Cortical Activation Reorganization of Cerebral Regions in Charcot– Marie–Tooth Patients: A Task-state Functional Magnetic Resonance Imaging Study
Zhifeng Wang1, Ce Han1, Chengjie Yuan1, Yiyuan Shen2, Xiang Geng1, Chen Wang1, Chao Zhang1, Jiazhang Huang1, Xin Ma1, Hanqiu Liu2* and Xu Wang1*
1Department of Orthopedics, Huashan Hospital, Fudan University, 12 Middle Wulumuqi Rd, Shanghai, 200040, China
2Department of Radiology, Huashan Hospital, Fudan University, 12 Middle Wulumuqi Rd, Shanghai, 200040, China
*Corresponding Author:Xu Wang, Department of Orthopedics, Huashan Hospital, Fudan University, 12 Middle Wulumuqi Rd, Shanghai, 200040, China
Hanqiu Liu, Department of Radiology, Huashan Hospital, Fudan University, 12 Middle Wulumuqi Rd, Shanghai, 200040, China
Received: October 10, 2022; Accepted: October 20, 2022; Published: October 29, 2022
Article Information
Citation:
Wang Z, Han C, Yuan C, Shen Y, Geng X, Wang C, Zhang C, Huang J, Ma X, Liu H, Wang X. Cortical Activation Reorganization of Cerebral Regions in Charcot–Marie–Tooth Patients: A Task-state Functional Magnetic Resonance Imaging Study. Journal of Orthopedics and Sports Medicine 4 (2022): 276-288.
View / Download Pdf Share at FacebookAbstract
Background: Evidence of altered brain functional activation in response to different tasks has been reported in some peripheral neuropathies. The aim of this study was to investigate possible central nervous system modifications using task-state Functional Magnetic Resonance Imaging (FMRI) in Charcot–Marie–Tooth patients.
Methods: A design of ankle dorsiflexion-plantarflexion and fist clutching paradigm was adopted at a frequency of 1 Hz in the FMRI portion in CMT patients and healthy controls. We acquired 3.0T MRI brain scans. The brain activation MR signals were recorded and a paired voxel-wise t-test was performed. The correlations between FMRI measurement and clinical variables were calculated.
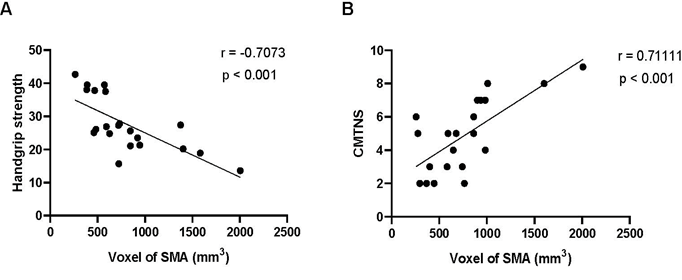
Results: Compared with control group during the ankle dorsiflexionplantarflexion movement, the voxel-wise independent sample t-test revealed that the CMT patients demonstrated statistically more activation of the contralateral precentral gyrus (M1, t=5.01, P<0.001), supplementary motor area (SMA, t=4.58, P=0.038), postcentral gyrus (PCG) (t=4.46, P=0.031), and ipsilateral PCG (t=4.14, P=0.002), temporal lobe (TL) (t=4.73, P<0.001). A significant positive correlation emerged between the CMTNS and increased activated voxels with SMA(r=0.71111, P<0.001), with a significant negative correlation between handgrip strength and increased activated voxels with SMA(r=-0.7073, P<0.001).
Conclusion: This study demonstrated the differences of cortical activation in CMT patients during ankle dorsiflexion-plantarflexion movement and its correlation with clinical severity. These findings give further understanding of the potential mechanism in central nervous system underlying the peripheral nerve pathology in CMT patients, and lay the foundation for longitud
Keywords
<p>Charcot-Marie-Tooth disease; Functional magnetic resonance imaging; Cortical activation, Peripheral nervous system diseases</p>
Article Details
Introduction
Charcot-Marie-Tooth Disease (CMT) also known as Hereditary Motor and Sensory Neuropathy (HMSN) is among the most common hereditary peripheral neuropathies [1], which are a genetically and phenotypically heterogeneous group of disorders [2,3]. This disease affects approximately 1 in 2000 individuals and decreases quality of life [4-6]. Typical clinical characteristics of CMT are muscle weakness, wasting, and sensory loss with
a distal predominance [7,8]. This symptom is most evident in the lower limbs and slowly progresses in a length dependent manner [9]. CMT is classically divided into two types: the more common type I, the demyelinating form, characterized by a slow Nerve Conduction Velocity (NCV), and type II, the axonal form, with only normal or slightly reduced NCV but mainly reduced amplitude of motor and sensory responses in neurographic recordings [9]. Conventionally, the diagnosis of CMT is based on a combination of clinical features, Nerve Conduction Studies (NCS) Electromyography (EMG) and genetic testing [8,10], but it may go unrecognized before overt clinical features such as pes cavus or hammer toe become evident because of its insidious onset [2,11].
Neuropathological studies show a distal damage consisting in segmental demyelination and remyelination, axonal degeneration, and Schwann cell proliferation in the form of onion bulbs [12]. Although CMT is primarily a peripheral nervous system disease, Central Nervous System (CNS) involvement have been anecdotally reported in different forms of CMT [13,14], including patients with peripheral myelin protein 22 (PMP22) duplication [15,16]. The duplication of PMP22 was confirmed to be the genetic aetiology of CMT1A [17]. However, PMP22 is also expressed in the Central Nervous System (CNS) [18], which implies the involvement of that system in CMT1A. Along with these anecdotal reports, previous studies demonstrated slight structural modifications in the CMT1A brain [19,20]. Furthermore, evidence of altered brain functional activation in response to different tasks has been reported in other peripheral neuropathies [21,22], along with modifications of resting-state networks, extending beyond the solely sensorimotor network involvement [23,24].
In a FMRI study, Pontillo et al. [20] showed evidence of structural reorganization in the brain of CMT1A patients, mostly involving the anterior cerebellum and possibly reflecting compensatory mechanisms in response to peripheral nerve pathology. Moreover, another study
[25] have reported that diffuse functional reorganization involving multiple large-scale networks in the CMT1A brain, independent of structural abnormalities and partially correlating with peripheral nerve damage and functional impairment. Nevertheless, conclusions regarding the change of supraspinal control in CMT patients remain controversial, and the underlying mechanism of how the motor cortex excitability affects neuromuscular function remains unclear.
In the present study, we intended to investigate differences in cortical activity during active ankle and hand movements between CMT patients and healthy controls, using task-state functional MRI, exploring the brain plasticity and providing more information regarding the relationship between CMT and central nervous system modifications. In addition, we explored the possible functional impact of these changes, correlating fMRI findings with clinical measurements.
Materials and Methods
Subjects
In this prospective cross-sectional study, from June 2019 to June 2021, we enrolled symptomatic patients with genetically diagnosed CMT along with a group of age- and sex-comparable healthy volunteers. Exclusion criteria for both groups were as followed: (1) a history of previous surgeries to the musculoskeletal structures (i.e., bones, joint structures and nerves) or a fracture requiring realignment in either lower extremity. (2) acute injury to the musculoskeletal structures of other joints of the lower extremity in the previous 3 months, which impacted joint integrity and function (i.e., sprains, fractures) resulting in at least 1 interrupted day of desired physical activity. (3) central nervous system diseases, muscular diseases and other conditions that may have influences on ankle and hand movements. (4) confirmed or suspected history of cardiopulmonary failure. (5) psychiatric disorders. (6) concurrent and contraindications to investigation by MRI. (7) the presence of other relevant neurological, psychiatric or systemic conditions that could affect peripheral nerves or CNS.
The current study was conducted in accordance with the Declaration of Helsinki, and all study procedures were carried out with adequate understanding and written consent of the participants. Formal approval from the Huashan Hospital Institutional Review Board was obtained before study initiation.
Clinical and electrophysiological evaluation
On the same day of the fMRI examination, CMT patients underwent clinical and electrophysiological examinations mainly oriented toward the assessment of motor and sensory domains. All the participants were strongly right-footed and right-handed based on participant self-review. Charcot- Marie-Tooth Neuropathy Score (CMTNS, second version) [26], considered as a global measure of clinical disability and defined as the sum of two distinct sub scores: the CMT Examination Score (CMTES), rating the patients’ symptoms and signs, and the CMTNS neurophysiological component, based on the assessment of ulnar Compound Motor Action Potential (CMAP) and radial Sensory Action Potential (SAP) on the non-dominant side as objective indexes of peripheral axonal damage. The total score ranges from 0 (no disability) to 36 (maximum disability). The scores of CMTNS were recorded to evaluate the ankle function. Moreover, a hand- held dynamometer was used to measure the hand-grip strength [27].
Design and motor paradigm
A short block design for ankle and hand movements was performed in the FMRI portion of the study. During the motor scans, the CMT patients were asked to execute two motor tasks: (1) ankle dorsiflexion-plantarflexion (2) fist clutching. As a control, the healthy volunteers were asked to perform the two tasks in the same way. Each experimental session consisted of three active ankle movements and three active fist clutching functional runs. The run order was counterbalanced across participants. Prior to scanning, subjects were trained the basic ankle dorsiflexion, plantarflexion and fist clutching movements until comfortable with the tasks. All ankle and hand movements were done with the left side.
During the ankle task, the participant’s left foot was firmly placed in a pedal of a special single-plane manipulator. And padding was placed under the popliteal fossa so the knee and hip joints were flexed about 20° each. The manipulator allowed for 15° of plantarflexion to 15° of dorsiflexion of ankle, with 0° defined as the foot perpendicular to the leg. Previous FMRI studies demonstrated that participants can complete similar ankle movement paradigms without muscle co-activation [28]. Likewise, participants performed a dynamic isometric fist clutching task with their left hands. And during the first clutching task, each participant completed it by squeezing the same grip ball [29].
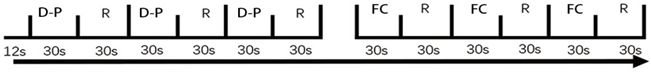
The beginning and completion of each section was communicated to the participants by visual instructions from a screen. For each stimulus presentation within a run, all the participants were instructed to see a series of 30 shining points presented at 1 Hz, and then performed the tasks at the same frequency as the shining points. All participants performed the motor tasks outside the scanner in order that they might be observed for the presence of associated movements and no movements of other limbs. During a continuous scanning session, subjects performed ankle movements/ fist clutching in blocks of 30 s, alternating with 30 s rest. A total of 3 blocks of ankle movements/ fist clutching and 3 rest blocks were performed per session, resulting in 90 task sets and 90 rest sets. The duration of each functional run was 192s (Figure 1).
FMRI data acquisition
All imaging data were obtained with a 3.0T GE Signa VH/I 3.0-T scanner (GE Healthcare, GE Asian Hub, Shanghai, China) equipped with 32-channel head coil. Their heads were immobilized by a pair of cushions that were fastened on both sides of the head to minimize head movements. Functional imaging (FMRI) data were measured with an echo-planar imaging sequence (TR/TE = 3000/35ms, FOV = 240 × 240 mm, 43 axial slices, acquisition matrix = 64 × 64, voxel size
= 3.4 × 3.4 × 3.2 mm, flip angle = 90°). Structural imaging
data were acquired with a multi-echo MPRAGE T1-weighted
pulse sequence (TR /TE = 1000/5ms, TI = 1200ms, flip angle = 200°, FOV = 240 × 240, acquisition matrix = 256 × 256, sagittal acquisition, spatial resolution = 1 × 1 × 1mm3, interslice space = 0 mm).
FMRI data analysis
The image data were analyzed with the Statistical Parametric Mapping Program (SPM 12, Wellcome Institute for Imaging Neuroscience, London, UK) implemented in MATLAB (MathWorks, Natick, MA). The EPI of each individual was realigned to the first image for each sequence separately for interscan movement artifacts. The aligned functional images from all sessions were normalized by DARTEL to the Montreal Neurological Institute (MNI) standard brain in order to report MNI coordinates. Finally, the images were spatially smoothed using a 6mm Full-Width-at- Half-Maximum (FWHM) Gaussian kernel. We made boxcar analysis with T-contrast for all sessions of every subject and retained only voxels for a p-value of 0.05 at voxel level with FDR (false discovery rate) correction for single-subject analysis. Statistical comparisons between groups regarding the two movements were performed with a p-value of 0.05 at voxel level with FDR.
Statistical analysis
The correlation analysis was performed between the clinical variables (CMTNS scores, hand-grip strength) and the activated voxel value in the cerebral regions with significant activation differences in addition to FMRI analysis. The voxels were extracted by WFU pickatlas tool and kept the same resolution and voxel size as the functional images. The so obtained residuals were standardized and their relationship with CMTNS scores and the hand-grip strength was assessed via Spearman rank correlation test. All the statistical analyses were conducted on SPSS (version 21) and P<0.05 was considered to be statistically significant.
Results
Subjects
A total of 21 (M/F 16/5) CMT patients aged 25.6 ± 5.4 years (range, 9-40 years), with average Body Mass Index (BMI) of 18.45 kg/m2 (range: 15.6-25.2kg/m2), were enrolled in the patient group between September 2019 and September 2021 from Huashan hospital, Fudan university, Shanghai, China. A group of 21 age- and sex-comparable healthy volunteers (27.8 ± 8.5 years; M/F 16/5) were enrolled as control group. The mean CMTNS of the patients was 9.92
(standard deviation 3.53). The average handgrip strength of the patients was 26.70 ± 17.65 (range 13.6–42.7). All the patients did not complain of any auditory, visual or cognitive symptoms except sensorimotor deficits. The demographic data was shown in Table 1.
Between-group FMRI analysis
Comparisons of ankle dorsiflexion-plantarflexion activation between two groups
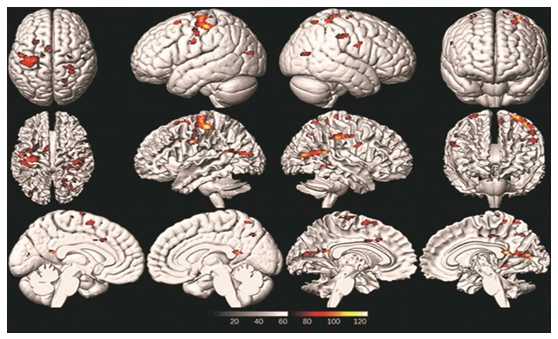
Compared with control group during the ankle dorsiflexion- plantarflexion movement, the voxel-wise independent sample t-test revealed that the CMT patients demonstrated statistically more activation of the contralateral precentral gyrus (M1, t=5.01, P<0.001), Supplementary Motor Area (SMA, t=4.58, P=0.038), Postcentral Gyrus (PCG) (t=4.46, P=0.031), and ipsilateral PCG (t=4.14, P=0.002), Temporal Lobe (TL) (t=4.73, P<0.001). In addition, the CMT patients activated more limbic lobe (cluster of 135 mm3), but there was no statistically significant difference compared with control group (t=2.03, P=0.932). The activation maps were created to delineate cerebral regions exhibiting activation difference between the CMT group and the control group during ankle dorsiflexion-plantarflexion movement (Figure 2, Figure 3 and Table 2).
Based on the voxel-wise independent sample t-test, the maximum signal intensity for the CMT group during ankle dorsiflexion-plantarflexion movement was mainly located in left paracentral lobule, SMA (cluster of 631 mm3). Morevoer, across the whole cerebrum, the control group activated relatively larger voxel in these cortical regions, including left M1, SMA, paracentral lobule (cluster of 2008 mm3), and PCG (cluster of 594 mm3).
Across other cortical regions, both the CMT patients and control group activated several regions without statistically significant difference, including right cerebellum anterior lobe
Table 1: Demographic data of all the subjects included in the study.
|
CMT |
Control |
P value |
|
|
Number |
21 |
21 |
- |
|
Gender (Male/Female) |
16M / 5F |
16M / 5F |
1.000a |
|
Age (Y) |
25.6 ± 5.4 |
27.8 ± 8.5 |
0.742b |
|
BMI (kg/m2) |
18.45 ± 4.22 |
20.82 ± 2.47 |
0.219b |
|
CMTNS score |
9.92 ± 3.53 |
0 |
<0.001b |
|
Handgrip strength (N) |
26.70 ±17.65 |
53.64 ± 12.83 |
<0.001b |
|
aPearson Chi-Square value, bindependent t-test. Data are shown as mean ± SD CMT: Charcot-Marie-Tooth; CMTNS: Charcot-Marie-Tooth Neuropathy Score; BMI: Body Mass Index |
|||
Figure 2: The cerebral regions with significantly greater activation during active ankle dorsiflexion-plantarflexion movement in CMT patients compared to healthy people (P<0.05, cluster > 100 mm3 across cerebral volume) and brain stem. Besides, the ankle dorsiflexion-plantarflexion of CMT patients was also associated with right cerebellar anterior lobe (cluster of 329 mm3), medial frontal gyrus (cluster of 631 mm3). However, the voxel-wise independent sample t-test did not show statistically significant difference between the two groups in these cerebral areas except for SMA, which indicated that the changes of contralateral paracentral lobule, ipsilateral cerebellum anterior lobe and lingual cerebellum may not be the influencing factors for CMT.
Comparisons of fist clutching activation between two groups
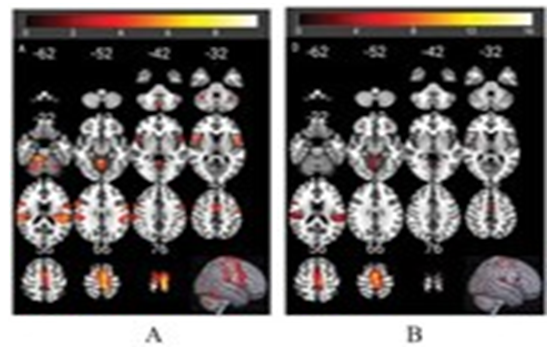
When looking at the cortical activation of fist clutching paradigm, no significant differences were observed in CMT patients compared to control group. The independent t-test was not statistically significant across four ROIs: left insula (t=2.72, P=0.306), inferior front gyrus (t=2.77, P=0.383), inferior parietal lobule (t=-2.41, P=0.376), and middle temporal gyrus (t=-2.41, P=0.762) (Figure 4 and Table 3). In addition, the fist clutching movement of CMT patients also activated other cortical regions, including right cerebellum anterior lobe, PCG, as well as the left brain stem, medial frontal gyrus and SMA. The significantly activated cerebral
regions during ankle dorsiflexion-plantarflexion and fist clutching movements in CMT and control group were shown in Table 4.
Relationship between cerebral activation changes and clinical parameters
According to the FMRI measurements, the number of significant voxels in the primary motor area and SMA was calculated. When investigating the clinical relevance of the observed cerebral activation changes, a significant positive correlation (r=0.71111, P<0.001) was found between the CMTNS score and the activated voxels of SMA. Furthermore, a significant negative correlation was found between the handgrip strength and the activated voxels in SMA (r=-0.7073, P<0.001) (Figure 5).
No significant correlations emerged between the CMTNS score or handgrip strength and the activated voxels of the primary motor area.
Discussion
CMT is a large group of disorders with a wide range of clinical presentations and abnormalities. First reported in 1886, the disease has been found to affect 1 in 2500-3300
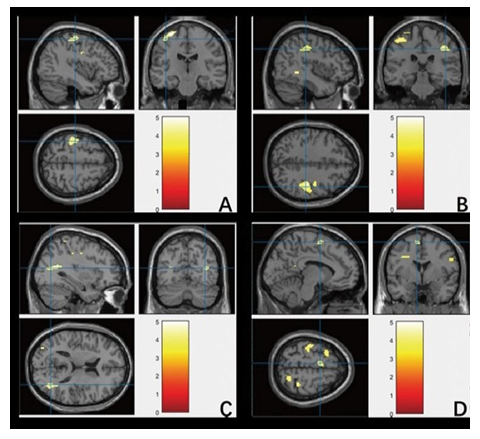
Figure 3: Regions with greater activation during active ankle dorsiflexion-plantarflexion movement in CMT patients compared with control participants. A: Right Precentral Gyrus (M1); B: Left and right Postcentral Gyrus (PCG); C: Left Temporal Lobe (TL); D: Left and right Supplementary Motor Area (SMA).
Table 2: Significantly activated cerebral regions during ankle dorsiflexion-plantarflexion paradigm (FWE corrected <0.05).
|
Regions |
Side |
BA/ subregion |
Peak MNI coordinates |
Volume (mm3) |
T |
||
|
x |
y |
z |
|||||
|
Control vs. CMT |
|||||||
|
Precentral gyrus |
L |
BA4 |
-32 |
-18 |
68 |
1927 |
5 |
|
Postcentral gyrus |
L |
BA3 |
-42 |
-20 |
60 |
4.5 |
|
|
Sub-gyral |
L |
BA6 |
-32 |
-6 |
34 |
4.4 |
|
|
Temporal lobe |
R |
BA22 |
34 |
-58 |
16 |
1278 |
4.7 |
|
Middle temporal gyrus |
R |
BA21 |
22 |
-46 |
16 |
4.6 |
|
|
Middle occipital gyrus |
R |
BA18 |
34 |
-44 |
18 |
4.3 |
|
|
Medial frontal gyrus |
L |
BA10 |
-18 |
52 |
-4 |
48 |
3.8 |
|
Front lobe |
R |
BA9 |
38 |
-26 |
40 |
831 |
4.7 |
|
Postcentral gyrus |
R |
BA3 |
36 |
-10 |
42 |
4.1 |
|
|
Supra Marginal |
R |
BA2 |
44 |
-28 |
52 |
3.7 |
|
|
SMA |
L |
BA6 |
-8 |
0 |
62 |
299 |
4.6 |
|
Middle frontal gyrus |
L |
BA9 |
-28 |
14 |
62 |
4 |
|
|
Precentral gyrus |
R |
BA2 |
28 |
-34 |
72 |
233 |
4.9 |
|
Somatosensory association cortex |
R |
BA5 |
30 |
-42 |
64 |
4.7 |
|
|
Superior frontal gyrus |
L |
BA9 |
-6 |
14 |
66 |
3.6 |
|
|
Superior parietal lobule |
R |
BA7 |
20 |
-58 |
62 |
88 |
4.2 |
|
Precuneus |
R |
BA7 |
20 |
-66 |
44 |
4 |
|
|
Precentral gyrus |
R |
BA4 |
28 |
-16 |
70 |
40 |
4 |
|
Insula |
L |
BA13 |
-46 |
2 |
14 |
18 |
3.7 |
|
Temporal lobe |
R |
BA22 |
40 |
-44 |
-2 |
88 |
4.8 |
|
Precentral gyrus |
R |
BA4 |
52 |
-4 |
26 |
156 |
4 |
|
Cerebellum posterior lobe |
R |
NA |
12 |
-64 |
-28 |
9 |
3.5 |
|
NA: not available; Non-relevant signal in ventricles or near an area of sinus drainage was not interpreted. |
|||||||
Table 3: Significantly activated cerebral regions during ankle dorsiflexion-plantarflexion and fist clutching movements in CMT and control
group (FWE corrected <0.05).
|
Regions |
Side |
BA/ subregion |
Peak MNI coordinates |
Volume (mm3) |
T |
||
|
x |
y |
z |
|||||
|
Control vs. CMT |
|||||||
|
Inferior front gyrus, triangular part |
R |
BA10 |
50 |
26 |
16 |
146 |
3.29 |
|
Inferior front gyrus |
R |
BA10 |
46 |
40 |
20 |
2.77 |
|
|
Extra-nuclear |
R |
BA 30 |
26 |
18 |
14 |
200 |
3 |
|
Caudate |
R |
NA |
20 |
26 |
12 |
2.94 |
|
|
Insula |
R |
BA13 |
28 |
30 |
14 |
2.72 |
|
|
Extra-nuclear |
L |
BA30 |
-18 |
24 |
16 |
89 |
2.77 |
|
Sub-gyral |
L |
BA6 |
-28 |
18 |
14 |
2.57 |
|
|
Parietal lobe |
L |
BA7 |
-36 |
-42 |
58 |
150 |
-2.4 |
|
Inferior parietal lobule |
L |
BA7 |
-34 |
-46 |
50 |
-2.41 |
|
|
Postcentral gyrus |
L |
BA3 |
-44 |
-44 |
56 |
-2.41 |
|
|
Insula |
L |
BA13 |
-34 |
-8 |
14 |
20 |
2.61 |
|
Middle temporal gyrus |
L |
BA21 |
-50 |
-68 |
26 |
22 |
-2.41 |
|
NA: not available; Non-relevant signal in ventricles or near areas of sinus drainage was not interpreted. |
|||||||
Figure 4: The activation pattern in CMT patients and healthy subjects during fist clutching movements. Cerebral activation pattern in healthy subjects (A): Cerebral activation pattern in CMT patients (B): The color bar is used to define the activation of the voxels. Red-yellow suggests a positive activation in the brain area. The numbers in the figures correspond to the slice numbers in the CH2 brain template.
Figure 5: The scatter diagram and fitting line of correlation analysis between the number of significant voxels extracted from the SMA of increased activation in CMT patients and handgrip strength [(A); r=-0.7073, p<0.001] and CMTNS [(B); r=0.71111, p<0.001] values, respectively. CMTNS: Charcot-Marie-Tooth Neuropathy Scorime; SMA: Supplementary Motor Area.
people and is linked to numerous gene mutations, resulting in over 25 subtypes [17,30,31]. Despite evidence of brain involvement in some forms of CMT [32,33], and, more widely, in other peripheral neuropathies [23,34,35], CMT is commonly considered as a purely peripheral nervous system disease. Indeed, PMP22 is produced primarily by Schwann cells and it is expressed in the compact portion of essentially all myelinated fibers in the peripheral nervous system [31,36]. Grooms et al. [37] reported in an FMRI study of patients after Anterior Cruciate Ligament (ACL) injury, that the function of specific areas of the bilateral cortex of the patient’s brain changes, which significantly increases the possibility of injury to the ACL of the other limb, indicating that there is a certain relationship between the ligament injury of lower limbs and the change of brain function. Actually, little structured evidence exists on the involvement of CNS in these CMT patients. There is a demand for expounding the underlying mechanism of how the motor cortex excitability affects neuromuscular function in CMT patients.
Based on the voxel-wise statistical analyses of FMRI activation during ankle dorsiflexion and plantarflexion, the results showed that the CMT patients clearly had a greater cortical representation than that one in healthy people, mainly including contralateral M1, SMA, TL. In addition, there were no statistically significant differences in the cortical activation during the fist clutching movement. Among these cortical areas, the primary motor cortex serves the purpose of executing neuromuscular control [38] and that SMA and PMA perform the function of advanced planning and preparation for voluntary movements [39]. Furthermore, the secondary sensory area extending to supramarginal gyrus, superior temporal gyrus, and rolandic operculum integrates visual, vestibular, and somatosensory information to navigate space and keep balance [40-42]. Hence, the different cortical representations can be attributed to the different functions of the two movements.
The M1 area is the cortical motor area, mainly located in precentral gyrus, which is to manage and control the random movement of the contralateral body [43,44]. In addition, ankle dorsiflexion and the resulting heel strike are necessary for the swing phase of erect bipedal gait and are unique to the human walking pattern [45].The range of motion (ROM) of ankle, especially dorsiflexion and plantarflexion, is important in biomechanical function. Typical clinical characteristics of CMT are muscle atrophy, sensory malfunction, and foot deformity (pes cavum) due to peripheral neuropathy [30]. CMT patients often complain of the abnormal gait and even difficulty walking because of limited ankle movements. As the CMT patients currently activated more contralateral M1 area, one interpretation is that the more activation of M1 area may relate to the need of balance. What’s more, the SMA is mainly involved in the motor-planning, preparation, starting and monitoring of complex motions [46,47]. SMA is associated with the anterior central gyrus, prefrontal cortex, basal ganglia, limbic system and inferior frontal gyrus [48,49]. Since the trapezoidal talus and ankle mortise is the best match when the ankle joint is dorsiflexed, the overall stiffness of the ankle joint is the highest, it could be regarded as a protective action during the gait [50]. And previous study has confirmed that when the talus moves 1 mm laterally, the contact area of the tibiotalar articulation decreases by 42% [51]. Therefore, the CMT patients with more cortical activation may facilitate the preparation for early ankle dorsiflexion during the GCP of the gait cycle, and then ankle joint is more prone to walk forward and keep balance in this state [52]. Dorsiflexion movement has the greatest impact during the heel strike of the gait cycle and navigation of the environment in bipedalism
[53]. Previous studies have demonstrated that the motor cortex appears to play an important role in gait modifications in response to obstacles [54,55]. Beloozerova and Sirota [56] reported that the cortical lesions in cats lead to inability of navigating obstacles such as repeated ladder rungs because they were unable to place their front foot accurately.
Another explanation is the coactivation of the Tibialis Anterior (TA) and Soleus (SOL). Even though the activation of antagonist was minimized by the paradigm we used, the possibility that an effort was made in the cortical level cannot be ruled out. Coactivation is a crucial feed forward strategy to increase the joint stiffness and impedance by reciprocal flexion-extension contractions in reaction to impaired ankle instability, which is accompanied by the enhanced corticomotor excitability [57-59]. Over time, the long-term accumulative effect of the changed strategies in gait led to the adaptive change of central nervous system. In brief, the larger activated extent in individuals with CMT during ankle dorsiflexion-plantarflexion paradigm was likely due to the functional reorganization of cerebral cortex, which might affect the motor control in turn.
Besides, the peak activation of contralateral PCG in CMT patients was significantly less than that one in control group. One interpretation of the less PCG activation during ankle dorsiflexion-plantarflexion paradigm in CMT patients is that the somatosensory information input decreased due to the existent of proprioception deficit. The appropriate neuromuscular control depends on the integration of the sensory inputs from peripheral structures and motor outputs from the central nervous system [60]. In this process, proprioception enables to perceive the body movement and position by the mechanoreceptors in supporting ligaments of ankle [61,62]. Previous study has reported the mechanisms for enhancing motor function with somatosensory stimulation, and suggests that network function cannot be thoroughly understood when weak ties are disregarded [63-65]. In CMT patients, the injured peripheral mechanoreceptors give rise to the differentiation and less sensory signal input to the central nervous system [62], causing the deactivation in this region. Therefore, the less activated PCG may interact with the less activated cortical motor areas, which may jointly lead to the occurrence and development of ankle instability, manifesting as ‘feeling of instability’ or ‘giving away’.
This FMRI analysis also found that the ipsilateral TL in CMT patients also showed greater activation than that in healthy volunteers. These findings are consistent with previous literatures [20]. The TL was responsible for processing auditory information, also related to memory and emotion [66]. The hippocampal formation and parahippocampal gyrus in the medial temporal lobe are important parts of limbic system, which is involved in emotion and memory storage [67]. The hippocampus, in particular, demonstrates
Table 4: Significantly activated cerebral regions during ankle dorsiflexion-plantarflexion and fist clutching movements in CMT and control
group (FWE corrected <0.05).
|
Regions |
Side |
BA/ subregion |
Peak MNI coordinates |
Volume (mm3) |
T |
||
|
x |
y |
z |
|||||
|
Dorsiflexion-Plantarflexion-Control group |
|||||||
|
Medial frontal gyrus |
L |
BA8 |
-8 |
-38 |
66 |
2008 |
8.9 |
|
Paracentral lobule |
L |
BA5 |
-8 |
-20 |
76 |
8.1 |
|
|
SMA |
L |
BA6 |
-6 |
-4 |
64 |
8 |
|
|
Cerebellum anterior lobe |
R |
NA |
2 |
-50 |
-6 |
446 |
8.6 |
|
Culmen |
R |
NA |
18 |
-38 |
-24 |
8.3 |
|
|
Vermis |
R |
NA |
4 |
-46 |
-18 |
6.2 |
|
|
Precentral gyrus |
L |
BA4 |
-46 |
0 |
12 |
294 |
6.5 |
|
Inferior frontal gyrus |
L |
BA10 |
-58 |
4 |
24 |
6.5 |
|
|
Insula |
L |
BA13 |
-56 |
10 |
6 |
4.9 |
|
|
Superior temporal gyrus |
L |
BA22 |
-46 |
-38 |
20 |
194 |
6.4 |
|
Inferior parietal lobule |
L |
BA40 |
-52 |
-26 |
24 |
5.8 |
|
|
Inferior frontal gyrus |
R |
BA44 |
54 |
10 |
12 |
232 |
6.3 |
|
Precentral gyrus |
R |
BA4 |
62 |
12 |
28 |
6 |
|
|
Middle frontal gyrus |
R |
BA9 |
54 |
4 |
46 |
5.8 |
|
|
Postcentral gyrus |
R |
BA3 |
58 |
-24 |
20 |
594 |
5.9 |
|
Dorsiflexion-Plantarflexion-CMT group |
|||||||
|
Paracentral lobule |
L |
BA5 |
-6 |
-38 |
64 |
631 |
4.3 |
|
Medial frontal gyrus |
L |
BA8 |
-2 |
-24 |
64 |
3.7 |
|
|
SMA |
L |
BA6 |
-6 |
-34 |
76 |
2.3 |
|
|
Cerebellum anterior lobe |
R |
NA |
20 |
-38 |
-24 |
329 |
4 |
|
Culmen |
R |
NA |
4 |
-46 |
-18 |
3.7 |
|
|
Vermis |
R |
NA |
4 |
-56 |
-2 |
2.4 |
|
|
Precentral gyrus |
L |
BA4 |
-58 |
8 |
28 |
102 |
3.4 |
|
Superior temporal gyrus |
L |
BA22 |
-46 |
-34 |
18 |
125 |
3 |
|
Inferior frontal gyrus |
R |
BA10 |
58 |
10 |
16 |
111 |
3 |
|
Cerebellum superior |
R |
NA |
36 |
-54 |
-30 |
30 |
3 |
|
Superior temporal gyrus |
L |
BA22 |
-48 |
0 |
4 |
50 |
2.7 |
|
Postcentral gyrus |
L |
BA3 |
-64 |
-26 |
16 |
29 |
2.4 |
|
Inferior parietal lobule |
R |
BA40 |
54 |
-32 |
30 |
26 |
2.6 |
|
Fist clutching-CMT group |
|||||||
|
Medial frontal gyrus |
L |
BA8 |
-2 |
-8 |
60 |
1798 |
7.5 |
|
Paracentral lobule |
L |
BA5 |
-4 |
-24 |
64 |
7.3 |
|
|
SMA |
L/R |
BA6 |
-6 |
-36 |
66 |
7.3 |
|
|
Cerebellum anterior lobe |
R |
NA |
4 |
-50 |
-8 |
172 |
5.4 |
|
Superior temporal gyrus |
L |
BA22 |
-46 |
-32 |
20 |
115 |
4.2 |
|
Postcentral gyrus |
R |
BA3 |
56 |
-20 |
22 |
110 |
4.1 |
|
Inferior frontal gyrus |
R |
BA10 |
58 |
8 |
22 |
9 |
3.7 |
|
Fist clutching-Control group |
|||||||
|
Medial frontal gyrus |
L |
BA8 |
-4 |
-8 |
60 |
852 |
6.5 |
|
Paracentral lobule |
L |
BA5 |
-4 |
-26 |
62 |
6 |
|
|
SMA |
L |
BA6 |
-10 |
-28 |
74 |
5 |
|
|
Cerebellum anterior lobe |
R |
NA |
4 |
-50 |
-10 |
130 |
5.4 |
|
Inferior frontal gyrus |
R |
BA10 |
58 |
8 |
20 |
93 |
5.3 |
|
Insula |
R |
BA13 |
34 |
18 |
12 |
37 |
4.2 |
|
Precentral gyrus |
R |
BA4 |
46 |
4 |
8 |
42 |
3.7 |
|
Precentral gyrus |
L |
BA4 |
-56 |
4 |
16 |
12 |
3.6 |
|
Precentral gyrus |
L |
BA4 |
-46 |
-6 |
12 |
14 |
3.6 |
|
NA: not available; Non-relevant signal in ventricles or near areas of sinus drainage was not interpreted. |
|||||||
unique cellular and synaptic flexibility in the adult brain [68], with evidence of activity-dependent reorganization in both healthy volunteers and neuropsychiatric conditions [69,70]. Indeed, a slight cognitive impairment has been described in CMT patients, predominantly involving executive functions, working memory, and verbal episodic memory [71], with minor depressive symptoms [71,72], which might prompt neural plasticity in the hippocampus as a putative mechanism of compensation.
Furthermore, based on the results of the whole brain analysis during the fist clutching movement, there were no differences in activation across four ROIs. While CMT disease is among the most common peripheral neuropathies, the symptoms of lower limbs are more serious than those of upper limbs in clinical work, as well the degree of deformity. The flexibility and mobility of upper limbs in many CMT patients are not significantly affected. De Salvo et al. [73] assessed the sensory and the nociceptive pathways of CMT patient by using Laser-Evoked Potentials (LEPs) recording associated to FMRI examination. Moreover, FMRI examination, by using laser stimuli which were applied at dorsum of feet and hands, was performed. They observed that the patient showed cortical activations, during fMRI laser stimuli of upper limbs, similar to Healthy Controls (HC). Instead, during stimulations to lower limbs, the cluster of activations was smaller respect to HC and a mild variation of cortical pain side was found. This result was consistent with lighter symptoms in the upper limbs than in the lower limbs of CMT patients. Our findings further confirm this clinical phenomenon. Although there were no statistically significant differences in the cortical activation between the two groups regarding the fist clutching movement, further studies of larger sample size, longer follow-up time, and more precise mission designs are needed to explore the cortical activation patterns of hand movements in CMT patients.
Another remarkable finding was the correlation between the motor-related cortex activity and the functional deficits. In our study we found an inverse correlation between the number of significant voxels in the primary motor area and SMA and the handgrip strength, considered as a measure of distal arm functional damage. A significant positive correlation was found between the CMTNS score and the activated voxels of SMA. In other words, this study revealed that there was a certain relationship between the peripheral nerve pathology and central nervous system reorganization. Distal limb weakness and atrophy is the main clinical manifestations in CMT patients [74]. Therefore, greater peripheral nerve pathology might lead to smaller handgrip strength and a greater compensatory neuroplasticity effort by the CNS, which modulates the effect of axonal degeneration on functional impairment. In this regard, the FMRI measurement can reflect the neural function and severity of symptoms of CMT patients. Hence, we speculate that brain function regulation may be involved in the pathogenesis of CMT disease, providing new insights into the mechanisms of CNS modifications associated to peripheral nerve pathology, and how these participate in the genesis of neurological dysfunction.
There were several limitations in this study. Firstly, the sample size was relatively small, reducing the power of test and probably making us miss undetected findings. Secondly, it was a cross-sectional study, preventing us from determining a casual interpretation. Thirdly, the ankle dorsiflexion-plantarflexion paradigm evaluated the cerebral activations only during the uniplanar and non-weight bearing ankle movement. Nevertheless, this paradigm is still the best option currently for this kind of fMRI studies because of its minimization of participant movements in the MRI scanner and resultant background noise [75]. In the study of lower extremity fMRI studies, especially when it comes to ankle movements, it is very important to use weight-bearing and multiplanar motions to simulate normal gait cycle in the future. In the following studies, the sample size will be enlarged and longitudinal investigations will be carried on. Moreover, the changes in task paradigms may correlate with more different brain functional outcomes, which would provide a more critical and comprehensive understanding to the CNS mechanism of CMT.
Conclusion
In conclusion, this study demonstrated the differences of cortical activation in CMT patients during ankle dorsiflexion- plantarflexion movement and its correlation with clinical severity. To the best of our knowledge, it is the first time to demonstrate statistically significant differences in cerebral activation between the CMT patients and healthy people by using task-state FMRI. These findings give further understanding of the potential mechanism in central nervous system underlying the peripheral nerve pathology in CMT patients, and lay the foundation for longitudinal research and further mechanism investigation. From a clinical perspective, interventions targeting the central nervous system changes are likely to be a new orientation in CMT management.
Ethics approval and consent to participate
The current study was conducted in accordance with the Declaration of Helsinki, and all study procedures were carried out with adequate understanding and written consent of the participants. Formal approval from the Huashan Hospital Institutional Review Board was obtained before study initiation.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' contributions
All authors were fully involved in the study and preparation of the manuscript. WZ contributed to the study design and preparation of manuscript. HC, YC, GX and SY contributed to the study design and data collection. MX, HJ, LH and WX contributed to statistical analysis.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (81772416), Science and Technology Commission of Shanghai Municipality Fund (17XD1401000 and 20ZR1409300). And no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- Krajewski KM, Lewis RA, Fuerst DR, et Neurological dysfunction and axonal degeneration in Charcot-Marie- Tooth disease type 1A. Brain 123 (2000): 1516-1527.
- Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol 8 (2009): 654-667.
- Bird TD, Ott J, Giblett ER, et Genetic linkage evidence for heterogeneity in Charcot-Marie-Tooth neuropathy (HMSN type I). Ann Neurol 14 (1983): 679-684.
- Skre Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet 6 (1974): 98-118.
- Cordeiro JL, Marques W, Hallak JE, et Charcot-Marie- Tooth disease, psychiatric indicators and quality of life: a systematic review. ASN Neuro 6 (2014): 185-192.
- Roberts-Clarke D, Fornusek C, Saigal N, et al. Relationship between physical performance and quality of life in Charcot-Marie-Tooth disease: a pilot study. J Peripher Nerv Syst 21 (2016): 357-364.
- Reilly MM, Murphy SM, Laurá M. Charcot-Marie-Tooth disease. J Peripher Nerv Syst 16 (2011): 1-14.
- Casasnovas C, Cano LM, Albertí A, et Charcot-Marie- Tooth disease. Foot Ankle Spec 1 (2008): 350-354.
- Harding AE, Thomas PK. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 103 (1980): 259-280.
- Szigeti K, Lupski Charcot-Marie-Tooth disease. Eur J Hum Genet 17 (2009): 703-710.
- Cornett KMD, Menezes MP, Shy RR, et al. Natural history of Charcot-Marie-Tooth disease during Ann Neurol 82 (2017): 353-359.
- Smith TW, Bhawan J, Keller RB, et al. Charcot-Marie- Tooth disease associated with hypertrophic neuropathy: a neuropathologic study of two cases. J Neuropathol Exp Neurol 39 (1980): 420-440.
- Paulson HL, Garbern JY, Hoban TF, et al. Transient central nervous system white matter abnormality in X-linked Charcot-Marie-Tooth disease. Ann Neurol 52 (2002): 429-434.
- Brockmann K, Dreha-Kulaczewski S, Dechent P, et al. Cerebral involvement in axonal charcot-marie-tooth neuropathy caused by mitofusin2 J Neurol 255 (2008): 1049-1058.
- Koros C, Evangelopoulos ME, Kilidireas C, et Central nervous system demyelination in a charcot-marie-tooth type 1A patient. Case Rep Neurol Med 2013 (2013): 243652.
- Daniel AGE, Carmen CR, Guillermo Charcot-marie- tooth disease type 1A and inflammatory-demyelinating lesions in the central nervous system. Int J Neurol Neurother 6 (2019): 080.
- McGrath MC. Charcot-Marie-Tooth 1A: A narrative review with clinical and anatomical perspectives. Clin Anat 29 (2016): 547-554.
- Ohsawa Y, Murakami T, Miyazaki Y, et al. Peripheral myelin protein 22 is expressed in human central nervous system. J Neurol Sci 247 (2006): 11-15.
- Chanson JB, Echaniz-Laguna A, Blanc F, et al. Central nervous system abnormalities in patients with PMP22 gene mutations: a prospective study. J Neurol Neurosurg Psychiatry 84 (2013): 392-397.
- Pontillo G, Dubbioso R, Cocozza S, et al. Brain plasticity in charcot-marie-tooth type 1A patients? A combined structural and diffusion MRI study. Front Neurol 11 (2020): 795.
- Li J, Zhang W, Wang X, et al. Functional magnetic resonance imaging reveals differences in brain activation in response to thermal stimuli in diabetic patients with and without diabetic peripheral PLoS One 13 (2018): e0190699.
- Selvarajah D, Wilkinson ID, Fang F, et al. Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal MRI study. Diabetes 68 (2019): 796-806.
- Rocca MA, Valsasina P, Fazio R, et Brain connectivity abnormalities extend beyond the sensorimotor network in peripheral neuropathy. Hum Brain Mapp 35 (2014): 513-526.
- Hsieh PC, Tseng MT, Chao CC, et al. Imaging signatures of altered brain responses in small-fiber neuropathy: reduced functional connectivity of the limbic system after peripheral nerve degeneration. Pain 156 (2015): 904-916.
- Pontillo G, Tozza S, Perillo T, et al. Diffuse brain connectivity changes in Charcot-Marie-Tooth type 1a patients: a resting-state functional magnetic resonance imaging study. Eur J Neurol 28 (2021): 305-313.
- Murphy SM, Herrmann DN, Mcdermott MP, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst 16 (2011): 191-198.
- Pareyson D, Reilly MM, Schenone A, et al. Ascorbic acid in Charcot–Marie–Tooth disease type 1A (CMT- TRIAAL and CMT-TRAUK): a double-blind randomised trial. Lancet Neurol 10 (2011):320-328.
- Ciccarelli O, Toosy AT, Marsden JF, et al. Identifying brain regions for integrative sensorimotor processing with ankle movements. Experimental Brain Research 166 (2005): 31-42.
- Ward NS, Brown MM, Thompson AJ, et al. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain 126 (2003): 1430-1448.
- Bird TD. Charcot–Marie–Tooth Hereditary Neuropathy Overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH et (eds). GeneReviews (R). Seattle (WA): University of Washington, Seattle (2022).
- van Paassen BW, van der Kooi AJ, van Spaendonck- Zwarts KY, et al. PMP22 related neuropathies: Charcot– Marie–Tooth disease type 1A and Hereditary Neuropathy with liability to Pressure Palsies. Orphanet J Rare Dis 9 (2014): 38.
- Paulson HL, Garbern JY, Hoban TF, et al. Transient central nervous system white matter abnormality in X-linked Charcot-Marie-Tooth disease. Ann Neurol 52 (2002): 429-434.
- Brockmann K, Dreha-Kulaczewski S, Dechent P, et al. Cerebral involvement in axonal charcot-marie-tooth neuropathy caused by mitofusin2 J Neurol 255 (2008): 1049-1058.
- Maeda Y, Kettner N, Sheehan J, et al. Altered brain morphometry in carpal tunnel syndrome is associated with median nerve pathology. Neuroimage Clin 2 (2013): 313-319.
- Selvarajah D, Wilkinson ID, Maxwell M, et al. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care 37 (2014): 1681-1688.
- Snipes GJ, Suter U, Welcher AA, et al. Characterization of a novel peripheral nervous system myelin protein (PMP-22/SR13). J Cell Biol 117 (1992): 225-238.
- Grooms D, Page S, Onate Brain Activation for Knee Movement Measured Days Before Second Anterior Cruciate Ligament Injury: Neuroimaging in Musculoskeletal Medicine. Journal of athletic training 50 (2015): 1005-1010.
- Jacobs JV, Horak F B. Cortical control of postural responses [J]. J Neural Transm (Vienna) 114 (2007): 1339-1348.
- Carlsen AN, Eagles JS, Mackinnon CD. Transcranial direct current stimulation over the supplementary motor area modulates the preparatory activation level in the human motor system [J]. Behav Brain Res 279 (2015): 68-75.
- Lopez C. A neuroscientific account of how vestibular disorders impair bodily self-consciousness [J]. Front Integr Neurosci 7 (2013):
- Fink A, Bay JU, Koschutnig K, et al. Brain and soccer: Functional patterns of brain activity during the generation of creative moves in real soccer decision-making situations [J]. Hum Brain Map 40 (2019): 755-764.
- Ventre-Dominey Vestibular function in the temporal and parietal cortex: distinct velocity and inertial processing pathways[J]. Front Integr Neurosci 8 (2014): 53.
- Balasubramanian K, Papadourakis V, Liang W, et Propagating Motor Cortical Dynamics Facilitate Movement Initiation. Neuron 106 (2020): 526-536.e4.
- Shibata S, Yamao Y, Kunieda T, et al, Intraoperative Electrophysiologic Mapping of Medial Frontal Motor Areas and Functional World neurosurgery 138 (2020): e389-e404.
- Capaday C. The special nature of human walking and its neural control. Trends in neurosciences 25 (2002): 370-376.
- Spedden M, Beck MM, Christensen MS, et al. Directed connectivity between primary and premotor areas underlying ankle force control in young and older NeuroImage 218 (2020): 116982.
- Terada M, Kosik KB, McCannc RS, et al. Gribbleb Corticospinal activity during a single-leg stance in people with chronic ankle instability. Journal of sport and health science 11 (2020): 58-66.
- Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. Journal of neurophysiology 80 (1998): 3247-3260.
- Bozkurt B, Yagmurlu K, Middlebrooks EH, et al. Microsurgical and Tractographic Anatomy of the Supplementary Motor Area Complex in Humans. World neurosurgery 95 (2016): 99-107.
- Hermans J, Beumer A, de Jong TAW, et Anatomy of the distal tibiofibular syndesmosis in adults: a pictorial essay with a multimodality approach. Journal of anatomy 217 (2010): 633-645.
- Ramsey P, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar J Bone Joint Surg Am 58 (1976): 356-357.
- Sahyoun C, Floyer-Lea A, Johansen-Berg H, et al. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot NeuroImage 21 (2004): 568-575.
- Johanson M, Allen JC, Matsumoto M, et Effect of heel lifts on plantarflexor and dorsiflexor activity during gait. Foot and ankle international 31(2010): 1014-1020.
- Drew T, Jiang W, Kably B, et Role of the motor cortex in the control of visually triggered gait modifications. Canadian Journal of Physiology and Pharmacology 74 (1996): 426-442.
- Drew T, Kalaska J, Krouchev Muscle synergies during locomotion in the cat: a model for motor cortex control. The Journal of physiology 586 (2008): 1239-1245.
- Beloozerova I, Sirota M. The role of the motor cortex in the control of vigour of locomotor movements in the The Journal of physiology 461 (1993): 27-46.
- Needle AR, Kaminski TW, Baumeister J, et al. The Relationship Between Joint Stiffness and Muscle Activity in Unstable Ankles and Copers [J]. J Sport Rehabil 26 (2017): 15-25.
- Kesar TM, Tan A, Eicholtz S, et al. Agonist-Antagonist Coactivation Enhances Corticomotor Excitability of Ankle Muscles [J]. Neural Plast 2019 (2019):
- Petersen N, Morita H, Nielsen Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man [J]. J Physiol 520 (1999): 605-619.
- Terada M, Kosik KB, Mccann RS, et al. Corticospinal activity during a single-leg stance in people with chronic ankle instability [J]. J Sport Health Sci (2020).
- Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability [J]. Clin Sports Med 27 (2008): 353-370.
- Willems T, Witvrouw E, Verstuyft J, et Proprioception and Muscle Strength in Subjects With a History of Ankle Sprains and Chronic Instability [J]. J Athl Train 37 (2002): 487-493.
- Wei P, Bao R, Lv Z, et Weak but Critical Links between Primary Somatosensory Centers and Motor Cortex during Movement. Frontiers in human neuroscience 12 (2018): 1.
- Beinert K, Mouthon A, Keller M, et al. Neural Correlates of Maladaptive Pain Behavior in Chronic Neck Pain - A Single Case Control fMRI Study. Pain physician 20 (2017): E115-E125.
- Karadimas S, Satkunendrarajah K, Laliberte AM, et al. Sensory cortical control of movement. Nature neuroscience 23 (2020): 75-84.
- Dai L, Zhou H, Xu X, et , Brain structural and functional changes in patients with major depressive disorder: a literature review. Peer J 7 (2019): e8170.
- Lai C. Task MRI-Based Functional Brain Network of Anxiety. Advances in experimental medicine and biology 1191 (2020): 3-20.
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15 (2012): 528-536.
- Arnone D, Mckie S, Elliott R, et al. Statedependent changes in hippocampal grey matter in depression. Mol Psychiatry 18 (2013): 1265-1272.
- Besteher B, Squarcina L, Spalthoff R, et al. Hippocampal volume as a putative marker of resilience or compensation to minor depressive symptoms in a nonclinical sample. Front Psychiatry 10 (2019):
- Chanson JB, Echaniz-Laguna A, Blanc F, et al. Central nervous system abnormalities in patients with PMP22 gene mutations: a prospective study. J Neurol Neurosurg Psychiatry 84 (2013): 392-397.
- Pfeiffer G, Wicklein EM, Ratusinski T, et al. Disability and quality of life in Charcot-Marie-Tooth disease type J Neurol Neurosurg Psychiatry 70 (2001): 548-550.
- De Salvo S, Bonanno L, Giorgianni R, et al. Functional MRI and laser-evoked potentials evaluation in Charcot- Marie-Tooth Neurol Sci 39 (2018): 1185-1189.
- Pareyson D. Differential diagnosis of Charcot-Marie- Tooth disease and related neuropathies. Neurol Sci 25 (2004): 72-82.
- Enzinger C, Johansen-Berg H, Dawes H, et Functional MRI correlates of lower limb function in stroke victims with gait impairment. Stroke 39 (2008): 1507-1513.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 