Eng Part A 16 (2010): 2861-2870.
- Lu SS, Zhang X, Soo C, et al. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J 7 (2007): 50-60.
- Ferrell Z, Grainger DW, Sinclair KD. Antibiotic-eluting resorbable bone-void filler evaluated in a large animal infection prevention model. Eur Cell Mater 37 (2019): 265-276.
- Jones Z, Brooks AE, Ferrell Z, et al. A resorbable antibiotic eluting bone void filler for periprosthetic joint infection prevention. J Biomed Mater Res B Appl Biomater 104 (2016): 1632-1642.
- Lyons FG, Gleeson JP, Partap S, et al. Novel microhydroxyapatite particles in a collagen scaffold: a bioactive bone void filler? Clin Orthop Relat Res 472 (2014): 1318-1328.
- Chang YL, Hsieh CY, Yeh CY, et al. Fabrication of Stromal Cell- Derived Factor-1 Contained in Gelatin/Hyaluronate Copolymer Mixed with Hydroxyapatite for Use in Traumatic Bone Defects. Micromachines (Basel) 12 (2021).
- Hofmann A, Gorbulev S, Guehring T, et al. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J Bone Joint Surg Am 102 (2020): 179-193.
- Nusselt T, Hofmann A, Wachtlin D, et al. CERAMENT treatment of fracture defects (CERTiFy): protocol for a prospective, multicenter, randomized study investigating the use of CERAMENT™ BONE VOID FILLER in tibial plateau fractures. Trials 15 (2014): 75.
- Chen M, Wang R, Jia P, et al. Stentoplasty with Resorbable Calcium Salt Bone Void Fillers for the Treatment of Vertebral Compression Fracture: Evaluation After 3 Years. Clin Interv Aging 16 (2021): 843-852.
- Friess W, Uludag H, Foskett S, et al. Characterization of absorbable collagen sponges as rhBMP-2 carriers. Int J Pharm 187 (1999): 91-99.
- Katsuura Y, Shafi K, Jacques C, et al. Cunningham, New Strategies in Enhancing Spinal Fusion. Hss j 16 (2020): 177-182.
- Buser Z, Hsieh P, Meisel HJ, et al. Use of Autologous Stem Cells in Lumbar Spinal Fusion: A Systematic Review of Current Clinical Evidence. Global Spine J 11 (2021): 1281-1298.
- Stark JR, Hsieh J, Waller D. Bone Graft Substitutes in Single- or Double-Level Anterior Cervical Discectomy and Fusion: A Systematic Review. Spine (Phila Pa 1976) 44 (2019): E618-E628.
- National Research Council Committee for the Update of the Guide for the C and A. Use of Laboratory, The National Academies Collection: Reports funded by National Institutes of Health, in Guide for the Care and Use of Laboratory Animals. National Academies Press (US) Copyright © 2011, National Academy of Sciences: Washington (DC). Sengupta, P, The Laboratory Rat: Relating Its Age With Human's. Int J Prev Med 4 (2013): 624-630.
- Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition 21 (2005): 775-777.
- Laboratories CR. Ovariectomy (2017).
- Laboratories CR. Surgical Pre- and Postoperative Care. (2016).
- Gazzeri R, Panagiotopoulos K, Galarza M, et al. Minimally invasive spinal fixation in an aging population with osteoporosis: clinical and radiological outcomes and safety of expandable screws versus fenestrated screws augmented with polymethylmethacrylate. Neurosurg Focus 49 (2020): E14.
- Jiron JM, Mendieta Calle JL, Castillo EJ, et al. Comparison of Isoflurane, Ketamine-Dexmedetomidine, and Ketamine-Xylazine for General Anesthesia during Oral Procedures in Rice Rats (Oryzomys palustris). J Am Assoc Lab Anim Sci 58 (2019): 40-49.
- Mees L, Fidler J, Kreuzer M, et al. Faster emergence behavior from ketamine/xylazine anesthesia with atipamezole versus yohimbine. PLoS One 13 (2018): e0199087.
- Miller AL, Golledge HD, Leach MC. The Influence of Isoflurane Anaesthesia on the Rat Grimace Scale. PLoS One 11 (2016): e0166652.
- Zhang EQ, Knight CG, Pang DS. Heating Pad Performance and Efficacy of 2 Durations of Warming after Isoflurane Anesthesia of Sprague-Dawley Rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 56 (2017): 786-791.
- Liu L, Lam WMR, Naidu M, et al. Synergistic Effect of NELL-1 and an Ultra-Low Dose of BMP-2 on Spinal Fusion. Tissue Eng Part A 25 (2019): 1677-1689.
- Kim BJ, Arai Y, Park EM, et al. Osteogenic Potential of Tauroursodeoxycholic Acid as an Alternative to rhBMP-2 in a Mouse Spinal Fusion Model. Tissue Eng Part A 24 (2018): 407-417.
- Zhu W, Qiu Y, Sheng F, et al. An effective delivery vehicle of demineralized bone matrix incorporated with engineered collagen-binding human bone morphogenetic protein-2 to accelerate spinal fusion at low dose. J Mater Sci Mater Med 29 (2017): 2.
- Marquardt N, Feja M, Hünigen H, et al. Euthanasia of laboratory mice: Are isoflurane and sevoflurane real alternatives to carbon dioxide? PLOS ONE 13 (2018): e0203793.
- Clark DP, Badea CT. Advances in micro-CT imaging of small animals. Phys Med 88 (2021): 175-192.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to Image J: 25 years of image analysis. Nature Methods 9 (2012): 671-675.
- Rosenberg AS, Puig M, Nagaraju K. et al. Immune-mediated pathology in Duchenne muscular dystrophy. Sci Transl Med 7 (2015): 299rv4.
- Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol 1180 (2014): 31-43.
- Jacques C, Floris I, Lejeune B. Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH(®) Micro-Immunotherapy Treatment. Int J Mol Sci 22 (2021).
- Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 32 (2012): 23-63.
- Peng S, Li W, Hou N, et al. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells 9 (2020).
- Zhou L, Wang F, Sun R, et al. SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep 17 (2016): 811-822.
- Almasy E, Szederjesi J, Grigorescu BL, et al. The Diagnostic and Prognostic Role of Vascular Endothelial Growth Factor C in Sepsis and Septic Shock. J Crit Care Med (Targu Mures) 6 (2020): 152-158.
- Chen C, Chen H, Sun J, et al. Smad1 expression and function during mouse embryonic lung branching morphogenesis. American Journal of Physiology-Lung Cellular and Molecular Physiology 288 (2005): L1033-L1039.
- Jämsä T, Jalovaara P, Peng Z, et al. Comparison of three- point bending test and peripheral quantitative computed tomography analysis in the evaluation of the strength of mouse femur and tibia. Bone 23 (1998): 155-61.
- Knop C, Lange U, Bastian L, et al. Biomechanical compression tests with a new implant for thoracolumbar vertebral body replacement. Eur Spine J 10 (2001): 30-37.
- Hvid I, Christensen P, Søondergaard J, et al. Compressive strength of tibial cancellous bone. Instron and osteopenetrometer measurements in an autopsy material. Acta Orthop Scand 54 (1983): 819-825.
- Murphy ME, McCutcheon MB, Grauberger J, et al. Allograft versus autograft in cervical and lumbar spinal fusions: an examination of operative time, length of stay, surgical site infection, and blood transfusions. J Neurosurg Sci, 63 (2019): 11-18.
- Noback PC, Donnelley CA, Yeatts NC, et al. Utilization of Orthobiologics by Sports Medicine Physicians: A Survey-based Study. J Am Acad Orthop Surg Glob Res Rev 5 (2021): e20.00185.
- Galimberti F, Lubelski D, Healy AT, et al. A Systematic Review of Lumbar Fusion Rates With and Without the Use of rhBMP-2. Spine (Phila Pa 1976) 40 (2015): 1132-1139.
- Le ADK, Enweze L, DeBaun MR, et al. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr Rev Musculoskelet Med 11 (2018): 624-634.
- West TA, Williams ML. Orthobiologics. Clin Podiatr Med Surg 36 (2019): 609- 626.
- Van Schaik KD, Lee KS. Orthobiologics: Diagnosis and Treatment of Common Tendinopathies. Semin Musculoskelet Radiol 25 (2021): 735-744.
- Kelly MP, Lenke LG, Bridwell KH, R. et al. Fate of the adult revision spinal deformity patient: a single institution experience. Spine (Phila Pa 1976) 38 (2013): E1196-200.
- Rajaee SS, Kanim LE, Bae HW. National trends in revision spinal fusion in the USA: patient characteristics and complications. Bone Joint J 96 (2014): 807-816.
- Zdeblick TA, Phillips FM. Interbody cage devices. Spine (Phila Pa 1976) 28 (2003): S2-7.
- Lee N, Kim KN, Yi S, et al. Comparison of Outcomes of Anterior, Posterior, and Transforaminal Lumbar Interbody Fusion Surgery at a Single Lumbar Level with Degenerative Spinal Disease. World Neurosurg, 101 (2017): 216-226.
- Itoh K, Udagawa N, Katagiri T, et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 142 (2001): 3656-3662.
- Choudhry OJ, Christiano LD, Singh R, et al. Bone morphogenetic protein-induced inflammatory cyst formation after lumbar fusion causing nerve root compression. J Neurosurg Spine 16 (2012): 296-301.
- Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J 9 (2009): 623-629.
- Pradhan BB, Bae HW, Dawson EG, et al. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 31 (2006): E277-284.
- Hansen SM, Sasso RC. Resorptive response of rhBMP2 simulating infection in an anterior lumbar interbody fusion with a femoral ring. Journal of spinal disorders & techniques 19 (2006): 130-134.
- Carragee EJ, Mitsunaga EA, Hurwitz EL. et al. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J 11 (2011): 511-516.
- Comer GC, Smith MW, Hurwitz EL, et al. Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a 10-year cohort controlled study. Spine J 12 (2012): 881-890.
- Chan DS, Garland J, Infante A, et al. Wound complications associated with bone morphogenetic protein-2 in orthopaedic trauma surgery. J Orthop Trauma 28 (2014): 599-604.
- Owens K, Glassman SD, Howard JM, et al. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J 20 (2011): 612-617.
- Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 31 (2006): 542-547.
- Glaeser JD, Salehi K, Kanim LEA, et al. Anti-Inflammatory Peptide Attenuates Edema and Promotes BMP-2-Induced Bone Formation in Spine Fusion. Tissue Eng Part A 24 (2018): 1641-1651.
- Bedair TM, Lee CK, Kim DS, et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. Journal of Tissue Engineering, 11 (2020): 2041731420967591.
- Gibson BHY, Duvernay MT, McKeithan LJ, et al. Variable Response to Antifibrinolytics Correlates with Blood-loss and Transfusion in Posterior Spinal Fusion. Spine Deform 10 (2022): 841-851.
- Nakagawa S, Okada R, Kushioka J, et al. Effects of rhBMP-2 Loaded Hydroxyapatite Granules/beta-Tricalcium Phosphate Hydrogel (HA/β-TCP hydrogel) Composite on a Rat Model of Coccygeal Intervertebral Fusion. Research Square (2021).
- Holmes C, Elder BD, Ishida W, et al. Comparing the efficacy of syngeneic iliac and femoral allografts with iliac crest autograft in a rat model of lumbar spinal fusion. J Orthop Surg Res 15 (2020): 410.
- Arlt H, Besschetnova T, Ominsky MS, et al. Effects of systemically administered abaloparatide, an osteoanabolic PTHrP analog, as an adjuvant therapy for spinal fusion in rats. JOR Spine 4 (2021): e1132.
- Harindhanavudhi T, Takahashi T, Petryk A, et al. An Adjunctive Use Of Asfotase Alfa And Zoledronic Acid After Spinal Surgery In Neurofibromatosis Type 1 Related Dystrophic Scoliosis. AACE Clin Case Rep 6 (2020): e305-e310.
- Metzger MF, Kanim LE, Zhao L, et al. The relationship between serum vitamin D levels and spinal fusion success: a quantitative analysis. Spine (Phila Pa 1976) 40 (2015): E458-E468.
- Kang Y, Liu C, Wang M, A novel rat model of interbody fusion based on Anterior Lumbar Corpectomy And Fusion (ALCF). BMC Musculoskeletal Disorders 22 (2021): 965.
- Yamaguchi T, Inoue N, Sah RL, et al. Micro-computed tomography-based three-dimensional kinematic analysis during lateral bending for spinal fusion assessment in a rat posterolateral lumbar fusion model. Tissue Eng Part C Methods 20 (2014): 578-587.
- Wu J, Shi L, Liu D, et al. Evaluating Screw Stability After Pedicle Screw Fixation With PEEK Rods. Global Spine Journal (2021): 2192568221996692.
- Wang ML, Massie J, Perry A, et al. A rat osteoporotic spine model for the evaluation of bioresorbable bone cements. Spine J 7 (2007): 466-474.
- Zhao X, Ma H, Han H, et al. Precision medicine strategies for spinal degenerative diseases: Injectable biomaterials with in situ repair and regeneration. Mater Today Bio 16 (2022): 100336.
- Xu J, Wang Y, Hsu CY, et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. Elife 8 (2019).
- Hsieh YY, Tsuang FY, Kuo YJ, et al. Biomechanical analysis of single-level interbody fusion with different internal fixation rod materials: a finite element analysis. BMC Musculoskeletal Disorders 21 (2020): 100.
- Overview of the Animal Model Qualification Program (2020).
- Drespe IH, Polzhofer GK, Turner AS, et al. Animal models for spinal fusion. Spine J 5 (2005): 209s-216s.
- Sandhu HS, Khan SN. Animal models for preclinical assessment of bone morphogenetic proteins in the spine. Spine (Phila Pa 1976) 27 (2002): S32-S38.
- Yousefzadeh N, Kashfi K, Jeddi S, et al. Ovariectomized rat model of osteoporosis: a practical guide. Excli j 19 (2020): 89-107.
- Nelson HD. Menopause. Lancet 371 (2008): 760-770.
- Li L, Wang Z. Ovarian Aging and Osteoporosis. Adv Exp Med Biol 1086 (2018): 199-215.
- Cauley JA. Estrogen and bone health in men and women. Steroids 99 (2015): 11-15.
- Sophocleous A, Idris AI. Rodent models of osteoporosis. Bonekey Rep 3 (2014): 614.
- Reisener MJ, Pumberger M, Shue J, et al. Trends in lumbar spinal fusion-a literature review. J Spine Surg 6 (2020): 752-761.
- Carreon LY, Djurasovic M, Glassman SD, et al. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine (Phila Pa 1976) 32 (2007): 892-895.
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 1 (2015): 2-18.
- Lestini WF, Fulghum JS, Whitehurst LA. Lumbar spinal fusion: advantages of posterior lumbar interbody fusion. Surg Technol Int 3 (1994): 577-590.
- Malham GM, Parker RM, Ellis NJ, et al. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: A prospective study of complications. J Neurosurg Spine 21 (2014): 851-860.
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 29 (2015): 705-711.
- Phan K, Rao PJ, Scherman DB, et al. Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J Clin Neurosci 22 (2015): 1714-1721.
- Audat Z, Moutasem O, Yousef K, et al. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J 53 (2012): 183-187.
- Lee JW, Lee S, Lee SH, et al. Improved spinal fusion efficacy by long-term delivery of bone morphogenetic protein-2 in a rabbit model. Acta Orthop 82 (2011): 756-760.
- Grauer JN, Bomback DA, Lugo R, et al. Posterolateral lumbar fusions in athymic rats: characterization of a model. Spine J 4 (2004): 281-286.
- von Scheidt M, Zhao Y, Kurt Z, et al. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab 25 (2017): 248-261.
- Hou J, Wang J, Cao L, et al. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Biomed Mater 7 (2012): 035002.
- Itoh T, Mochizuki M, Nishimura R, et al. Repair of ulnar segmental defect by recombinant human bone morphogenetic protein-2 in dogs. J Vet Med Sci 60 (1998): 451-458.
- Hwang CJ, Lee JH, Baek HR, et al. Evaluation of the efficacy of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in a mini-pig spinal anterior interbody fusion model. Bone Joint J 95 (2013): 217-223.
- Liu X, Manzano G, Kim HT, et al. A rat model of massive rotator cuff tears. J Orthop Res 29 (2011): 588-595.
- Emmert MY, Schmitt BA, Loerakker S, et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med 10 (2018).
- Hosper NA, Eggink AJ, Roelofs LA, et al. Intra-uterine tissue engineering of full-thickness skin defects in a fetal sheep model. Biomaterials 31 (2010): 3910-3919.
- Andersen ML, Winter LMF. Animal models in biological and biomedical research - experimental and ethical concerns. An Acad Bras Cienc 91 (2019): e20170238.
- Harding J, Roberts RM, et al. Large animal models for stem cell therapy. Stem Cell Res Ther 4 (2013): 23.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 